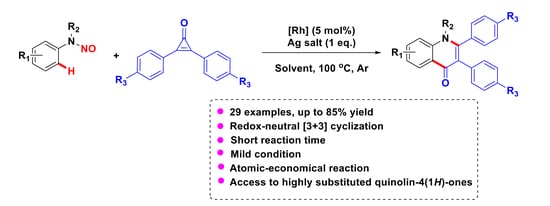
Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds
Abstract
:1. Introduction
2. Results and Discussions
3. Materials and Methods
3.1. General Information
3.2. General Procedures for Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones (3 and 4)
3.3. General Procedures for 1-Methyl-2,3-diphenylquinoline-4(1H)-thione (5)
3.4. General Procedures for 4-((2-Ethoxy-2-oxoethyl)thio)-1-methyl-2,3-diphenylquinolin-1-ium (6)
3.5. Analytical Characterization Data of Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carta, D.; Ferlin, M.G. An Overview on 2-arylquinolin-4(1H)-ones and Related Structures as Tubulin Polymerisation Inhibitors. Curr. Top. Med. Chem. 2014, 14, 2322–2345. [Google Scholar] [CrossRef] [PubMed]
- Hadida, S.; Van Goor, F.; Zhou, J.; Arumugam, V.; McCartney, J.; Hazlewood, A.; Decker, C.; Negulescu, P.; Grootenhuis, P.D. Discovery of N-(2,4-di-tert-butyl-5-hydroxyphenyl)-4-oxo-1,4-dihydroquinoline-3-carboxamide (VX-770, ivacaftor), a potent and orally bioavailable CFTR potentiator. J. Med. Chem. 2014, 57, 9776–9795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.P.; Gogliotti, R.D.; Domagala, J.M.; Gracheck, S.J.; Huband, M.D.; Sesnie, J.A.; Cohen, M.A.; Shapiro, M.A. The synthesis, structure-activity, and structure-side effect relationships of a series of 8-alkoxy- and 5-amino-8-alkoxyquinolone antibacterial agents. J. Med. Chem. 1995, 38, 4478–4487. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Motomura, T.; Aramaki, H.; Matsuda, T.; Yamashita, M.; Ito, Y.; Kawakami, H.; Matsuzaki, Y.; Watanabe, W.; Yamataka, K.; et al. Novel HIV-1 integrase inhibitors derived from quinolone antibiotics. J. Med. Chem. 2006, 49, 1506–1508. [Google Scholar] [CrossRef]
- Wentland, M.P.; Bailey, D.M.; Cornett, J.B.; Dobson, R.A.; Powles, R.G.; Wagner, R.B. Novel amino-substituted 3-quinolinecarboxylic acid antibacterial agents: Synthesis and structure-activity relationships. J. Med. Chem. 1984, 27, 1103–1108. [Google Scholar] [CrossRef]
- Xiao, Z.P.; Li, H.Q.; Shi, L.; Lv, P.C.; Song, Z.C.; Zhu, H.L. Synthesis, antiproliferative activity, and structure-activity relationships of 3-aryl-1H-quinolin-4-ones. ChemMedChem 2008, 3, 1077–1082. [Google Scholar] [CrossRef]
- Soural, M.; Hradil, P.; Krupkova, S.; Hlavac, J. An Interesting Synthetic Pathway to Some Quinolin-4(1H)ones: Phenacylanthranilates Rearrangement-Limits and Scopes. Mini-Rev. Org. Chem. 2012, 9, 426–432. [Google Scholar] [CrossRef]
- Coffman, K.C.; Palazzo, T.A.; Hartley, T.P.; Fettinger, J.C.; Tantillo, D.J.; Kurth, M.J. Heterocycle-heterocycle strategies: (2-nitrophenyl)isoxazole precursors to 4-aminoquinolines, 1H-indoles, and quinolin-4(1H)-ones. Org. Lett. 2013, 15, 2062–2065. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Park, S.; Kim, K.S.; Song, C.; Lee, Y. Copper-Catalyzed Aza-Michael Addition of 2-Aminobenzoate to beta-Substituted α,β-Unsaturated Ketones: One-Pot Synthesis of 3-Carbonyl-2-Substituted Quinolin-4(1H)-ones. J. Org. Chem. 2018, 83, 2694–2705. [Google Scholar] [CrossRef]
