Continuous Flow Photochemistry for the Preparation of Bioactive Molecules
Abstract
:1. Introduction
2. Reactor Design and Technology
3. Greener and More Sustainable Approaches
4. Synthesis of Bioactives: Drugs and Natural Products
5. Remaining Challenges for Effectively Integrating Photoreactions within Multistep Sequences
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bach, T.; Hehn, J.P. Photochemical Reactions as Key Steps in Natural Product Synthesis. Angew. Chem. Int. Ed. 2011, 50, 1000–1045. [Google Scholar] [CrossRef]
- Hoffmann, N. Photochemical Reactions as Key Steps in Organic Synthesis. Chem. Rev. 2008, 108, 1052–1103. [Google Scholar] [CrossRef] [PubMed]
- Kärkäs, M.D.; Porco, J.A., Jr.; Stephenson, C.R.J. Photochemical Approaches to Complex Chemotypes: Applications in Natural Product Synthesis. Chem. Rev. 2016, 116, 9683–9747. [Google Scholar]
- Ravelli, D.; Protti, S.; Fagnoni, M. Carbon-Carbon Bond Forming Reactions via Photogenerated Intermediates. Chem. Rev. 2016, 116, 9850–9913. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.A.; Nicewicz, D.A. Organic Photoredox Catalysis. Chem. Rev. 2016, 116, 10075–10166. [Google Scholar] [CrossRef] [PubMed]
- Skubi, K.L.; Blum, T.R.; Yoon, T.P. Dual Catalysis Strategies in Photochemical Synthesis. Chem. Rev. 2016, 116, 10035–10074. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-Flow Technology—A Tool for the Safe Manufacturing of Active Pharmaceutical Ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6729. [Google Scholar] [CrossRef]
- Movsisyan, M.; Delbeke, E.I.P.; Berton, J.K.E.T.; Battilocchio, C.; Ley, S.V.; Stevens, C.V. Taming hazardous chemistry by continuous flow technology. Chem. Soc. Rev. 2016, 45, 4892–4928. [Google Scholar] [CrossRef]
- Baumann, M.; Baxendale, I.R. The synthesis of active pharmaceutical ingredients (APIs) using continuous flow chemistry. Beilstein J. Org. Chem. 2015, 11, 1194–1219. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.F. Flow chemistry—Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Fitzpatrick, D.E.; Ley, S.V. Engineering chemistry for the future of organic synthesis. Tetrahedron 2018, 74, 3087–3100. [Google Scholar] [CrossRef] [Green Version]
- Baumann, M. Integrating continuous flow synthesis with in-line analysis and data generation. Org. Biomol. Chem. 2018, 16, 5946–5954. [Google Scholar] [CrossRef] [PubMed]
- Cambie, D.; Bottecchia, C.; Straathof, N.J.W.; Hessel, V.; Noël, T. Applications of Continuous-Flow Photochemistry in Organic Synthesis, Material Science, and Water Treatment. Chem. Rev. 2016, 116, 10276–10341. [Google Scholar] [CrossRef] [PubMed]
