Effects of Oil Droplet Size and Interfacial Protein Film on the Properties of Fish Myofibrillar Protein–Oil Composite Gels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Droplet Size and Distribution of Emulsions
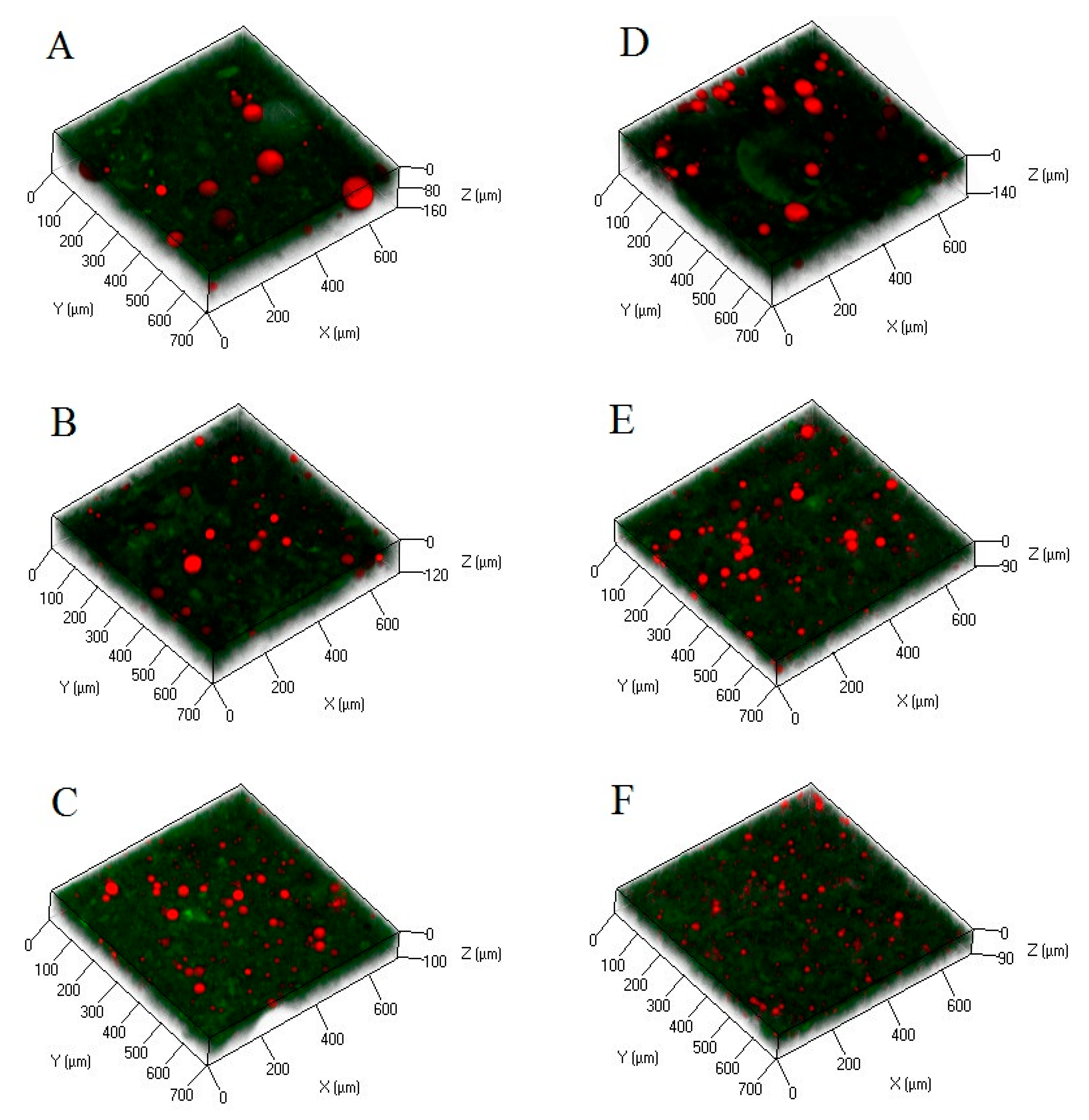
2.2. Microstructures of the Composite Gels
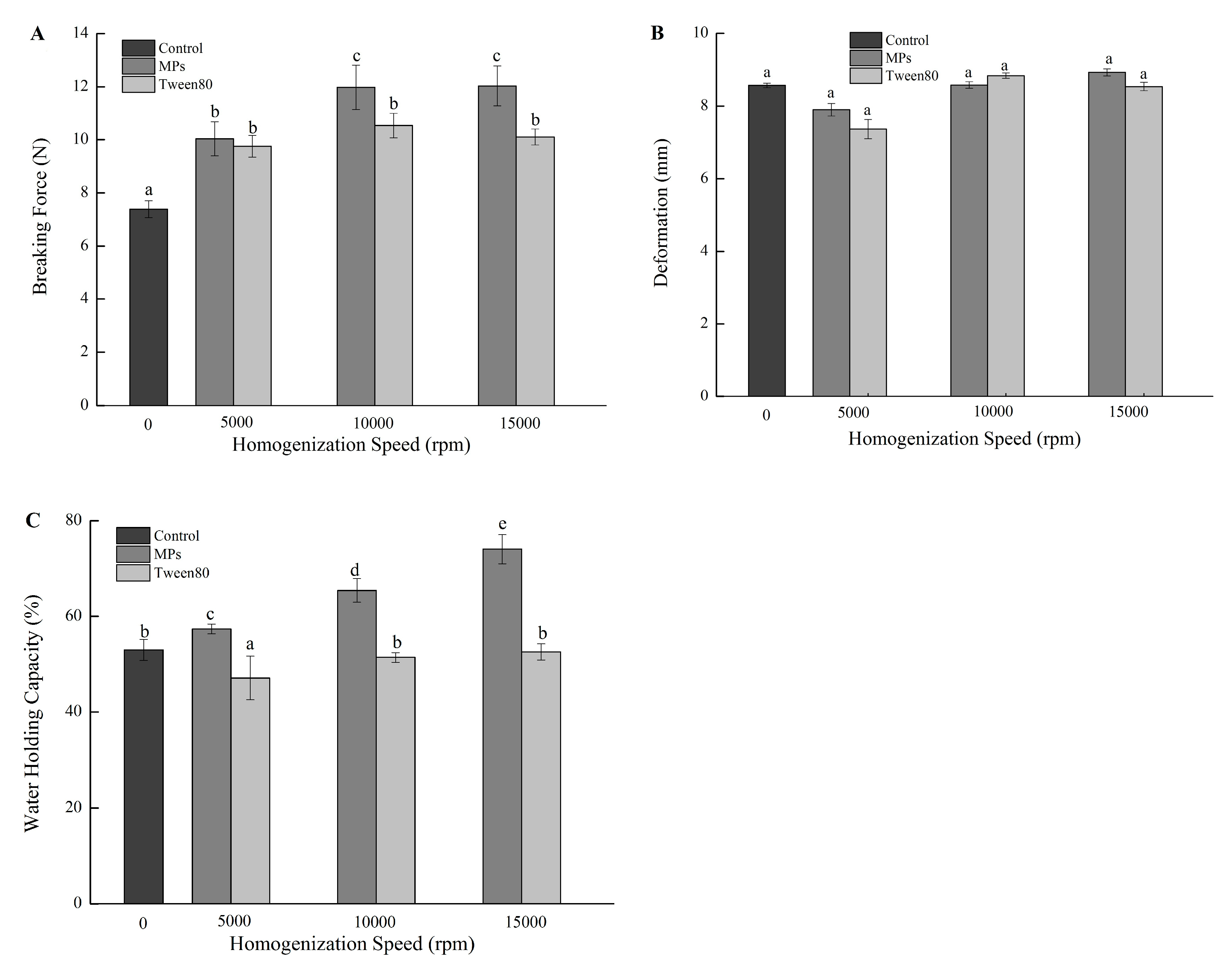
2.3. Effect of Oil Droplet Size and IPF on Textural Characteristics of Gels
2.4. Effect of Oil Droplet Size and IPF on the WHC of Gels
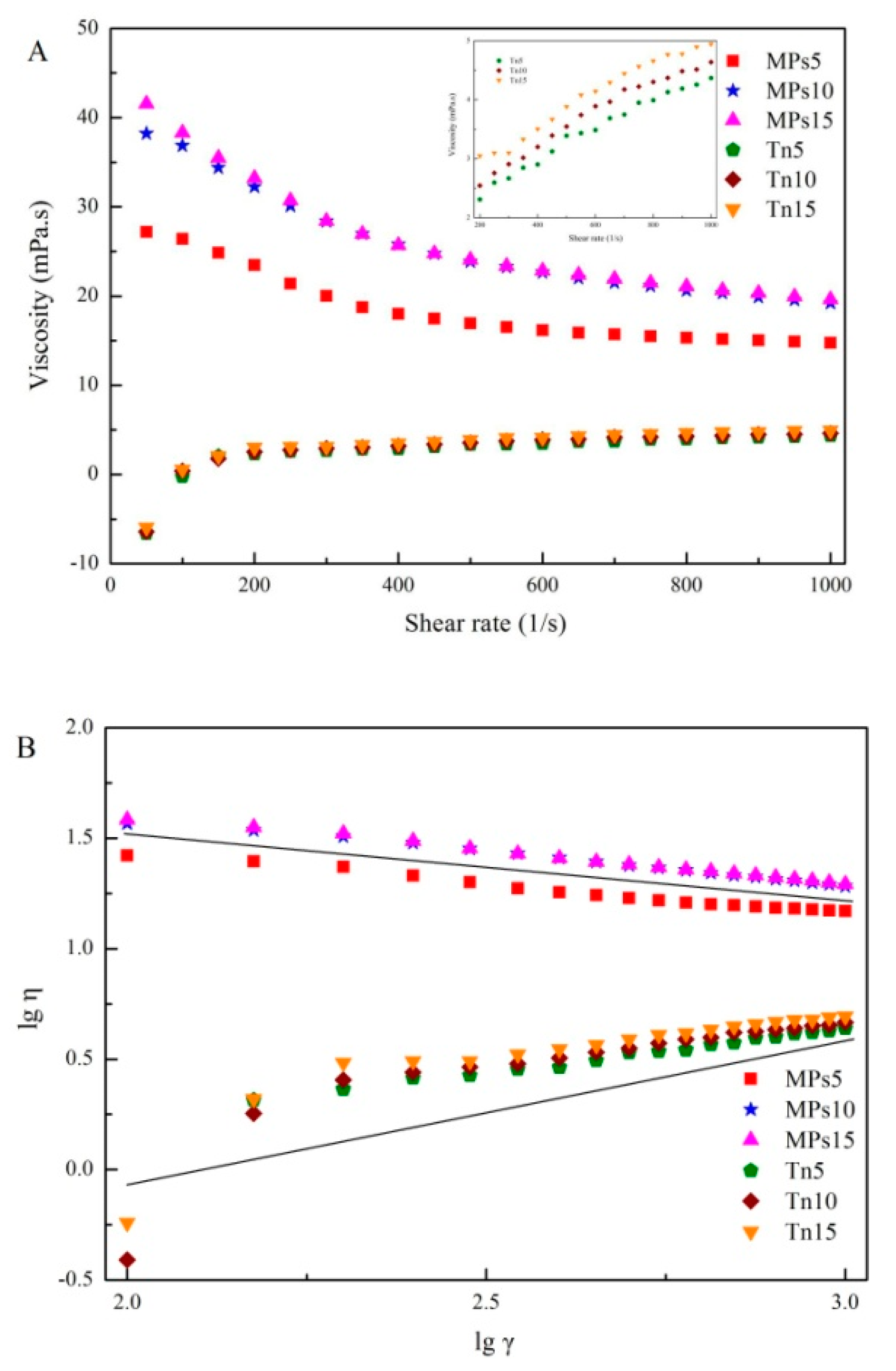
2.5. Effect of Oil Droplet Size and IPF on the Dynamic Rheological Behavior of Gels
2.6. Interfacial Shear Rheology of Gels
3. Materials and Methods
3.1. Materials
3.2. MP Preparation
3.3. Oil Emulsion Preparation
3.4. Composite Gel Preparation
3.5. Determination of Oil Droplet Size
3.6. Texture Analysis
3.7. WHC Measurements
3.8. Dynamic Rheological Measurements
3.9. Interfacial Shear Rheological Measurements
3.10. Confocal Laser Scanning Microscopy (CLSM)
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xiong, Y.L. Muscle Proteins. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing: London, UK, 2004; Volume 5, pp. 100–118. [Google Scholar]
- Zhao, Y.; Wang, P.; Zou, Y.; Li, K.; Kang, Z.; Xu, X.; Zhou, G. Effect of pre-emulsification of plant lipid treated by pulsed ultrasound on the functional properties of chicken breast myofibrillar protein composite gel. Food Res. Int. 2014, 58, 98–104. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Role of disulphide linkages between protein-coated lipid droplets and the protein matrix in the rheological properties of porcine myofibrillar protein-peanut oil emulsion composite gels. Meat Sci. 2011, 88, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.D.; Holley, R.A. Factors influencing gel formation by myofibrillar proteins in muscle foods. Compr. Rev. Food Sci. Food Saf. 2011, 10, 33–51. [Google Scholar] [CrossRef]
