Abstract
New four isomeric chair architectures of 1:1 H-bonded supramolecular complexes were prepared through intermolecular interactions between 4-(2-(pyridin-4-yl)diazenyl-(2-(or 3-)chlorophenyl) 4-alkoxybenzoates and 4-n-alkoxybenzoic acids. The H-bond formation of all complexes was confirmed by differential scanning calorimetry (DSC) and Fourier-transform infrared spectroscopy (FTIR). Mesomorphic characterization was carried by DSC and polarized optical microscopy (POM). It was found that all prepared laterally chloro-substituted supramolecular complexes were nematogenic, and exhibited nematic phase and low melting temperature. The thermal stability of the nematic mesophase observed depends upon the location and spatial orientation of the lateral Cl− atom in as well as the length of terminal chains. Theoretical calculations were carried out within the paradigm of the density functional theory (DFT) in order to establish the molecular conformation for the formed complexes and estimate their thermal parameters. The results of the computational calculations revealed that the H-bonded complexes were in a chair form molecular geometry. Additionally, out of the acquired data, it was possible to designate the influence of the position and orientation of the lateral group as well as the alkoxy chain length on the stability of the nematic phase.
1. Introduction
Recently, supramolecular liquid crystals (SMLCs) attracted a vivid attention of the scientific community [1,2,3,4,5]. These systems combine the supramolecular chemistry [6] and liquid crystals [7,8] with efficient properties for optical and technological potential applications [9]. H-bonding intermolecular interactions are a well-established strategy to design self-assembly LCs through several non-covalent bonds [10,11,12,13,14]. Among the hydrogen bond acceptors and donors, the pair of a carboxylic acid and a pyridine derivative is the best choice in several studies. Moreover, the use of multifunctional components in the formation of a non-covalent interaction can produce better characteristics of supramolecular LC network architectures [6,7]. The supramolecular systems could be photosensitive host–guest complexes and they are of significant interest where the light can be applied in a remote manner as an external stimulus and offers excellent control with the wavelength [15,16,17]. Azopyridine molecules are incorporated into liquid-crystal materials to make them photoresponsive [18,19] due to their ability for trans–cis-isomerization upon thermal and photo-irradiation. Modifying the core structure or adding lateral substituents to azopyridine-based derivatives can lead to marked changes in photophysical and photochemical properties. [18,19] An incorporation of lateral groups with different size and polarity widely improves many characteristics of liquid crystalline materials. It could be attributed to the disturbance in the molecular packing that decreases the melting temperature and thermal stability of liquid crystal mesophases [20,21,22,23,24,25,26,27]. Lately, azopyridines have been used in the formation of nano-fiber supramolecular self-assembling and hydrogen/halogen-bonding LCs with photo-induced transition phenomena [28,29,30,31,32]. Designing of photosensitive SMLCs through intermolecular interactions using the suitable H-bond donors and acceptors are concerns of our area of interest [33,34,35,36,37,38,39]. Anisotropic structures are produced from the overall molecular shape of architectures and the combination of rigid (aromatic) and flexible segments (alkyl chains). Changes in the structure of molecules forming liquid crystalline phases impacts the mesomorphism as well as the properties essential for technical uses. Recently, construction of materials according to computational prediction has a high attention of many researchers [21,40,41,42,43,44,45,46,47,48,49]. Mutual influence of the many optical parameters requires stimulated information about the energies of molecular orbitals as well as the molecular geometries of the LCs. Moreover, density functional theory (DFT) is a powerful tool for taking an insight into features of the molecules at ease [21,43,50,51,52,53,54,55,56].
The goal of the present work was focused on designing the new series of liquid crystalline forming H-bonded supramolecular architectures and examines their physicochemical properties. Also, [55] to study the stability of different spatial oriented lateral polar groups on the thermal and optical behavior of prepared intermolecular H-bonded complexes, which oriented with different angles on the central ring of the azopyridine-based moiety, Scheme 1. Moreover, DFT theoretical calculations will be discussed to predict the molecular conformation for the formed complexes as well as their thermal parameters. In addition, these calculations will be used to explain the effect of the position and orientation of the lateral group as well as the length of the alkoxy chain on the type and the stability of the observed mesophase. Finally, to investigate the impact of the estimated thermal parameters of H-bonded complexes and how these parameters could affect their thermal and optical properties.

Scheme 1.
Prepared compounds.