- Sambiago, C.; Noël, T. Flow Photochemistry: Shine Some Light on Those Tubes! Trends Chem. 2019. [Google Scholar] [CrossRef] [Green Version]
- Knowles, J.P.; Elliott, L.D.; Booker-Milburn, K.I. Flow photochemistry: Old light through new windows. Beilstein J. Org. Chem. 2012, 8, 2025–2052. [Google Scholar] [CrossRef] [Green Version]
- Elliott, L.D.; Knowles, J.P.; Koovits, P.J.; Maskil, K.G.; Ralph, M.J.; Lejeune, G.; Edwards, L.J.; Robinson, R.I.; Clemens, I.R.; Cox, B.; et al. Batch versus Flow Photochemistry: A Revealing Comparison of Yield and Productivity. Chem. Eur. J. 2014, 20, 15226–15232. [Google Scholar] [CrossRef] [Green Version]
- Politano, F.; Oksdath-Mansilla, G. Light on the Horizon: Current Research and Future Perspectives in Flow Photochemistry. Org. Process Res. Dev. 2018, 22, 1045–1062. [Google Scholar] [CrossRef] [Green Version]
- Staveness, D.; Bosque, I.; Stephenson, C.R.J. Free Radical Chemistry Enabled by Visible Light-Induced Electron Transfer. Acc. Chem. Res. 2016, 49, 2295–2306. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Organ, M.G. Flow Chemistry as a Drug Discovery Tool: A Medicinal Chemistry Perspective. In Flow Chemistry for the Synthesis of Heterocycles; Sharma, U., Van der Eycken, E., Eds.; Springer: Cham, Switzerland, 2018; p. 56. [Google Scholar]
- Rehm, T.H. Reactor technology concepts for flow photochemistry. Chem. Photo. Chem. 2019. [Google Scholar] [CrossRef]
- Shvydkiv, O.; Jähnisch, K.; Steinfeldt, N.; Yavorskyy, A.; Oelgemöller, M. Visible-light photooxygenation of α-terpinene in a falling film microreactor. Catal. Today 2018, 308, 102–118. [Google Scholar] [CrossRef]
- Elgue, S.; Aillet, T.; Loubiere, K.; Conté, A.; Dechy-Cabaret, O.; Prat, L.E.; Horn, C.R.; Lobet, O.; Vallon, S. Flow photochemistry: A meso-scale reactor for industrial applications. Chimica. Oggi. 2015, 33, 58–62. [Google Scholar]
- Emmanuel, N.; Mendoza, C.; Winter, M.; Horn, C.R.; Vizza, A.; Dreesen, L.; Heinrichs, B.; Monbaliu, J.-C.M. Scalable Photocatalytic Oxidation of Methionine under Continuous-Flow Conditions. Org. Process Res. Dev. 2017, 21, 1435–1438. [Google Scholar] [CrossRef]
- Al-Rawashdeh, M.; Hessel, V.; Löb, P.; Mevissen, K.; Schönfeld, F. Pseudo 3-D simulation of a falling film microreactor based on realistic channel and film profiles. Chem. Eng. Sci. 2008, 63, 5149–5159. [Google Scholar] [CrossRef]
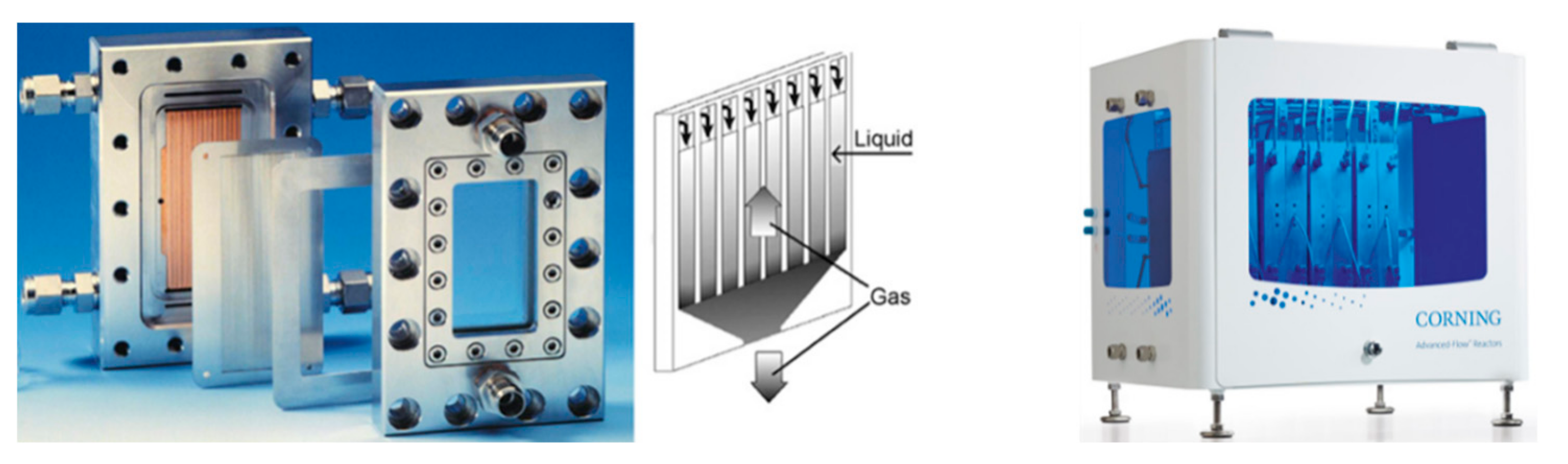
- CORNING. Available online: https://www.corning.com/emea/en/innovation/corning-emerging-innovations/advanced-flow-reactors.html (accessed on 18 December 2019).