- Gani, A.; Benjakul, S. Impact of virgin coconut oil nanoemulsion on properties of croaker surimi gel. Food Hydrocoll. 2018, 82, 34–44. [Google Scholar] [CrossRef]
- Pramualkijja, T.; Pirak, T.; Kerdsup, P. Effect of salt, rice bran oil and malva nut gum on chemical, physical and physico-chemical properties of beef salt-soluble protein and its application in low fat salami. Food Hydrocoll. 2016, 53, 303–310. [Google Scholar] [CrossRef]
- Sun, F.; Huang, Q.; Hua, T.; Xiong, S.; Zhao, S. Effects and mechanism of modified starches on the gel properties of myofibrillar protein from grass carp. Int. J. Biol. Macromol. 2014, 64, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.; Barbut, S. Effect of chloride salts on protein extraction and interfacial protein film formation in meat batters. J. Sci. Food Agric. 1992, 58, 227–238. [Google Scholar] [CrossRef]
- Sala, G.; van Vliet, T.; Stuart, M.C.; van de Velde, F.; van Aken, G.A. Deformation and fracture of emulsion-filled gels: Effect of gelling agent concentration and oil droplet size. Food Hydrocoll. 2009, 23, 1853–1863. [Google Scholar] [CrossRef]
- Wu, M.; Xiong, Y.L.; Chen, J. Rheology and microstructure of myofibrillar protein-plant lipid composite gels: Effect of emulsion droplet size and membrane type. J. Food Eng. 2011, 106, 318–324. [Google Scholar] [CrossRef]