2. Results and Discussion
2.1. FT-IR Spectroscopic Confirmation of SMHB Complexes Formation
The formation of the supramolecular complexes has been confirmed by Fourier-transform infrared spectroscopy (FTIR) spectral data. The measurements were performed for the individual components as well as their H-bonded supramolecular complexes. The FTIR spectrum of acids, azopyridine bases and their complexes (A12/I16 and A12II16 as representative examples) are given in Figure 1. It has been reported that, no significant effect of the length of the alkoxy chain on the wavenumber of the C=O group stretching vibration either for the individual acids or the H-bonded complexes [39,57,58]. The signal at 1678.2 cm−1 was assigned to the stretching vibration of the C=O group of the alkoxy acid, experimentally and theoretically, respectively. The H-bonding between the nitrogen of azopyridines and the carboxylic group of alkoxybenzoic acid of the supramolecular complexes An/Im and An/IIm replaces the bis H-bonds of the dimeric form of the alkoxybenzoic acid. One of the most important evidences of the H-bonded supramolecular complexes formation is the stretching vibration of the C=O carboxylic group either experimentally or theoretically. The sharing of carboxylic group OH-group in H-bonding formation will decreases the strength O-H bond. Theoretically, (Table 1), the OH-bond length increased from 0.97588 Å for the free acid to 1.04046 Å and 1.03154 Å for H-bonded complex A12/I16 and A12/II16, respectively. Moreover, the wave-number of their stretching vibration decreases from 3660.9 cm−1 of the free acid to 2508.8 cm−1 for isomer A12/I16 and 2572.5 cm−1 for the other isomer, A12/II16. Similarly, the strength of the C=O bond of the COOH group decreases upon the H-bonding formation, where, the stretching vibration decreases to 1687.0 and 1666.6 cm−1 for H-bonded isomers A12/I16 and A12/II16 instead of 1691.0 cm−1 for the free acid. Obviously, from the theoretical results, the position of the Cl-atom has an intensive effect on the H-bond strength of the H-bonded complex. The presence of the electronegative Cl-atom near the pyridine ring responsible for the H-bond formation for A12/I16 complex (the Cl-atom in meta position with respect to the ester group) will disrupt the H-bond formation by decreasing the availability of the lone pair on the N-atom of the pyridine ring. Experimentally, the results of the FT-IR revealed that no significant effect of the H-bond formation on the C=O group of the free carboxylic acid, only 2 cm−1 decreasing, (ύC=O = 1681.7 cm−1). However, the supramolecular complex formation has a high stretching vibration effect on the C=O of the ester linkage of the azopyridine base, their wave number increases from 1727.8 to 1743.4 cm−1 for complex A12/I16 and 15.9 cm−1 for the other complex A12/II16. Moreover, it has been reported [49,59,60,61,62,63,64] that a major piece of evidence for the formation H-bond supramolecular complex is the presence of three vibration bands of Fermi resonance of the H-bonded OH groups A-, B- and C-types. The vibrational peak assigned to A-type Fermi band of complex A12/I16 and A12/II16 presented under the C-H vibrational peaks at 2915 to 2855 cm−1. Moreover, the peak at 2329 (A12/I16) and 2356 cm−1 (A12/II16) could be attributed to the O–H in-plane bending vibration as well as its fundamental stretch (B-type). However, 1899.3 and 1906.8 cm−1 were assigned to C-type Fermi band due to the interaction between the overtone of the torsional effect and the fundamental stretching vibration of the OH.
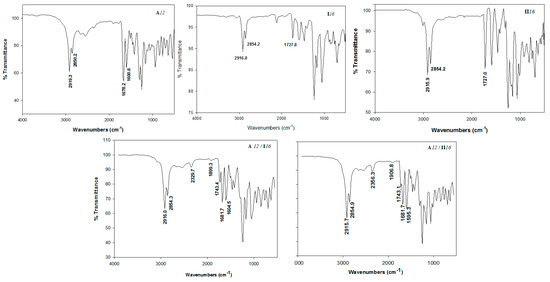
Figure 1.
The Fourier-transform infrared spectroscopy (FTIR) spectrum of acid A12, azopyridine bases, I16 and II16, as well as their 1:1 supramolecular complexes A12/I16 and A12II16.

Table 1.
The calculated bond length (Å) wavenumbers (cm−1) of characteristic groups of A12, I16, II16, A12/I16 and A12/II16.
2.2. Mesomorphic and Optical Behavior
All 1:1 molar ratio complex, An/Im and An/IIm, were prepared from two homologs of the azopyridine base (Im and IIm) and four homologs of the acid, An. The prepared complexes were characterized by DSC and POM. The textures observed by POM were verified by the DSC measurements and types of mesophases were identified for all prepared supramolecular complexes An/Im and An/IIm. DSC thermograms of the 1:1 supramolecular complexes A12/I16 and A12/II16, as examples, are depicted in Figures S1 and S2 (see Supplementary Data). DSC behavior was observed for the prepared mixtures by subjecting them to repeat heating/cooling cycles.
Transition temperatures and their associated enthalpies of transition values were measured by DSC for all prepared complexes and are summarized in Table 2. The effect of terminal alkoxy chain length of the acid component (n) represented graphically, as a function of m of the two isomeric groups of base moieties (Im and IIm) in Figure 2 and Figure 3, respectively. The results of Table 2 and Figure 2 and Figure 3 showed that neither terminal alkoxy chains of acid nor the base, n and m, respectively, effected the type of the mesophase, (nematic (N)), observed for all prepared lateral Cl complexes. In most cases, the N phase stability (TN-I) was found to decrease with the increment of n. As shown from Figure 2, the complexes An/I8 exhibit an enantiotropic nematic phase and the nematic enhancement is slightly increases with the increment of n (Figure 2a). While, the longer base terminal (m = 16, Figure 2b), the prepared complexes An/I16 showed a stable nematic phase upon heating and cooling except for A12/I16 exhibits monotropic N phase behavior. Upon heating, A12/I16 converts to isotropic liquid at 80.5 °C without showing any LC phase, whereas, in the cooling scan it exhibits a nematic mesophase start from 71.5 °C.

Table 2.
Phase transition temperatures (°C), enthalpy of transitions (kJ/mol) and normalized entropy change for the supramolecular complexes An/Im and An/IIm.