- Ioannou, G.I.; Montagnon, T.; Kalaitzakis, D.; Pergantis, S.A.; Vassilikogiannakis, G. A Novel Nebulizer-Based Continuous Flow Reactor: Introducing the Use of Pneumatically Generated Aerosols for Highly Productive Photooxidations. ChemPhotoChem 2017, 1, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.A.; Lee, D.S.; Pickering, S.J.; Poliakoff, M.; George, M.W. A Simple and Versatile Reactor for Photochemistry. Org. Process Res. Dev. 2016, 20, 1792–1798. [Google Scholar] [CrossRef]
- Gandy, M.N.; Raston, C.L.; Stubbs, K.A. Photoredox catalysis under shear using thin film vortex microfluidics. Chem. Commun. 2015, 51, 11041–11044. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.; Stubbs, K.A.; Weiss, G.A.; Raston, C.L. Vortex fluidic chemical transformations. Chem. Eur. J. 2017, 23, 13270–13278. [Google Scholar] [CrossRef]
- Elliott, L.D.; Berry, M.; Harji, B.; Klauber, D.; Leonard, J.; Booker-Milburn, K.I. A small-footprint, high-capacity flow reactor for UV photochemical synthesis on the kilogram scale. Org. Process Res. Dev. 2016, 20, 1806–1811. [Google Scholar] [CrossRef]
- Chen, Y.; Cantillo, D.; Kappe, C.O. Visible Light-Promoted Beckmann Rearrangements: Separating Sequential Photochemical and Thermal Phenomena in a Continuous Flow Reactor. Eur. J. Org. Chem. 2019, 2019, 2163–2171. [Google Scholar] [CrossRef]
- Grainger, R.; Heightman, T.D.; Ley, S.V.; Lima, F.; Johnson, C.N. Enabling synthesis in fragment-based drug discovery by reactivity mapping: Photoredox-mediated cross-dehydrogenative heteroarylation of cyclic amines. Chem. Sci. 2019, 10, 2264–2271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcoran, E.B.; Levesque, F.; McMullen, J.P.; Naber, J.R. Studies Toward the Scaling of Gas-Liquid Photocycloadditions. ChemPhotoChem 2018, 2, 931–937. [Google Scholar] [CrossRef]
- Lima, F.; Grunenberg, L.; Rahman, H.B.A.; Labes, R.; Sedelmeier, J.; Ley, S.V. Organic photocatalysis for the radical couplings of boronic acid derivatives in batch and flow. Chem. Commun. 2018, 54, 5606–5609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; May, O.; Blakemore, D.C.; Ley, S.V. A Photoredox Coupling Reaction of Benzylboronic Esters and Carbonyl Compounds in Batch and Flow. Org. Lett. 2019, 21, 6140–6144. [Google Scholar] [CrossRef] [PubMed]
- Ley, S.V. On being green: Can flow chemistry help? Chem. Rec. 2012, 12, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Dallinger, D.; Kappe, C.O. Why flow means green—Evaluating the merits of continuous processing in the context of sustainability. Curr. Opin. Green Sust. Chem. 2017, 7, 6–12. [Google Scholar] [CrossRef]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Lummis, J.A.M.; Morse, P.D.; Beingessner, R.L.; Jamison, T.F. Towards more efficient, greener syntheses through flow chemistry. Chem. Rec. 2017, 7, 667–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verschueren, R.H.; De Borggraeve, W.M. Electrochemistry and photoredox catalysis: A comparative evaluation in organic synthesis. Molecules 2019, 24, 2122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuyama, T.; Hino, Y.; Kamata, N.; Ryu, I. Quick execution of [2+2] type photochemical cycloaddition reaction by continuous flow system using a glass-made microreactor. Chem. Lett. 2004, 33, 1430–1431. [Google Scholar] [CrossRef]
