γ-Tocotrienol and α-Tocopherol Ether Acetate Enhance Docetaxel Activity in Drug-Resistant Prostate Cancer Cells
Abstract
:1. Introduction
2. Results
2.1. IC50 Determination
2.2. Cell Viability Responses for MDRSM Combinations
2.3. Chou-Talalay Analysis
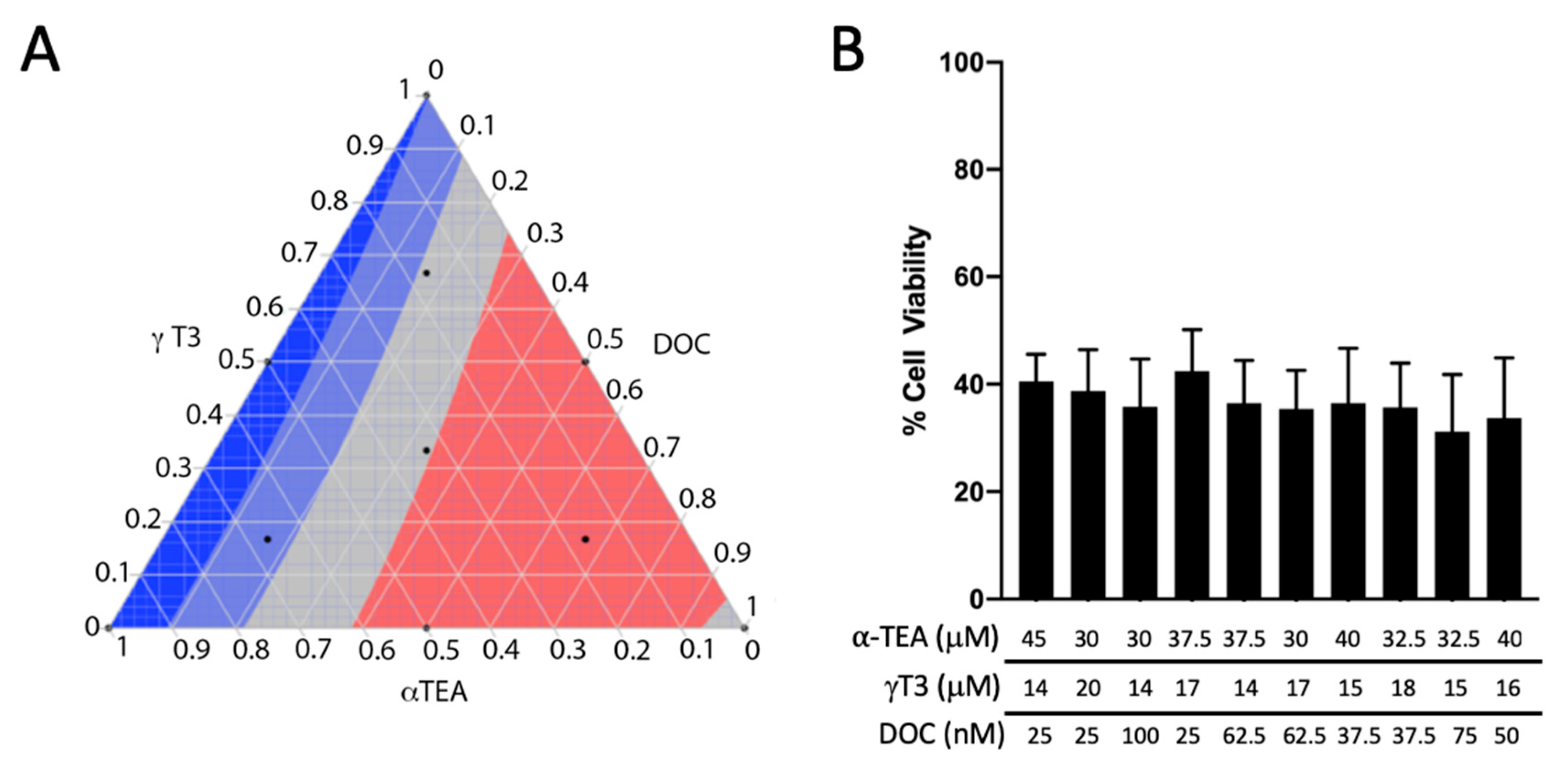
2.4. Cell Viability Response in DOC-Resistant PC-3 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Selection of Docetaxel-Resistant Clones
4.3. Reagents
4.4. Cell Viability
4.5. IC50 Calculation
4.6. Mixture Design and Response Surfaces
4.7. Chou-Talalay Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Gundem, G.; Van Loo, P.; Kremeyer, B.; Alexandrov, L.B.; Tubio, J.M.C.; Papaemmanuil, E.; Brewer, D.S.; Kallio, H.M.L.; Hognas, G.; Annala, M.; et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015, 520, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.B.; Matulewicz, R.S.; Eggener, S.E.; Schaeffer, E.M. Increasing incidence of metastatic prostate cancer in the United States (2004–2013). Prostate Cancer Prostatic Dis. 2016, 19, 395–397. [Google Scholar] [CrossRef] [Green Version]
- Singer, E.A.; Golijanin, D.J.; Miyamoto, H.; Messing, E.M. Androgen deprivation therapy for prostate cancer. Expert Opin. Pharmacol. 2008, 9, 211–228. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, J.; George, D.J.; Armstrong, A.J. Enzalutamide versus abiraterone acetate for the treatment of men with metastatic castration-resistant prostate cancer. Expert Opin. Pharmacol. 2015, 16, 473–485. [Google Scholar] [CrossRef]
- Liang, X.J.; Meng, H.; Wang, Y.; He, H.; Meng, J.; Lu, J.; Wang, P.C.; Zhao, Y.; Gao, X.; Sun, B.; et al. Metallofullerene nanoparticles circumvent tumor resistance to cisplatin by reactivating endocytosis. Proc. Natl. Acad. Sci. USA 2010, 107, 7449–7454. [Google Scholar] [CrossRef] [Green Version]
- Theyer, G.; Schirmbock, M.; Thalhammer, T.; Sherwood, E.R.; Baumgartner, G.; Hamilton, G. Role of the MDR-1-encoded multiple drug resistance phenotype in prostate cancer cell lines. J. Urol. 1993, 150, 1544–1547. [Google Scholar] [CrossRef]
- Ho, M.Y.; Mackey, J.R. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef]
- Baker, J.; Ajani, J.; Scotté, F.; Winther, D.; Martin, M.; Aapro, M.S.; von Minckwitz, G. Docetaxel-related side effects and their management. Eur. J. Oncol. Nurs. 2009, 13, 49–59. [Google Scholar] [CrossRef]