Figure 2.
Dependence of the alkoxy-chain length of the acid component (n) of the lateral Cl azopyridines (Im) on the mesophase behavior of the 1:1 supramolecular hydrogen-bonded complexes (a) m = 8; (b) m = 16.
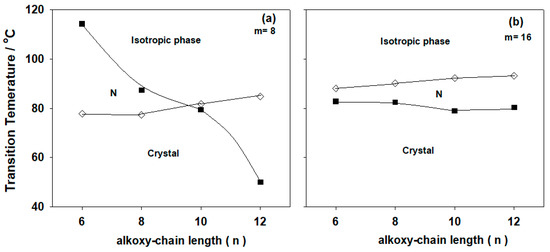
Figure 3.
Dependence of the alkoxy-chain length of the acid component (n) of the lateral Cl azopyridines (IIm) on the mesophase behavior of the 1:1 supramolecular hydrogen-bonded complexes (a) m = 8; (b) m = 16.
Figure 3 shows the mesomorphic behavior of base moiety IIm (the lateral Cl group introduced at the meta-position with respect to the ester carbonyl core) with variable alkoxy chains. It could be seen from Figure 3a, the supramolecular complexes An/II8 exhibit different nematic behaviors than the corresponding isomeric complexes An/I8, whereas, An/II8 have relatively wide enantiotropic nematic ranges with higher value for the complex A6/II8 (~36.4 °C) and the wide nematic range value for A12/II8 monotropically. Moreover, the nematic stability decreases with the alkoxy chain length (n) of the acid component. In addition, the supramolecular complexes melting temperature is slightly affected by the length of the alkoxy chain of the acid. Finally, it is obvious from Figure 3b (An/II16) that, an independent effect of the alkoxy chain length of the acid on a monotropic nematic phase covered all supramolecular complexes. From the present investigation, it would be expected that the increment of the molecular anisotropy due to the orientation of the lateral electron-withdrawing Cl atom in the supramolecular geometry impacted the stability of nematic phase that agrees with our previous work [34,65].
Furthermore, the addition of lateral Cl atom in supramolecular architectures weakens the side by side cohesion interactions thus enhances a nematic phase for all 1:1 complex. Furthermore, the molecular geometry and size of the lateral substituent impact the mesophase stability and the polarizability of the whole molecule [22,23,66]. It was found that the length of the alkoxy chain, the polarity as well as the position (or orientation) of the lateral group are important factors determining the type and the range of the mesophase. Images of the mesophase as representative examples from POM are shown in Figure 4. Schlieren texture of the nematic phase was observed for all prepared complex.

Figure 4.
Nematic phase textures under polarized-optical microscopy (POM) of the supramolecular complexes (a) A10/I8 at 72.0 °C upon heating; and (b) A12/II16 at 77.0 °C upon cooling.
2.3. Effect of Polarity and Orientation of Lateral Substituent on the Supramolecular Hydrogen-Bonded Complexes Stability
In order to study the effect of the polarity and the position (spatial orientation) of the lateral group on the mesophase thermal stability (TC) of 1:1 the prepared supramolecular H-bonded complex, a comparative study was constructed between mesophase stabilities (clearing temperature, TC) of the present lateral Cl complexes (An/Im and An/IIm) and their corresponding lateral CH3 supramolecular H-bonded complexes (An/IIIm and An/IVm) [8,67], as well as the laterally neat one (An/Vm) [36]. The data are represented graphically in Figure 5a–d. It had been found that the location and the inductive effect of the lateral substituent incorporated in base complement impact the polarizability between H-donors and H-acceptor and thus affect the strength of the H-bond [59]. However, the polarity of both components was not affected by the length of the terminal alkoxy chain (Figure 5a–d). Moreover, the laterally neat supramolecular H-bonded complexes (An/Vm) have the highest thermal stability with respect to the derivatives of electron-donating CH3 and electron-withdrawing Cl lateral substituents. In addition, the nematic mesophase in the present investigation (lateral Cl complexes, An/Im and An/IIm) is observed instead of the smectic C of the lateral CH3 and neat supramolecular complexes. Thus, the nature of intermolecular interactions between molecules affects the stability as well as the type of the mesophase. The lateral electron withdrawing Cl-atom of the complexes An/Im and An/IIm predominates the end to end interaction to enhance a less ordered nematic phase, while the strong backing side by side interactions in the case of lateral CH3 (An/IIIm and An/IVm) and laterally neat (An/Vm) complexes to give more ordered mesophase, smectic C, Scheme 2.
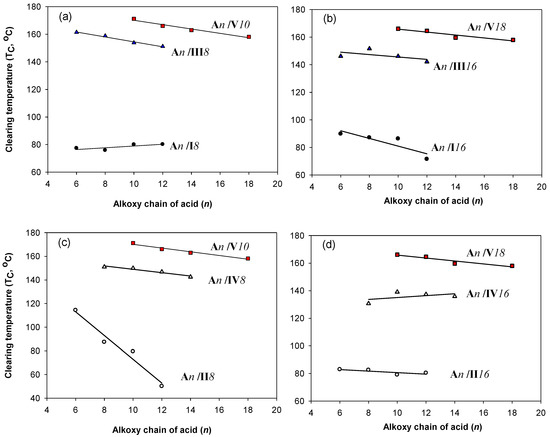
Figure 5.