- Cambie, D.; Noël, T. Solar Photochemistry in flow. Top. Curr. Chem. 2018, 376, 45. [Google Scholar] [CrossRef] [Green Version]
- Cambie, D.; Dobbelaar, J.; Riente, P.; Vanderspikken, J.; Shen, C.; Seeberger, P.H.; Gilmore, K.; Debije, M.G.; Noël, T. Energy-efficient solar photochemistry with luminescent solar concentrator based photomicroreactors. Angew. Chem. Int. Ed. 2019, 58, 14374–14378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oelgemöller, M. Solar photochemical synthesis: From the beginnings of organic photochemistry to the solar manufacturing of commodity chemicals. Chem. Rev. 2016, 116, 9664–9682. [Google Scholar] [CrossRef] [PubMed]
- Roth, H.D. The beginnings of organic photochemistry. Angew. Chem. Int. Ed. 1989, 28, 1193–1207. [Google Scholar] [CrossRef]
- Roth, H.D. Twentieth century developments in photochemistry. Brief historical sketches. Pure Appl. Chem. 2001, 73, 395–403. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Cambie, D.; Janse, J.; Wieland, E.W.; Kuijpers, K.P.L.; Hessel, V.; Debije, M.G.; Noël, T. Scale-up of a luminescent solar concentrator-based photomicroreactor via numbering-up. ACS Sustain. Chem. Eng. 2018, 6, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Pieber, B.; Shalom, M.; Antonietti, M.; Seeberger, P.H.; Gilmore, K. Continuous heterogeneous photocatalysis in serial micro-batch reactors. Angew. Chem. Int. Ed. 2018, 57, 9976–9979. [Google Scholar] [CrossRef]
- Levesque, F.; Seeberger, P.H. Continuous-flow synthesis of the anti-malaria drug artemisinin. Angew. Chem. Int. Ed. 2012, 51, 1706–1709. [Google Scholar] [CrossRef]
- Gilmore, K.; Kopetzki, D.; Lee, J.W.; Horvath, Z.; McQuade, D.T.; Seidel-Morgenstern, A.; Seeberger, P.H. Continuous synthesis of artemisinin-derived medicines. Chem. Commun. 2014, 50, 12652–12655. [Google Scholar] [CrossRef] [Green Version]
- Fuse, S.; Tanabe, N.; Yoshida, M.; Yoshida, H.; Doi, T.; Takahashi, T. Continuous-flow synthesis of vitamin D3. Chem. Commun. 2010, 46, 8722–8724. [Google Scholar] [CrossRef]
- Fuse, S.; Mifune, Y.; Tanabe, N.; Takahashi, T. Continuous-flow synthesis of activated vitamin D3 and its analogues. Org. Biomol. Chem. 2012, 10, 5205–5211. [Google Scholar] [CrossRef] [PubMed]
- Escriba-Gelonch, M.; Noël, T.; Hessel, V. Microflow high-p,T intensification of vitamin D3 synthesis using an ultraviolet lamp. Org. Process Res. Dev. 2018, 22, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lainchbury, M.D.; Medley, M.I.; Taylor, P.M.; Hirst, P.; Dohle, W.; Booker-Milburn, K.I. A Protecting Group Free Total Synthesis of (±)-Neostenine via the [5+2] Photocycloaddition of Maleimides. J. Org. Chem. 2009, 73, 6497–6505. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M.; Ng, S.; Booker-Milburn, K.I. Short flow-photochemistry enabled synthesis of the cytotoxic lactone (+)-goniofufurone. Org. Lett. 2016, 18, 968–971. [Google Scholar] [CrossRef] [PubMed]