- Chen, J.R.; Hu, X.Q.; Lu, L.Q.; Xiao, W.J. Exploration of Visible-Light Photocatalysis in Heterocycle Synthesis and Functionalization: Reaction Design and Beyond. Acc. Chem. Res. 2016, 49, 1911–1923. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lokman Hossain, M.; Xiao, T. Synthesis of N-Containing Heterocyclic Compounds Using Visible-light Photoredox Catalysis. Chem. Rec. 2016, 16, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.O.; Banerjee, S.; Saswade, S.S.; Bedi, V.; Patil, N.T. Oxidant-free oxidative gold catalysis: The new paradigm in cross-coupling reactions. Chem. Commun. 2018, 54, 11069–11083. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Mo, J.; Wang, L.; Liu, Y. Transition-Metal-Catalyzed Direct C–H Functionalization under External-Oxidant-Free Conditions. Synthesis 2015, 47, 439–459. [Google Scholar] [CrossRef]
- Xu, P.; Li, W.; Xie, J.; Zhu, C. Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond. Acc. Chem. Res. 2018, 51, 484–495. [Google Scholar] [CrossRef]
- Li, D.D.; Cao, Y.X.; Wang, G.W. Palladium-catalyzed ortho-acyloxylation of N-nitrosoanilines via direct sp2 C-H bond activation. Org. Biomol. Chem. 2015, 13, 6958–6964. [Google Scholar] [CrossRef]
- Liu, Z.; Zeng, H.; Zhang, W.; Song, C.; Yang, F.; Liu, Y.; Zhu, J. Rh(III)-catalyzed N-nitroso-directed C-H olefination polymerization. Polymer 2019, 172, 152–159. [Google Scholar] [CrossRef]
- Peng, Q.; Hu, J.; Huo, J.; Yuan, H.; Xu, L.; Pan, X. Cp*Rh(iii) catalyzed ortho-halogenation of N-nitrosoanilines by solvent-controlled regioselective C-H functionalization. Org. Biomol. Chem. 2018, 16, 4471–4481. [Google Scholar] [CrossRef]
- Li, L.; Wang, G.-W. Solvent-free rhodium(III)-catalyzed synthesis of 2-aminoanilides via C−H amidation of N -nitrosoanilines under ball-milling conditions. Tetrahedron 2018, 74, 4188–4196. [Google Scholar] [CrossRef]
- Liu, B.; Fan, Y.; Gao, Y.; Sun, C.; Xu, C.; Zhu, J. Rhodium(III)-catalyzed N-nitroso-directed C-H olefination of arenes. High-yield, versatile coupling under mild conditions. J. Am. Chem. Soc. 2013, 135, 468–473. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, L.J.; Lu, X.; Kwong, F.Y.; Luo, H.B. Palladium-catalyzed oxidative C-H bond acylation of N-nitrosoanilines with toluene derivatives: A traceless approach to synthesize N-alkyl-2-aminobenzophenones. Chem. Commun. 2014, 50, 15352–15354. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Sun, L.; Chen, Y.; Zhou, Q.; Huang, J.W.; Miao, H.; Luo, H.B. Palladium-Catalyzed Decarboxylative Acylation of N-Nitrosoanilines with alpha-Oxocarboxylic Acids. J. Org. Chem. 2016, 81, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, X.; Shao, Y.; Xie, H.; Deng, Y.; Ke, Z.; Jiang, H.; Zeng, W. Co(III)-Catalyzed Coupling-Cyclization of Aryl C–H Bonds with α-Diazoketones Involving Wolff Rearrangement. ACS Catal. 2018, 8, 1308–1312. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, N. Cationic Cobalt(III) Catalyzed Indole Synthesis: The Regioselective Intermolecular Cyclization of N-Nitrosoanilines and Alkynes. Angew. Chem. Int. Ed. 2016, 55, 4035–4039. [Google Scholar] [CrossRef]
- Liu, B.; Song, C.; Sun, C.; Zhou, S.; Zhu, J. Rhodium(III)-catalyzed indole synthesis using N-N bond as an internal oxidant. J. Am. Chem. Soc. 2013, 135, 16625–16631. [Google Scholar] [CrossRef]
- Yu, S.; Li, X. Rhodium(III)-catalyzed C-C coupling of arenes with 2-vinyloxiranes: Synthesis of allylic alcohols. Org. Lett. 2014, 16, 1200–1203. [Google Scholar] [CrossRef]