Mesophase stability temperature (TC) dependency on the terminal alkoxy chain length (n) of the acid complement; (●); An/IIm (○); An/IIIm (▲); An/IVm (∆); An/IVm (■), (a) An/I8, An/III8, An/V10 (b) An/I16, An/III16, An/V18 (c) An/II8, An/IV8, An/V10, (d) An/II16, An/IV16, An/V18.

Scheme 2.
Previously reported analogues compounds.
2.4. DFT Calculations
2.4.1. Relationship between Experimental and Theoretical Parameters
The theoretical DFT calculations were performed in the gas phase by DFT/B3LYP method at 6-31G (d,p) basis set. All optimum compounds are stable, and this is approved in the term of the absence of the imaginary frequency. The results of the theoretical DFT calculations for lateral complexes of ortho chloro-derivatives with respect to the ester group (An/Im) as well as the other isomeric supramolecular complex (meta-chloro with respect to the ester group) A12/II16 and A16/II16 showed a chair geometry for all investigated compounds. The three phenyl rings (two of the azopyridine base and one of the 4-alkoxybenzoic acid) of the H-bonded complexes are completely planar for both supramolecular H-bonded complexes. Recently, our group reported that [39], the chair forms conformation do not permit a strong lateral interaction leaving the end to end aggregation of the chains to be the predominant interaction. The pronounced terminal interaction could be a good explanation for the enhancement of the nematic mesophases observed for all alkoxy chain lengths of the H-bonded complexes over the parallel interaction that enhances the smectic phase formation, Figure 6. The estimated DFT calculations for thermal parameters, dipole moment and the polarizability of the prepared supramolecular hydrogen bonding liquid crystal complexes A12/I16 and An/IIm are summarized in Table 3.
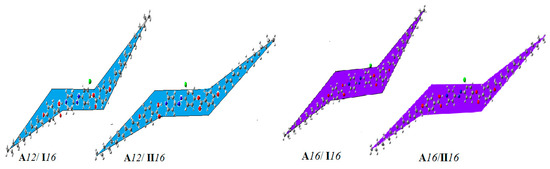
Figure 6.
Optimized chair geometrical structures of A12/I16, A12/II16, A16/I16, and A16/II16.

Table 3.
Parameters (Hartree/particle) and dipole moment (Debye) of A12/I16, A16/I16, and An/IIm.
As shown from Table 3 and Figure 6, the length of the alkoxy chain of the homologs series enhancement the calculated thermal energy. As the chain length increases more packing of the molecules is permitted and consequently, the stability of the molecules increases [21,39,50,52,57,61,68]. Obviously, there is no significant effect of the alkoxy chain length on the dipole moment. However, the position and the spatial orientation of the Cl-atom has a high impact on the magnitude of the dipole moment, 6.8408 and 8.8598 Debye for ortho (A12/I16) and meta (A12/II16) chloro with respect to the carboxylate linkage, respectively. On the other hand, Figure 7 illustrates the relationship between the alkoxy chain length of acid moiety (n) and the polarizability. As the chain length increases the polarizability increases, and so, the candidate of the highest chain length showed the maximum polarizability and could be predicted to have the best characteristics in NLO applications. Moreover, the position and the orientation of the chloro-atom affects the predicted stability as well as the polarizability, the ortho chloro-derivative with respect to the ester group (An/Im) showed higher polarizability and lower stability rather than that of the other isomer (An/IIm), the difference was 38.4 Bohr3 and 424.36 Kcal/mole, respectively, for n = 12, m = 16. The higher stability of the ortho chloro-derivatives could be illustrated in the term of its high degree of interaction of the molecules which permits more packing of the compounds rather than that of the meta derivatives.

Figure 7.
Dependence of the acid akoxy chain-length of SMHB complexes An/IIm on the (a) the calculated polarizability and (b) thermal energies.
Figure 8 shows the relationship between the length of the acid alkoxy groups and the mesophase nematic stability of 1:1 mixture An/Im against the calculated thermal energy (Etot) and the polarizability (α). As shown from the figure, the length terminal alkoxy chain has a high effect on the mesophases stability of the nematic phase. The calculated thermal energy decreases with the length of the chain and mesophase stability decreases, the similar behavior was noticed with polarizability. The mesophase stability gradually decreases with the chain length up to n = 10 then sharp decrements were observed either with the estimated energy or polarizability. This result could be attributed to the high degree of the terminal aggregation at shorter chain lengths rather than that of the longer one which permits more parallel. The chair conformer structure of the H-bonded supramolecular compounds under investigation could permit the maximum end to end interaction for shorter chain lengths while for the longer one this interaction could be decreased with enhancement of side-side aggregation of alkoxy chains and the ester carbonyl moieties, that decreases the mesophase stability of the formed mesophase.
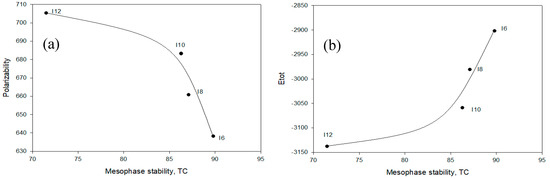
Figure 8.
The relationship of the akoxy chain-length/mesophase stability of 1:1 complex An/II16 against (a) the calculated polarizability and (b) the total predicted Etot.