- Baumann, M.; Baxendale, I.R. Continuous photochemistry: The flow synthesis of ibuprofen via a photo-Favorskii rearrangement. React. Chem. Eng. 2016, 1, 147–150. [Google Scholar] [CrossRef] [Green Version]
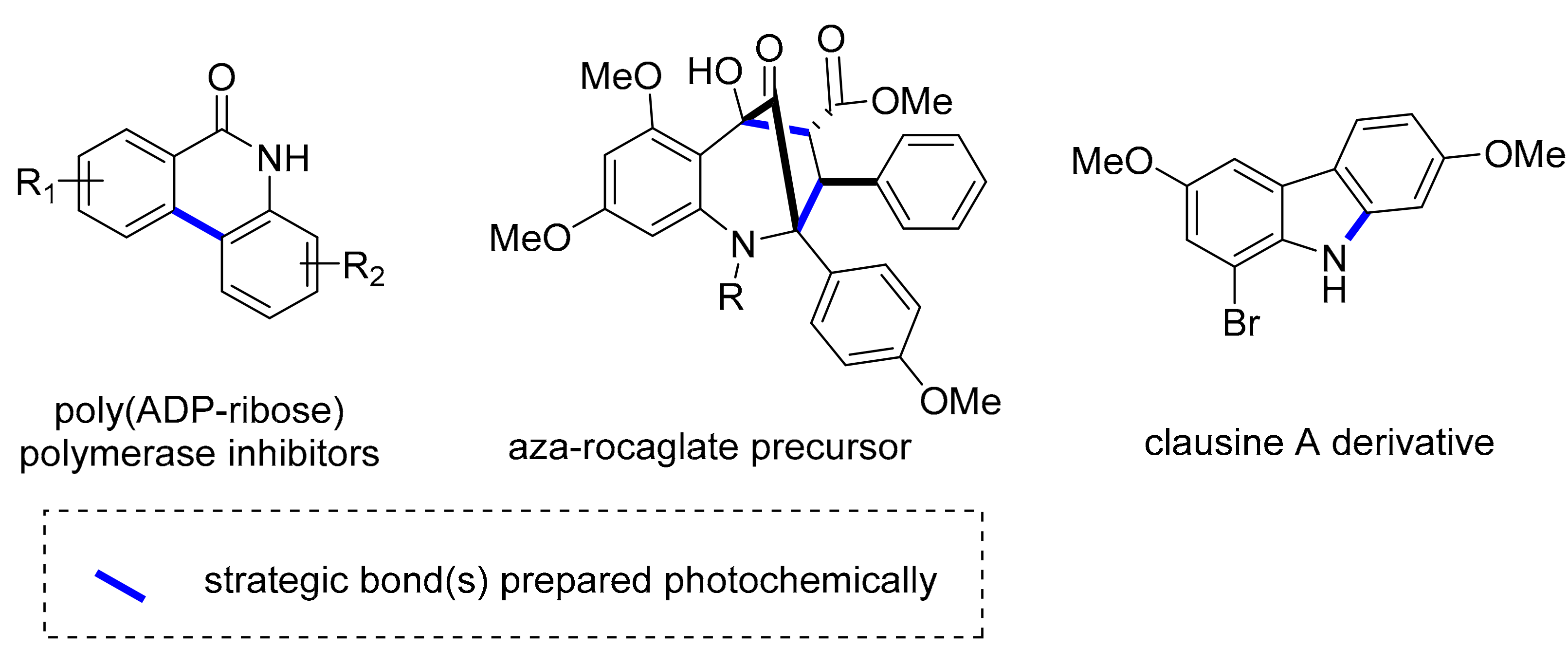
- Fang, Y.; Tranmer, G.K. Continuous flow photochemistry as an enabling synthetic technology: Synthesis of substituted-6(5H)-phenanthridinones for use as poly(ADP-ribose) polymerase inhibitors. Med. Chem. Commun. 2016, 7, 720–724. [Google Scholar] [CrossRef]
- Wang, W.; Cencic, R.; Whitesell, L.; Pelletier, J.; Porco, J.A., Jr. Synthesis of aza-rocaglates via ESIPT-mediated (3+2) photocycloaddition. Chem. Eur. J. 2016, 22, 12006–12010. [Google Scholar] [CrossRef] [Green Version]
- Parisien-Collette, S.; Collins, S.K. Exploiting photochemical processes in multi-step continuous flow: Derivatization of the natural product Clausine, C. ChemPhotoChem 2018, 2, 855–859. [Google Scholar] [CrossRef]
- Mortzfeld, F.B.; Pietruszka, J.; Baxendale, I.R. A simple and efficient flow preparation of pyocyanine a virulence factor of Pseudomonas aeruginosa. Eur. J. Org. Chem. 2019, 5424–5433. [Google Scholar] [CrossRef]
- Levterov, V.V.; Michurin, O.; Borysko, P.O.; Zozulya, S.; Sadkova, I.V.; Tolmachev, A.A.; Mykhailiuk, P.K. Photochemical in-flow synthesis of 2,4-methanopyrrolidines: Pyrrolidine analogues with improved water solubility and reduced lipophilicity. J. Org. Chem. 2018, 83, 14350–14361. [Google Scholar] [CrossRef]
- Elliott, L.D.; Booker-Milburn, K.I. Photochemically produced aminocyclobutanes as masked dienes in thermal electrocyclic cascade reactions. Org. Lett. 2019, 21, 1463–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruggeri, M.; Dombrowski, A.W.; Djuric, S.W.; Baxendale, I.R. Photochemical flow synthesis of 3-hydroxyazetidines. ChemPhotoChem 2019, 3, 1212–1218. [Google Scholar] [CrossRef]
- Mumtaz, S.; Robertson, M.J.; Oelgemöller, M. Continuous flow photochemical and thermal multi-step synthesis of bioactive 3-arylmethylene-2,3-dihydro-1H-isoindolin-1-ones. Molecules 2019, 24, 4527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strauss, F.J.; Cantillo, D.; Guerra, J.; Kappe, C.O. A laboratory-scale continuous flow chlorine generator for organic synthesis. React. Chem. Eng. 2016, 1, 472–476. [Google Scholar] [CrossRef]
- Baker, J.R.; Gilbert, J.; Paula, S.; Zhu, X.; Sakoff, J.A.; McCluskey, A. Dichlorophenylacrylonitriles as AhR ligands that display selective breast cancer cytotoxicity in vitro. ChemMedChem 2018, 13, 1447–1458. [Google Scholar] [CrossRef]
- Rehm, T.H. Photochemical fluorination reactions—A promising research field for continuous flow synthesis. Chem. Eng. Technol. 2016, 39, 66–80. [Google Scholar] [CrossRef]