- Zhao, D.; Shi, Z.; Glorius, F. Indole synthesis by rhodium(III)-catalyzed hydrazine-directed C-H activation: Redox-neutral and traceless by N-N bond cleavage. Angew. Chem. Int. Ed. 2013, 52, 12426–12429. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, J.; Zhang, F.; Song, C.; Zhu, J. A Versatile, Traceless C-H Activation-Based Approach for the Synthesis of Heterocycles. Org. Lett. 2016, 18, 2427–2430. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Chen, K.; Zha, S.; Song, C.; Zhu, J. C-H Activation-Based Traceless Synthesis via Electrophilic Removal of a Directing Group. Rhodium(III)-Catalyzed Entry into Indoles from N-Nitroso and alpha-Diazo-beta-keto Compounds. Org. Lett. 2016, 18, 1178–1181. [Google Scholar] [CrossRef]
- Song, X.; Gao, C.; Li, B.; Zhang, X.; Fan, X. Regioselective Synthesis of 2-Alkenylindoles and 2-Alkenylindole-3-carboxylates through the Cascade Reactions of N-Nitrosoanilines with Propargyl Alcohols. J. Org. Chem. 2018, 83, 8509–8521. [Google Scholar] [CrossRef]
- Fang, F.; Zhang, C.; Zhou, C.; Li, Y.; Zhou, Y.; Liu, H. Rh(III)-Catalyzed C-H Activation of Benzoylacetonitriles and Tandem Cyclization with Diazo Compounds to Substituted Benzo[ de]chromenes. Org. Lett. 2018, 20, 1720–1724. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, J.; Wu, X.; Zhou, Y.; Liu, H. Rh(III)-Catalyzed C-H Cyclization of Arylnitrones with Diazo Compounds: Access to 3-Carboxylate Substituted N-Hydroxyindoles. J. Org. Chem. 2017, 82, 8984–8994. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, J.; Fang, F.; Xu, B.; Liu, H.; Zhou, Y. Rhodium(III)-Catalyzed C-H Activation of alpha-Iminonitriles or alpha-Imino Esters and Cyclization with Acrylates to 2 H-Isoindoles. J. Org. Chem. 2018, 83, 11736–11746. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, B.; Zhou, S.; Zhou, Y.; Liu, H. Ruthenium-Catalyzed Redox-Neutral [4 + 1] Annulation of Benzamides and Propargyl Alcohols via C–H Bond Activation. ACS Catal. 2017, 7, 2494–2499. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, C.; Zhou, J.; He, G.; Liu, H.; Zhou, Y. Highly selective C–H bond activation of N-arylbenzimidamide and divergent couplings with diazophosphonate compounds: A catalyst-controlled selective synthetic strategy for 3-phosphorylindoles and 4-phosphorylisoquinolines. Org. Chem. Front. 2019, 6, 393–398. [Google Scholar] [CrossRef]
- Zhou, J.; Li, J.; Li, Y.; Wu, C.; He, G.; Yang, Q.; Zhou, Y.; Liu, H. Direct Synthesis of 3-Acylindoles through Rhodium(III)-Catalyzed Annulation of N-Phenylamidines with alpha-Cl Ketones. Org. Lett. 2018, 20, 7645–7649. [Google Scholar] [CrossRef]
- Kondo, T.; Taniguchi, R.; Kimura, Y. Ruthenium- and Rhodium-Catalyzed Ring-Opening Coupling Reactions of Cyclopropenones with Alkenes or Alkynes. Synlett 2018, 29, 717–722. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Zhou, X.; Xu, Y.; Li, X. Rhodium(III)-Catalyzed Acylation of C(sp3)-H Bonds with Cyclopropenones. Org. Lett. 2017, 19, 3644–3647. [Google Scholar] [CrossRef]
- Xie, F.; Yu, S.; Qi, Z.; Li, X. Nitrone Directing Groups in Rhodium(III)-Catalyzed C-H Activation of Arenes: 1,3-Dipoles versus Traceless Directing Groups. Angew. Chem. Int. Ed. 2016, 55, 15351–15355. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, Y.; Su, K.; Wang, P.; Guo, X.; Chen, B. Rhodium(iii)-catalyzed [3 + 3] annulation reactions of N-nitrosoanilines and cyclopropenones: An approach to functionalized 4-quinolones. Org. Chem. Front. 2019, 6, 3973. [Google Scholar] [CrossRef]