2.4.2. Entropy Change of SMHB Complexes
Terminal alkoxy chains have a pronounced role as they are flexible and can easily make multi-conformational changes. An enhancement of the entropy change is observed in all supramolecular H-bonded complexes due to the increment in the conformational and orientation changes of the whole complex. A comparison of the normalized entropy changes for SMHB complexes An/I16, An/II16, An/III16, and An/IV16 was depicted in Figure 9. Entropy of transitions (∆S/R) was constructed graphically as a function of the terminal alkoxy-chain length of acid component (n) for different lateral substituted supramolecular complexes. Figure 9 shows that, independent of the terminal flexible chains, an irregular entropy change was observed. That irregular change may be explained to the intermolecular interactions due to the location and rotation as well as the polarity of lateral substituent effect on the ordering of the whole complex [69,70]. The high dipole moment of An/II16 than An/I16 is accompanied by more conformational entropy changes due to good packing of lateral meta Cl supramolecular complexes molecules than the ortho Cl isomers. In contrast to the lateral electron-donating CH3 group, lower entropy transitions observed for meta CH3 SMHB complexes than the ortho CH3 isomeric complexes. These results could be explained in terms of the high degree of alignment of the molecules in the case of electron-donating lateral substituent (CH3) in the smectic mesophase that highly decreases the entropy with respect to the less ordered nematic mesophase in case of lateral electron-withdrawing group (Cl). The large value of entropy in many cases may be explained by the intermolecular interactions due to the location and rotation as well as the polarity of the lateral Cl-atom which enhancement the ordering of whole supramolecular complex. Moreover, non-correlation between the entropies and the terminal alkoxy-chain length may be due to the irregular change of lateral adhesion upon the increase of the total molecular length.
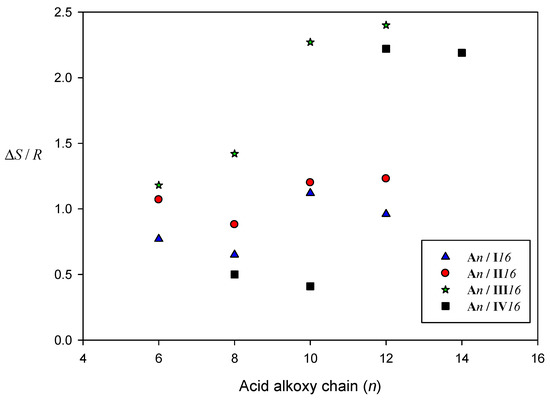
Figure 9.
Comparison of the entropy changes of nematic transitions for SMHB complexes An/I16 (▲); An/II16 (●); An/III16 (*); An/IV16 (■).
2.4.3. Frontier Molecular Orbitals and Molecular Electrostatic Potential
Figure 10 summarizes the predicted ground state isodensity surface plots for the FMOs HOMO (highest occupied molecular orbital) and LUMO (lowest unoccupied molecular orbital)) as well as their energies difference (ΔE) of the compounds under investigation An/IIm and A12/I16 as examples. As shown from Table 4, the FMO energy gap and the global softness (S) were not significantly affected by the length of the terminal alkoxy chain of compounds An/IIm. However, the position and the orientation of the lateral Cl atom have a high impact on the energy difference between the FMOs. The attachment of the Cl atom at the ortho position with respect to the ester linkage increases the energy difference between FMOs (HOMO and LUMO) than that at the meta position. This result could be helpful in the building of the molecules in a certain isomerism (positional and/or orientational) that would improve their characteristics to offer proper applications.

Figure 10.
The calculated ground state isodensity surface plots for frontier molecular orbitals of A12/I16, A16/I16 and An/IIm.

Table 4.
Molecular orbital energies and global softness (S) of A12/I16, A16/I16 and An/IIm.
The charge distribution map for the complexes A12/I16, A16/I16 and An/IIm was calculated under the same basis sets according to molecular electrostatic potential (MEP) (Figure 11). The red region (negatively charged atomic sites) was distributed on the aromatic moiety and the maximum was carbonyl oxygen of the H-bonded carboxylic group, while alkoxy chains showed the least negatively charged atomic sites (blue regions). As shown in Figure 11, there is no significant effect of either the location, the orientation of the Cl atom or the alkoxy-chain length on the charge distribution. This could explain the reason of alteration of the type of the mesophase of the compounds under investigation in the term of the competitive interaction between end-to-end and side-side interaction by increasing of the chain length rather than the change of the charge distribution.

Figure 11.
Molecular electrostatic potentials (MEP) of A12/I16, A16/I16 and An/IIm.
3. Experimental
3.1. Preparation of 1:1 Supramolecular Complexes
The 4-n-Alkoxy benzoic acids were obtained from Merck (Nuremberg, Germany). All the solvents used were of pure grade and purchased from Aldrich (St. Louis, WI, USA).
The 4-n-Alkoxy benzoic acids (An), and lateral chloro–pyridine-based derivatives (Im and IIm) were checked to exhibit identical transition temperatures as previously reported [8,71]. The 1:1 molar ratio of any two complementary components SMHBLCs (Supramolecular H-bonded complexes) complexes (An/Im and An/IIm) were prepared by melting the appropriate amounts of each component, stirring to give an intimate blend and then, cooling with stirring to room temperature (Scheme 3). For example, to prepare the supramolecular complex A10/I8: 0.0278 g of 4-decyloxybenzoic acid A10 and 0.0466 g of 4-(2-(pyridin-4-yl)diazenyl-(2-chlorophenyl) 4-octyloxy benzoate I8 were melted together to form the complex.