- Ben-Harb, S.; Panouill, M.; Huc-Mathis, D.; Moulin, G.; Saint-Eve, A.; Irlinger, F.; Bonnarme, P.; Michon, C.; Souchon, I. The rheological and microstructural properties of pea, milk, mixed pea/milk gels and gelled emulsions designed by thermal, acid, and enzyme treatments. Food Hydrocoll. 2018, 77, 75–84. [Google Scholar] [CrossRef]
- Abhyankar, A.R.; Mulvihill, D.M.; Auty, M.A.E. Combined microscopic and dynamic rheological methods for studying the structural breakdown properties of whey protein gels and emulsion filled gels. Food Hydrocoll. 2011, 25, 275–282. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, H.; Lyu, F.; Lin, H.; Zhang, Q.; Ding, Y. Physicochemical properties and microstructure of fish myofibrillar proteinlipid composite gels: Effects of fat type and concentration. Food Hydrocoll. 2019, 90, 433–442. [Google Scholar] [CrossRef]
- Joseph, D.; Lee, H.; Huh, Y.S.; Han, Y.K. Cylindrical core-shell tween 80 micelle templated green synthesis of gold-silver hollow cubic nanostructures as efficient nanocatalysts. Mater. Des. 2018, 160, 169–178. [Google Scholar] [CrossRef]
- Liu, K.; Stieger, M.; van der Linden, E.; van de Velde, F. Fat droplet characteristics affect rheological, tribological and sensory properties of food gels. Food Hydrocoll. 2015, 44, 244–259. [Google Scholar] [CrossRef]
- Kaler, E.W.; Prager, S. A model of dynamic scattering by microemulsions. J. Colloid Interface Sci. 1982, 86, 359–369. [Google Scholar] [CrossRef]
- Gülseren, I.; Corredig, M. Interactions at the interface between hydrophobic and hydrophilic emulsifiers: Polyglycerol polyricinoleate (PGPR) and milk proteins, studied by drop shape tensiometry. Food Hydrocoll. 2012, 29, 193–198. [Google Scholar] [CrossRef]
- Oppermann, A.K.L.; Noppers, J.M.E.; Stieger, M.; Scholten, E. Effect of outer water phase composition on oil droplet size and yield of (w1/o/w2) double emulsions. Food Res. Int. 2018, 107, 148–157. [Google Scholar] [CrossRef]