- Zimmermann, G.R.; Lehar, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Oblad, R.; Doughty, H.; Lawson, J.; Christensen, M.; Kenealey, J. Application of Mixture Design Response Surface Methodology for Combination Chemotherapy in PC-3 Human Prostate Cancer Cells. Mol. Pharmacol. 2018, 94, 907–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; de Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Galsky, M.D.; Dritselis, A.; Kirkpatrick, P.; Oh, W.K. Cabazitaxel. Nat. Rev. Drug Discov. 2010, 9, 677–678. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef]
- Jia, L.; Yu, W.; Wang, P.; Sanders, B.G.; Kline, K. In vivo and in vitro studies of anticancer actions of alpha-TEA for human prostate cancer cells. Prostate 2008, 68, 849–860. [Google Scholar] [CrossRef]
- Curti, B.D.; Akporiaye, E.; Sutcliffe, K.; Bahjat, K.S.; Koguchi, Y.; Cramer, J.; Urba, W. Phase I study of alpha-tocopherlyoxyacetic acid in patients with advanced cancer. J. Immunother. Cancer 2015, 3, 148. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Gao, P.; Xu, Y.; Li, Z. alpha-TEA inhibits the growth and motility of human colon cancer cells via targeting RhoA/ROCK signaling. Mol. Med. Rep. 2016, 14, 2534–2540. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Shun, M.C.; Anderson, K.; Chen, H.; Sanders, B.G.; Kline, K. α-TEA inhibits survival and enhances death pathways in cisplatin sensitive and resistant human ovarian cancer cells. Apoptosis 2006, 11, 1813–1823. [Google Scholar] [CrossRef] [PubMed]
- Hahn, T.; Jagadish, B.; Mash, E.A.; Garrison, K.; Akporiaye, E.T. α-Tocopheryloxyacetic acid: A novel chemotherapeutic that stimulates the antitumor immune response. Breast Cancer Res. 2011, 13, R4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Yu, W.; Tiwary, R.; Park, S.K.; Xiong, A.; Sanders, B.G.; Kline, K. α-TEA-induced death receptor dependent apoptosis involves activation of acid sphingomyelinase and elevated ceramide-enriched cell surface membranes. Cancer Cell Int. 2010, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahsan, H.; Ahad, A.; Siddiqui, W.A. A review of characterization of tocotrienols from plant oils and foods. J. Chem. Biol. 2015, 8, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Kanchi, M.M.; Shanmugam, M.K.; Rane, G.; Sethi, G.; Kumar, A.P. Tocotrienols: The unsaturated sidekick shifting new paradigms in vitamin E therapeutics. Drug Discov. Today 2017, 22, 1765–1781. [Google Scholar] [CrossRef]
- Conte, C.; Floridi, A.; Aisa, C.; Piroddi, M.; Floridi, A.; Galli, F. Gamma-tocotrienol metabolism and antiproliferative effect in prostate cancer cells. Ann. N. Y. Acad. Sci. 2004, 1031, 391–394. [Google Scholar] [CrossRef]