- Bume, D.D.; Harry, S.A.; Pitts, C.R.; Lectka, T. Sensitized aliphatic fluorination directed by terpenoidal enones: A “visible light” approach. J. Org. Chem. 2018, 83, 1565–1575. [Google Scholar] [CrossRef]
- Barthelemy, A.-L.; Dagousset, G.; Magnier, E. Metal-free visible-light-mediated hydrotrifluoromethylation of unactivated alkenes and alkynes in continuous flow. Eur. J. Org. Chem. 2019. [Google Scholar] [CrossRef]
- Abdiaj, I.; Bottecchia, C.; Alcazar, J.; Noël, T. Visible-light-induced trifluoromethylation of highly functionalized arenes and heteroarenes in continuous flow. Synthesis 2017, 49, 4978–4985. [Google Scholar]
- Beatty, J.W.; Douglas, J.J.; Cole, K.P.; Stephenson, C.R.J. A scalable and operationally simple radical trifluoromethylation. Nat. Commun. 2015, 6, 7919. [Google Scholar] [CrossRef]
- Ushakov, D.B.; Gilmore, K.; Seeberger, P.H. Consecutive oxygen-based oxidations convert amines to a-cyanoepoxides. Chem. Commun. 2014, 50, 12649–12651. [Google Scholar] [CrossRef] [PubMed]
- Kouridaki, A.; Huvaere, K. Singlet oxygen oxidations in homogeneous continuous flow using a gas–liquid membrane reactor. React. Chem. Eng. 2017, 2, 590–597. [Google Scholar] [CrossRef]
- Radjagobalou, R.; Blanco, J.-F.; Dechy-Cabaret, O.; Oelgemöller, M.; Loubiere, K. Photooxygenation in an advanced led-driven flow reactor module: Experimental investigations and modelling. Chem. Eng. Process Process Intensif. 2018, 130, 214–228. [Google Scholar] [CrossRef] [Green Version]
- Babra, J.S.; Russell, A.T.; Smith, C.D.; Zhang, Y. Combining C-H functionalisation and flow photochemical heterocyclic metamorphosis (FP-HM) for the synthesis of benzo[1,3]oxazepines. Tetrahedron 2018, 74, 5351–5357. [Google Scholar] [CrossRef] [Green Version]
- Cantillo, D.; Mateos, C.; Rincon, J.A.; de Frutos, O.; Kappe, C.O. Light-induced C-H arylation of (hetero)arenes by in situ generated diazo anhydrides. Chem. Eur. J. 2015, 21, 12894–12898. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Ferlin, F.; Ackermann, L.; Vaccaro, L. C–H functionalization reactions under flow conditions. Chem. Soc. Rev. 2019, 48, 2767–2782. [Google Scholar] [CrossRef]
- Lange, H.; Carter, C.F.; Hopkin, M.D.; Ley, S.V. A breakthrough method for the accurate addition of reagents in multi-step segmented flow processing. Chem. Sci. 2011, 2, 765–769. [Google Scholar] [CrossRef]









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Filippo, M.; Bracken, C.; Baumann, M. Continuous Flow Photochemistry for the Preparation of Bioactive Molecules. Molecules 2020, 25, 356. https://doi.org/10.3390/molecules25020356
Di Filippo M, Bracken C, Baumann M. Continuous Flow Photochemistry for the Preparation of Bioactive Molecules. Molecules. 2020; 25(2):356. https://doi.org/10.3390/molecules25020356
Chicago/Turabian StyleDi Filippo, Mara, Cormac Bracken, and Marcus Baumann. 2020. "Continuous Flow Photochemistry for the Preparation of Bioactive Molecules" Molecules 25, no. 2: 356. https://doi.org/10.3390/molecules25020356