- Robins, M.M.; Watson, A.D.; Wilde, P.J. Emulsions-creaming and rheology. Curr. Opin. Colloid Interface Sci. 2002, 7, 419–425. [Google Scholar] [CrossRef]
- Wilde, P.; Mackie, A.; Husband, F.; Gunning, P.; Morris, V. Proteins and emulsifiers at liquid interfaces. Adv. Colloid Interface Sci. 2004, 108–109, 63–71. [Google Scholar] [CrossRef]
- Zhuang, X.; Jiang, X.; Zhou, H.; Han, M.; Liu, Y.; Bai, Y.; Xu, X.; Zhou, G. The effect of insoluble dietary fiber on myofibrillar protein emulsion gels: Oil particle size and protein network microstructure. LWT-Food Sci. Technol. 2019, 101, 534–542. [Google Scholar] [CrossRef]
- Chen, J.; Dickinson, E. Effect of surface character of filler particles on rheology of heat-set whey protein emulsion gels. Colloid Surf. B 1999, 12, 373–381. [Google Scholar] [CrossRef]
- Wiedenmann, V.; Oehlke, K.; van der Schaaf, U.; Hetzer, B.; Greiner, R.; Karbstein, H.P. Impact of the incorporation of solid lipid nanoparticles on β-lactoglobulin gel matrices. Food Hydrocoll. 2018, 84, 498–507. [Google Scholar] [CrossRef]
- Chojnicka, A.; Sala, G.; de Kruif, C.G.; de Velde, F. The interactions between oil droplets and gel matrix affect the lubrication properties of sheared emulsion-filled gels. Food Hydrocoll. 2009, 23, 1038–1046. [Google Scholar] [CrossRef]
- Zhou, X.; Jiang, S.; Zhao, D.; Zhang, J.; Gu, S.; Pan, P.; Ding, Y. Changes in physicochemical properties and protein structure of surimi enhanced with camellia tea oil. LWT-Food Sci. Technol. 2017, 84, 562–571. [Google Scholar] [CrossRef]