Scheme 3.
Preparation of 1:1 SMHB complexes (An/Im and An/IIm).
3.2. Characterizations
Supramolecular complexes formations were confirmed by TA Instruments Co. Q20 Differential Scanning Calorimeter (DSC; TA Instruments Co. Q20, DSC, New Castle, DE, USA), polarized-optical microscopy (POM, Wild, Humborg, Germany) and FT-IR (PerkinElmer, Inc., Shelton, CT, USA) spectroscopic analysis.
Calorimetric measurements were carried out using a PL-DSC of Polymer Laboratories, London, England. The instrument was calibrated for temperature, heat and heat flow according to the method recommended by Cammenga, et. al. [72] Measurements were carried out for small samples (2–3 mg) placed in sealed aluminum pans. All measurements were conducted at a heating rate of 10°C/min in an inert atmosphere of nitrogen gas (10 mL/min). For DSC, the sample was heated from room temperature to 280 °C at a heating rate of 10 °C/min under a nitrogen atmosphere, and then cooled in the cell to 0 °C. All weighed samples were made using an ultra-microbalance, Mettler Toledo, London, England, with accuracy ± 0.0001 mg.
Transition temperatures for the complexes (An/Im and An/IIm) were investigated by DSC in heating and cooling cycles. The types of the mesophase were identified using standard POM (Wild, Germany), attached with Mettler FP82HT hot stage. Measurements were made twice, and the results were found to have accuracy in transition temperature and enthalpy within ± 0.2 °C.
3.3. Computational Methods and Calculations
The theoretical calculations for the investigated compounds were carried out by Gaussian 09 software [73]. DFT/B3LYP methods using a 6-31G (d,p) basis set was selected for the calculations. The geometries were optimized by minimizing the energies with respect to all geometrical parameters without imposing any molecular symmetry constraints. The structures of the optimized geometries had been drawn with Gauss view [74]. Moreover, the calculated frequencies were carried out using the same level of theory. The frequency calculations showed that all structures were stationary points in the geometry optimization method with none imaginary frequency.
4. Conclusions
Four new isomeric series of 1:1 SMHB complexes in chair-shaped liquid crystalline were constructed based on laterally Cl azopyridine derivatives and 4-alkoxybenzoic acids. All investigated complexes were confirmed by DSC, POM and FT-IR Fermi bands. It was found that all present 1:1 mixture is purely nematogenic with low melting temperatures. The experimental and DFT theoretical calculations results revealed that the H-bonded complexes were in a chair form molecular geometry. Moreover, the results of the DFT show that the position and orientation of the lateral group, as well as the alkoxy chain length, affects the type and the stability of the nematic mesophase. The position and the spatial orientation of the Cl-atoms have a high impact on the magnitude of the dipole moment as well as the polarizability. FMO energy gap and the global softness (S) were not significantly affected by the length of the terminal alkoxy chain of compounds. However, the position of the lateral Cl atom has a high impact on the energy difference between the FMOs. The higher stability of the ortho chloro-derivatives was illustrated in the term of its high degree of interaction of the molecules which permits more packing of the compounds rather than that of the meta derivatives. It could be concluded that the designing of new nematogenic supramolecular H-bonded conformers with certain molecular geometry offers suitable criteria that could be promising for proper optical applications. In addition, alteration of thermal and optical parameters by H-bonded complex formations and showing how could play an important role in improving the optical properties.
Supplementary Materials
The following are available online, Figure S1. DSC thermograms of A12/I16 supramolecular complex upon heating and cooling cycles with heating rate 10 °C/min. Figure S2. DSC thermograms of A12/II16 supramolecular complex upon heating and cooling cycles with heating rate 10 °C/min. Figure S3: Some textures (Size: 41.8 × 106 nm) under POM of the supramolecular complexes upon heating (1) solid phase of A10/I8 at 52.0 °C; (2) nematic phase of A6/I8 at 75.0 °C; (3) nematic phase of A6/II8 at 112.0 °C; and (4) nematic phase of A8/II8 at 85.0 °C. Table S1: Normalized entropy change (∆S/R) for the supramolecular complexes An/III16 and An/IV16.