- Mochida, S.; Hirano, K.; Satoh, T.; Miura, M. Synthesis of Functionalized α-Pyrone and Butenolide Derivatives by Rhodium-Catalyzed Oxidative Coupling of Substituted Acrylic Acids with Alkynes and Alkenes. J. Org. Chem. 2009, 74, 6295–6298. [Google Scholar] [CrossRef]
- Tamura, Y.; Bayomi, S.M.; Tsunekawa, M.; Ikeda, M. Nucleophilic Reactions of N-Unsubstituted Sulfoximines with Activated Acetylenes, Olefins, and Diphenylcyclopropenone. Chem. Pharm. Bull. 1979, 27, 2137–2142. [Google Scholar] [CrossRef] [Green Version]
- Simmons, E.M.; Hartwig, J.F. On the interpretation of deuterium kinetic isotope effects in C-H bond functionalizations by transition-metal complexes. Angew. Chem. Int. Ed. 2012, 51, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zeng, J.; Li, Y.; Liu, X.-W. Polysubstituted pyrrole derivatives via 1,2-alkenyl migration of novel γ-amino-α,β-unsaturated aldehydes and α-diazocarbonyls. RSC Adv. 2014, 4, 7275–7278. [Google Scholar] [CrossRef]
- Li, X.; Han, C.; Yao, H.; Lin, A. Organocatalyzed [3 + 2] Annulation of Cyclopropenones and beta-Ketoesters: An Approach to Substituted Butenolides with a Quaternary Center. Org. Lett. 2017, 19, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Vanos, C.M.; Lambert, T.H. Development of a catalytic platform for nucleophilic substitution: Cyclopropenone-catalyzed chlorodehydration of alcohols. Angew. Chem. Int. Ed. 2011, 50, 12222–12226. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |








| Entry | [Rh] | [Ag] | Solvent | Yield (%) b |
|---|---|---|---|---|
| 1 | [Cp*RhCl2]2 | AgBF4 | DCE | 13 |
| 2 | Rh(PPh3)3Cl | AgBF4 | DCE | 0 |
| 3 | Rh(COD)2(BF4) | AgBF4 | DCE | 0 |
| 4 | / | AgBF4 | DCE | 0 |
| 5 c | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 6 d | [Cp*RhCl2]2 | AgBF4 | DCE | 72 (70) j |
| 7 d | [Cp*RhCl2]2 | AgSbF6 | DCE | 40 |
| 8 d | [Cp*RhCl2]2 | AgOTf | DCE | 23 |
| 9 d | [Cp*RhCl2]2 | / | DCE | 0 |
| 10 d | [Cp*RhCl2]2 | AgBF4 | THF | 69 |
| 11 d | [Cp*RhCl2]2 | AgBF4 | Acetone | 66 |
| 12 d,e | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 13 d,f | [Cp*RhCl2]2 | AgBF4 | DCE | 49 |
| 14 d,g | [Cp*RhCl2]2 | AgBF4 | DCE | 67 |
| 15 d,h | [Cp*RhCl2]2 | AgBF4 | DCE | 57 |
| 16 d,i | [Cp*RhCl2]2 | AgBF4 | DCE | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, J.; Dai, W.; Gao, F.; Chen, K.; Zhou, Y.; Liu, H. Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds. Molecules 2020, 25, 268. https://doi.org/10.3390/molecules25020268
Liu L, Li J, Dai W, Gao F, Chen K, Zhou Y, Liu H. Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds. Molecules. 2020; 25(2):268. https://doi.org/10.3390/molecules25020268
Chicago/Turabian StyleLiu, Lingjun, Jiyuan Li, Wenhao Dai, Feng Gao, Kaixian Chen, Yu Zhou, and Hong Liu. 2020. "Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds" Molecules 25, no. 2: 268. https://doi.org/10.3390/molecules25020268
APA StyleLiu, L., Li, J., Dai, W., Gao, F., Chen, K., Zhou, Y., & Liu, H. (2020). Rhodium(III)-Catalyzed Redox-Neutral [3+3] Annulation of N-nitrosoanilines with Cyclopropenones: A Traceless Approach to Quinolin-4(1H)-One Scaffolds. Molecules, 25(2), 268. https://doi.org/10.3390/molecules25020268