- Sánchez-González, I.; Rodríguez-Casado, A.; Careche, M.; Carmona, P. Raman analysis of surimi gelation by addition of wheat dietary fibre. Food Chem. 2009, 112, 162–168. [Google Scholar] [CrossRef]
- Kirimlidou, M.; Matsakidou, A.; Scholten, E.; Nikiforidis, C.V.; Kiosseoglou, V. Composite gels structured by a gelatin protein matrix filled with oil bodies. Food Struct. 2017, 14, 46–51. [Google Scholar] [CrossRef]
- Ching, S.H.; Bansal, N.; Bhandari, B. Rheology of emulsion-filled alginate microgel suspensions. Food Res. Int. 2016, 80, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.F.; Dol, S.S.; Wee, S.K.; & Chua, H.B. Miri light crude water-in-oil emulsions characterization-Rheological behaviour, stability and amount of emulsions formed. J. Petrol. Sci. Eng. 2018, 165, 58–66. [Google Scholar] [CrossRef]
- Meng, W.; Wul, L.; Chen, D.; Zhong, A. Ambient self-crosslinkable acrylic microemulsion in the presence of reactive surfactants. Iran. Polym. J. 2008, 17, 555–564. [Google Scholar]
- Zhang, Y.; Zhang, Q.; Lu, J.; Xu, J.; Zhang, H.; Wang, J. Physicochemical properties of Tremella fuciformis polysaccharide and its interactions with myofibrillar protein. Bioact. Carbohydr. Diet. Fibre 2017, 11, 18–25. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.R.; Cunha, R.L. Impact of oil type and WPI/Tween 80 ratio at the oil-water interface: Adsorption, interfacial rheology and emulsion features. Colloid Surf. B 2018, 164, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Chronakis, I.S.; Piculell, L.; Borgstrom, J. Rheology of kappa-carrageenan in mixtures of sodium and cesium iodide: Two types of gels. Carbohydr. Polym. 1996, 31, 215–225. [Google Scholar] [CrossRef]