- Yap, W.N.; Chang, P.N.; Han, H.Y.; Lee, D.T.; Ling, M.T.; Wong, Y.C.; Yap, Y.L. Gamma-tocotrienol suppresses prostate cancer cell proliferation and invasion through multiple-signalling pathways. Br. J. Cancer 2008, 99, 1832–1841. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms. IUBMB Life 2019, 71, 495–506. [Google Scholar] [CrossRef]
- Tang, K.D.; Liu, J.; Russell, P.J.; Clements, J.A.; Ling, M.-T. Gamma-Tocotrienol Induces Apoptosis in Prostate Cancer Cells by Targeting the Ang-1/Tie-2 Signalling Pathway. Int. J. Mol. Sci. 2019, 20, 1164. [Google Scholar] [CrossRef] [Green Version]
- Luk, S.U.; Yap, W.N.; Chiu, Y.T.; Lee, D.T.; Ma, S.; Lee, T.K.; Vasireddy, R.S.; Wong, Y.C.; Ching, Y.P.; Nelson, C.; et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer 2011, 128, 2182–2191. [Google Scholar] [CrossRef]
- Anderson, K.; Simmons-Menchaca, M.; Lawson, K.A.; Atkinson, J.; Sanders, B.G.; Kline, K. Differential response of human ovarian cancer cells to induction of apoptosis by vitamin E Succinate and vitamin E analogue, alpha-TEA. Cancer Res. 2004, 64, 4263–4269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilley, W.D.; Bentel, J.M.; Aspinall, J.O.; Hall, R.E.; Horsfall, D.J. Evidence for a novel mechanism of androgen resistance in the human prostate cancer cell line, PC-3. Steroids 1995, 60, 180–186. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Yuan, J.; Qin, W.; Liu, F.; Wang, F.; Zhang, G.; Yang, X. Toll-like receptor 4 ligation confers chemoresistance to docetaxel on PC-3 human prostate cancer cells. Cell Biol. Toxicol. 2012, 28, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.E.; Stone, W.L.; Lee, S.; Whaley, S.; Yang, H.; Qui, M.; Goforth, P.; Sherman, D.; McHaffie, D.; Krishnan, K. Comparative effects of RRR-alpha- and RRR-gamma-tocopherol on proliferation and apoptosis in human colon cancer cell lines. BMC Cancer 2006, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Meganathan, P.; Jabir, R.S.; Fuang, H.G.; Bhoo-Pathy, N.; Choudhury, R.B.; Taib, N.A.; Nesaretnam, K.; Chik, Z. A new formulation of Gamma Delta Tocotrienol has superior bioavailability compared to existing Tocotrienol-Rich Fraction in healthy human subjects. Sci. Rep. 2015, 5, 13550. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, Y.; Yu, X.; Yin, L.; Peng, Y.; Gao, Y.; Zhu, Q.; Cao, T.; Yang, Y.; Fan, X.; et al. FOXM1 contributes to taxane resistance by regulating UHRF1-controlled cancer cell stemness. Cell Death Dis. 2018, 9, 562. [Google Scholar] [CrossRef] [Green Version]
- McAnally, J.A.; Gupta, J.; Sodhani, S.; Bravo, L.; Mo, H. Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp. Biol. Med. 2007, 232, 523–531. [Google Scholar]