Author Contributions
O.A.A. and H.A.A.; Formal analysis, H.A.A. and M.H.; Funding acquisition, O.A.A., H.A.A. and M.H.; Investigation, H.A.A. and M.H.; Methodology, O.A.A., H.A.A. and M.H.; Project administration, O.A.A.; Resources, H.A.A. and M.H.; Software, M.H.; Writing—original draft, H.A.A. and M.H.; Writing—review & editing, H.A.A. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
Authors gratefully acknowledge the Deanship of Scientific Research, Taibah University for the support of this research work, research group No. 60333, 60334.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shen, P.; Zhang, X.; Lu, H.; Su, Z.; Zhou, Y.; Song, B.; Li, X.; Yang, X.; Tu, Y.; Li, C.Y. Effect of Fullerene Volume Fraction on Two-Dimensional Crystal-Constructed Supramolecular Liquid Crystals. Chem. Asian J. 2019, 14, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.; Dechant, M.; Gerbig, L.; Baumann, M. Supramolecular click procedures in liquid crystals. Liq. Cryst. 2019, 46, 1–10. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the mesomorphic behaviour of supramolecular liquid crystals by resonance-assisted hydrogen bonding. J. Mater. Chem. C 2019. [Google Scholar] [CrossRef]
- Sharma, V.S.; Shah, A.P.; Sharma, A.S. A new class of supramolecular liquid crystals derived from azo calix [4] arene functionalized 1, 3, 4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Kong, S.; Song, Y.; Meng, F. Star-shaped supramolecular ionic liquid crystals based on pyridinium salts. Liq. Cryst. 2019, 46, 512–522. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Supramolecular liquid-crystalline networks built by self-assembly of multifunctional hydrogen-bonding molecules. Chem. Mater. 1996, 8, 961–968. [Google Scholar] [CrossRef]
- Kihara, H.; Kato, T.; Uryu, T.; Frechet, J.M. Induction of a cholesteric phase via self-assembly in supramolecular networks built of non-mesomorphic molecular components. Liq. Cryst. 1998, 24, 413–418. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Gowda, A.; Jacob, L.; Joy, N.; Philip, R.; Pratibha, R.; Kumar, S. Thermal and nonlinear optical studies of newly synthesized EDOT based bent-core and hockey-stick like liquid crystals. New J. Chem. 2018, 42, 2047–2057. [Google Scholar] [CrossRef]
- Gray, G.W.; Jones, B. The Mesomorphic Transition Points of the Para-Normal-Alkoxybenzoic Acids-A Correction; Royal Society of Chemistry: Cambridge, UK, 1953; pp. 4179–4180. [Google Scholar]
- Kato, T.; Frechet, J.M. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Kato, T.; Wilson, P.G.; Fujishima, A.; Fréchet, J.M. Hydrogen-bonded liquid crystals. A novel mesogen incorporating nonmesogenic 4,4′-bipyridine through selective recognition between hydrogen bonding donor and acceptor. Chem. Lett. 1990, 19, 2003–2006. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.; Wilson, P.G.; Saito, T.; Uryu, T.; Fujishima, A.; Jin, C.; Kaneuchi, F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem. Mater. 1993, 5, 1094–1100. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M. Hydrogen Bonding and the Self-Assembly of Supramolecular Liquid-Crystalline Materials; Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1995; pp. 311–326. [Google Scholar]
- Yagai, S.; Kitamura, A. Recent advances in photoresponsive supramolecular self-assemblies. Chem. Soc. Rev. 2008, 37, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Jiao, D.; Biedermann, F.; Scherman, O.A. Orthogonal switching of a single supramolecular complex. Nat. Commun. 2012, 3, 1207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Grubert, L.; Hecht, S.; Bléger, D. Orthogonal switching in four-state azobenzene mixed-dimers. Chem. Commun. 2017, 53, 3323–3326. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Quan, M.; Zhang, L.; Yang, H.; Lu, Y. Photothermal effect of azopyridine compounds and their applications. RSC Adv. 2015, 5, 4675–4680. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Cuadrado, A.; Ortega, M.; Nonell, S.; Velasco, D. A photoswitchable bis-azo derivative with a high temporal resolution. Chem. Commun. 2014, 50, 11462–11464. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff Base/Ester Liquid Crystals with Different Lateral Substituents: Mesophase Behaviour and DFT Calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Naoum, M.M.; Metwally, N.H.; Abd Eltawab, M.M.; Ahmed, H.A. Polarity and steric effect of the lateral substituent on the mesophase behaviour of some newly prepared liquid crystals. Liq. Cryst. 2015, 42, 1351–1369. [Google Scholar] [CrossRef]
- Ahmed, H.; Saad, G. Mesophase behaviour of laterally di-fluoro-substituted four-ring compounds. Liq. Cryst. 2015, 42, 1765–1772. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Effect of the relative orientation of the two fluoro-substituents on the mesophase behavior of phenylazophenyl benzoates. Mol. Cryst. Liq. Cryst. 2012, 562, 43–65. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Ahmed, H. Liquid crystalline behaviour of model compounds di-laterally substituted with different polar groups. Liq. Cryst. 2011, 38, 511–519. [Google Scholar] [CrossRef]
- Naoum, M.; Ahmed, H. Effect of dipole moment and conformation on the mesophase behavior of di-laterally substituted phenylazophenyl benzoate liquid crystals. Thermochim. Acta 2011, 521, 202–210. [Google Scholar] [CrossRef]
- Naoum, M.; Mohammady, S.; Ahmed, H. Lateral protrusion and mesophase behaviour in pure and mixed states of model compounds of the type 4-(4′-substituted phenylazo)-2-(or 3-) methyl phenyl-4’-alkoxy benzoates. Liq. Cryst. 2010, 37, 1245–1257. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, H. Different morphologies of self-assembled nanofibers fabricated with amphiphilic low-molecular-weight azopyridinium salts. RSC Adv. 2013, 3, 22155–22159. [Google Scholar] [CrossRef]