- Torres, O.; Murray, B.; Sarkar, A. Emulsion microgel particles: Novel encapsulation strategy for lipophilic molecules. Trends Food Sci. Technol. 2016, 55, 98–108. [Google Scholar] [CrossRef]
- Amiri, A.; Sharifian, P.; Soltanizadeh, N. Application of ultrasound treatment for improving the physicochemical, functional and rheological properties of myofibrillar proteins. Int. J. Biol. Macromol. 2018, 111, 139–147. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Index | MPs5 | MPs10 | MPs15 | Tn5 | Tn10 | Tn15 |
|---|---|---|---|---|---|---|
| DS (nm) | 683.60 ± 37.26 e | 474.50 ± 8.30 d | 265.61 ± 10.15 c | 165.02 ± 38.15 b | 15.79 ± 0.75 a | 12.36 ± 1.25 a |
| PDI | 17.14 ± 2.78 c | 5.31 ± 0.63 b | 0.29 ± 0.02 a | 1.46 ± 0.51 a | 0.07 ± 0.01 a | 0.17 ± 0.02 a |
| K | 95.06 ± 5.62 b | 167.9 ± 10.44 c | 173.3 ± 9.35 c | 0.25 ± 0.002 a | 0.33 ± 0.01 a | 0.28 ± 0.002 a |
| n | 0.73 ± 0.03 a | 0.70 ± 0.01 a | 0.71 ± 0.05 a | 1.50 ± 0.02 b | 1.62 ± 0.03 c | 1.68 ± 0.02 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Chen, H.; Zhang, Q.; Lyu, F.; Ding, Y.; Zhou, X. Effects of Oil Droplet Size and Interfacial Protein Film on the Properties of Fish Myofibrillar Protein–Oil Composite Gels. Molecules 2020, 25, 289. https://doi.org/10.3390/molecules25020289
Xu X, Chen H, Zhang Q, Lyu F, Ding Y, Zhou X. Effects of Oil Droplet Size and Interfacial Protein Film on the Properties of Fish Myofibrillar Protein–Oil Composite Gels. Molecules. 2020; 25(2):289. https://doi.org/10.3390/molecules25020289
Chicago/Turabian StyleXu, Xia, Hong Chen, Qi Zhang, Fei Lyu, Yuting Ding, and Xuxia Zhou. 2020. "Effects of Oil Droplet Size and Interfacial Protein Film on the Properties of Fish Myofibrillar Protein–Oil Composite Gels" Molecules 25, no. 2: 289. https://doi.org/10.3390/molecules25020289