- Jayakumar, S.; Kunwar, A.; Sandur, S.K.; Pandey, B.N.; Chaubey, R.C. Differential response of DU145 and PC3 prostate cancer cells to ionizing radiation: Role of reactive oxygen species, GSH and Nrf2 in radiosensitivity. BBA Gen. Subj. 2014, 1840, 485–494. [Google Scholar] [CrossRef]
- Wolf, G. How an increased intake of alpha-tocopherol can suppress the bioavailability of gamma-tocopherol. Nutr. Rev. 2006, 64, 295–299. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.M.; Tangen, C.M.; Crowley, J.J.; Lucia, M.S.; Goodman, P.J.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; et al. Vitamin E and the risk of prostate cancer: The Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2011, 306, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Li, G.; Yang, Z.; Guan, F.; Chen, A.; Ju, J. Cancer prevention by tocopherols and tea polyphenols. Cancer Lett. 2013, 334, 79–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Not available. |




| 0.25 × IC50 | 0.5 × IC50 | 1 × IC50 | 2 × IC50 | 4 × IC50 | |
|---|---|---|---|---|---|
| γ-T3/α-TEA | 25,798.9 | 28,716.9 | 31,965.0 | 34,381.7 | 36,211.6 |
| α-TEA/DOC | 1.09516 | 1.05744 | 1.02102 | 0.99697 | 0.98021 |
| γ-T3/DOC | 0.94101 | 0.93797 | 0.93494 | 0.93288 | 0.93142 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asay, S.; Graham, A.; Hollingsworth, S.; Barnes, B.; Oblad, R.V.; Michaelis, D.J.; Kenealey, J.D. γ-Tocotrienol and α-Tocopherol Ether Acetate Enhance Docetaxel Activity in Drug-Resistant Prostate Cancer Cells. Molecules 2020, 25, 398. https://doi.org/10.3390/molecules25020398
Asay S, Graham A, Hollingsworth S, Barnes B, Oblad RV, Michaelis DJ, Kenealey JD. γ-Tocotrienol and α-Tocopherol Ether Acetate Enhance Docetaxel Activity in Drug-Resistant Prostate Cancer Cells. Molecules. 2020; 25(2):398. https://doi.org/10.3390/molecules25020398
Chicago/Turabian StyleAsay, Spencer, Andrew Graham, Sydney Hollingsworth, Bradley Barnes, Richard V. Oblad, David J. Michaelis, and Jason D. Kenealey. 2020. "γ-Tocotrienol and α-Tocopherol Ether Acetate Enhance Docetaxel Activity in Drug-Resistant Prostate Cancer Cells" Molecules 25, no. 2: 398. https://doi.org/10.3390/molecules25020398
APA StyleAsay, S., Graham, A., Hollingsworth, S., Barnes, B., Oblad, R. V., Michaelis, D. J., & Kenealey, J. D. (2020). γ-Tocotrienol and α-Tocopherol Ether Acetate Enhance Docetaxel Activity in Drug-Resistant Prostate Cancer Cells. Molecules, 25(2), 398. https://doi.org/10.3390/molecules25020398