- Zhou, W.; Kobayashi, T.; Zhu, H.; Yu, H. Electrically conductive hybrid nanofibers constructed with two amphiphilic salt components. Chem. Commun. 2011, 47, 12768–12770. [Google Scholar] [CrossRef]
- Zhang, H.; Hao, R.; Jackson, J.K.; Chiao, M.; Yu, H. Janus ultrathin film from multi-level self-assembly at air–water interfaces. Chem. Commun. 2014, 50, 14843–14846. [Google Scholar] [CrossRef]
- Mamiya, J.-I.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Aoki, K.I.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular hydrogen-bonded liquid crystals formed from 4-(4′-pyridylazophenyl)-4″-alkoxy benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2008, 487, 74–91. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. I. 4-(4′-Pyridylazophenyl)-4″-substituted benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2009, 506, 22–33. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.G.A.; Almllal, W.A. Supramolecular Liquid Crystals Induced by Hydrogen-Bonding Interactions between Non-Mesomorphic Compounds. II. Effect of Lateral Substitution. Mol. Cryst. Liq. Cryst. 2010, 518, 109–128. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl) benzo [d] thiazol-6-yl) methylene) malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical study of hydrogen-bonded supramolecular liquid crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Quinazolin-4-yl-sulfanylacetyl-hydrazone derivatives; Synthesis, molecular structure and electronic properties. J. Mol. Struct. 2013, 1049, 177–188. [Google Scholar] [CrossRef]
- Soliman, S.M.; Hagar, M.; Ibid, F.; El Sayed, H. Experimental and theoretical spectroscopic studies, HOMO–LUMO, NBO analyses and thione–thiol tautomerism of a new hybrid of 1, 3, 4-oxadiazole-thione with quinazolin-4-one. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Hagar, M.; Soliman, S.M.; Ibid, F.; El Sayed, H. Synthesis, molecular structure and spectroscopic studies of some new quinazolin-4 (3H)-one derivatives; an account on the N-versus S-Alkylation. J. Mol. Struct. 2016, 1108, 667–679. [Google Scholar] [CrossRef]
- Aboelnaga, A.; Hagar, M.; Soliman, S.M. Ultrasonic Synthesis, Molecular Structure and Mechanistic Study of 1, 3-Dipolar Cycloaddition Reaction of 1-Alkynylpyridinium-3-olate and Acetylene Derivatives. Molecules 2016, 21, 848. [Google Scholar] [CrossRef]
- Paterson, D.A.; Gao, M.; Kim, Y.-K.; Jamali, A.; Finley, K.L.; Robles-Hernández, B.; Diez-Berart, S.; Salud, J.; de la Fuente, M.R.; Timimi, B.A. Understanding the twist-bend nematic phase: The characterisation of 1-(4-cyanobiphenyl-4′-yloxy)-6-(4-cyanobiphenyl-4′-yl) hexane (CB6OCB) and comparison with CB7CB. Soft Matter 2016, 12, 6827–6840. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino) phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A New Chiral Boron–dipyrromethene (BODIPY)–based Fluorescent Probe: Molecular docking, DFT, Antibacterial and Antioxidant approaches. J. Biomol. Struct. Dyn. 2019. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef] [PubMed]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2019. [Google Scholar] [CrossRef]
- Alnoman, R.; Ahmed, H.A.; Hagar, M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef]
- Hagar, M.; Chaieb, K.; Parveen, S.; Ahmed, H.; Alnoman, R. N-alkyl 2-pyridone versus O-alkyl 2-pyridol: Ultrasonic synthesis, DFT, docking studies and their antimicrobial evaluation. J. Mol. Struct. 2020, 1199, 126926. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Cleland, W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef] [PubMed]
- Lizu, M.; Lutfor, M.; Surugau, N.; How, S.; Arshad, S.E. Synthesis and characterization of ethyl cellulose–based liquid crystals containing azobenzene chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR investigation of two alkyl-p-terphenyl-4, 4 ″-dicarboxylates in their crystalline, smectic and isotropic phases. Mol. Cryst. Liq. Cryst. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; Marcelis, A.T.M.; Storey, J.M.D.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.; Henderson, P.; Storey, J.; Gorecka, E. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Thaker, B.; Kanojiya, J.; Tandel, R. Effects of different terminal substituents on the mesomorphic behavior of some azo-schiff base and azo-ester-based liquid crystals. Mol. Cryst. Liq. Cryst. 2010, 528, 120–137. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Saad, G. Impact of the proportionation of dialkoxy chain length on the mesophase behaviour of Schiff base/ester liquid crystals; experimental and theoretical study. Liq. Cryst. 2019, 46, 1–10. [Google Scholar] [CrossRef]
- Imrie, C. Laterally substituted dimeric liquid crystals. Liq. Cryst. 1989, 6, 391–396. [Google Scholar] [CrossRef]
- Schroeder, J.; Bristol, D. Liquid crystals. IV. Effects of terminal substituents on the nematic mesomorphism of p-phenylene dibenzoates. J. Org. Chem. 1973, 38, 3160–3164. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1–12. [Google Scholar] [CrossRef]
- Cammenga, H.K.; Eysel, W.; Gmelin, E.; Hemminger, W.; Höhne, G.W.; Sarge, S.M. The temperature calibration of scanning calorimeters: Part 2. Calibration substances. Thermochim. Acta 1993, 219, 333–342. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.B.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision A 02; Gaussian Inc.: Wallingford, CT, USA, 2009; p. 200. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
Sample Availability: Samples of all compounds are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).