Homochiral Supramolecular Thin Film from Self-Assembly of Achiral Triarylamine Molecules by Circularly Polarized Light
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Self-Assembly
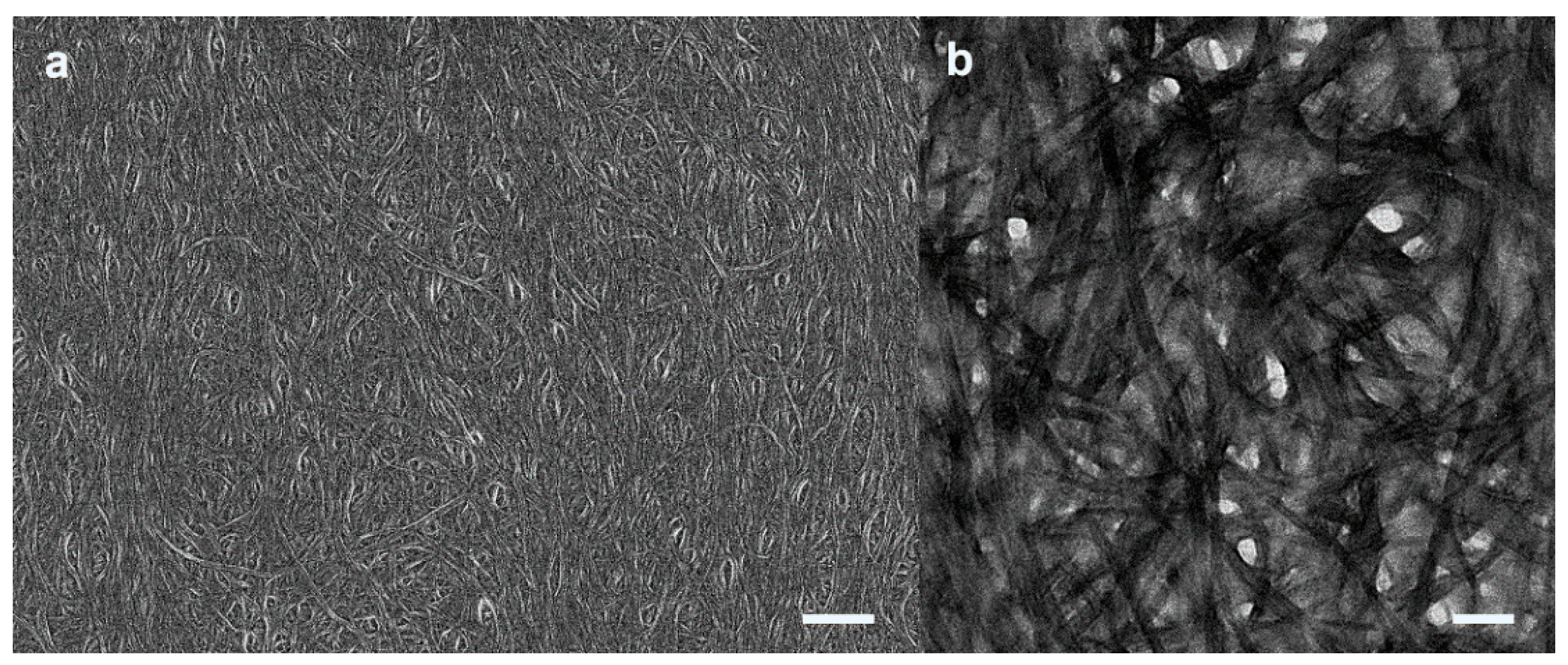
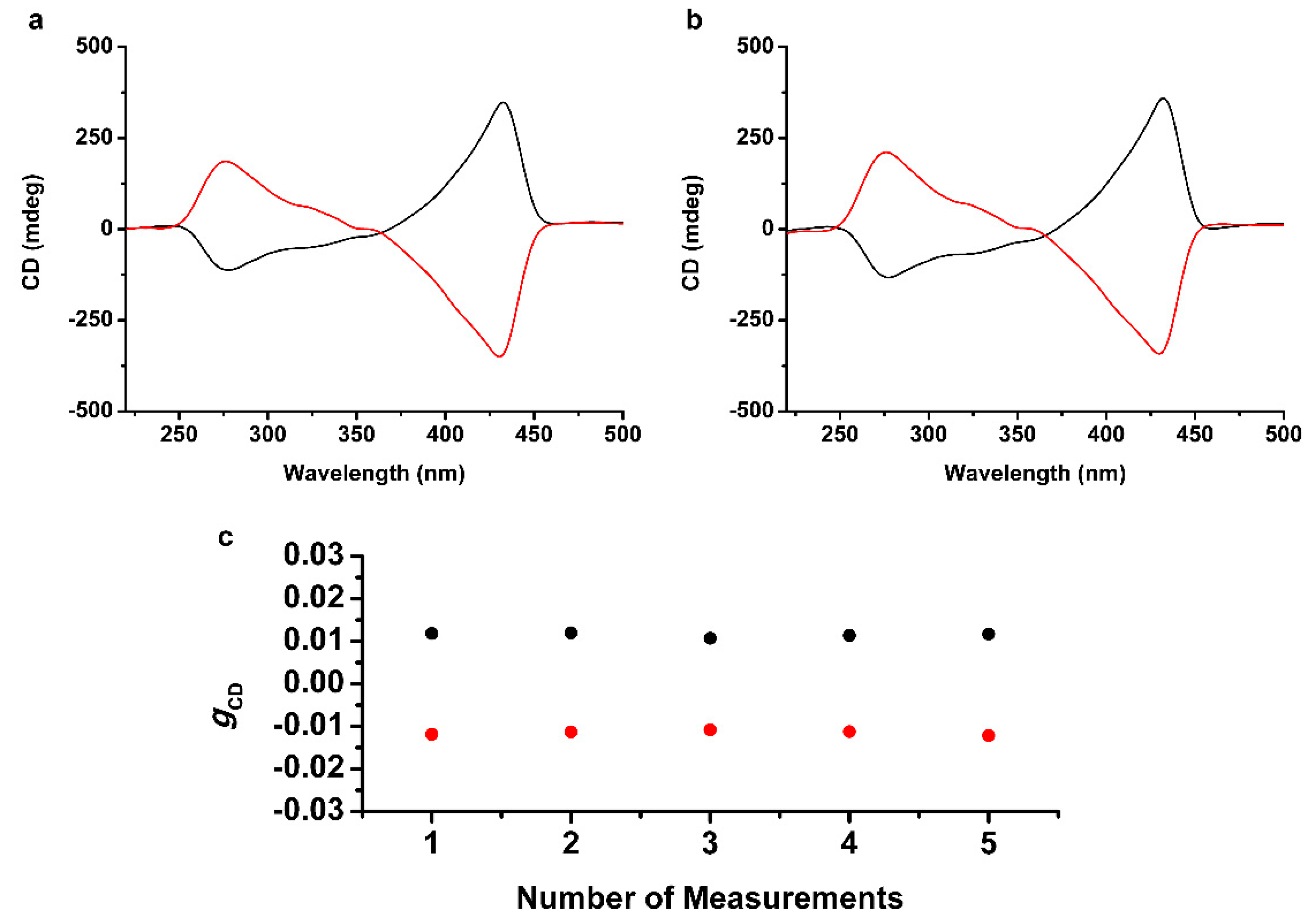
2.2. Formation of Homochiral Supramolecular Thin Film by CPL
2.3. Chiral Stability of the Photopolymerized Homochiral Thin Film
3. Materials and Methods
3.1. Materials and Characterization
3.2. Synthetic Procedures and Methods
3.2.1. Synthesis of an Amine-Substituted TAA Compound 2
3.2.2. Synthesis of TSADA
3.2.3. Density-Functional-Tight-Binding (DFTB) Simulation
3.2.4. Formation of Homochiral Supramolecular Thin Film and Polymerization by Light
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hegstrom, R.A.; Kondepudi, D.K. The Handedness of the Universe. Sci. Am. 1990, 262, 108–115. [Google Scholar] [CrossRef]
- Kondepudi, D.K.; Kaufman, R.J.; Singh, N. Chiral Symmetry Breaking in Sodium Chlorate Crystallizaton. Science 1990, 250, 975–976. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.F. Origins of biomolecular handedness. Nature 1984, 311, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhang, L.; Wang, T. Supramolecular chirality in self-Assembled systems. Chem. Rev. 2015, 115, 7304–7397. [Google Scholar] [CrossRef]
- Yashima, E.; Ousaka, N.; Taura, D.; Shimomura, K.; Ikai, T.; Maeda, K. Supramolecular Helical Systems: Helical Assemblies of Small Molecules, Foldamers, and Polymers with Chiral Amplification and Their Functions. Chem. Rev. 2016, 116, 13752–13990. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Yan, F.; Liu, C.; Sang, Y.; Tian, F.; Feng, Q.; Duan, P.; Zhang, L.; Shi, X.; et al. Control over the emerging chirality in supramolecular gels and solutions by chiral microvortices in milliseconds. Nat. Commun. 2018, 9, 2599. [Google Scholar] [CrossRef]
- Sang, Y.; Yang, D.; Duan, P.; Liu, M. Towards homochiral supramolecular entities from achiral molecules by vortex mixing-accompanied self-assembly. Chem. Sci. 2019, 10, 2718–2724. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.W.; Izumi, T.; Hoshino, Y.; Takanishi, Y.; Ishikawa, K.; Watanabe, J.; Takezoe, H. Circular-polarization-induced enantiomeric excess in liquid crystals of an achiral, bent-shaped mesogen. Angew. Chem. Int. Ed. 2006, 45, 1382–1385. [Google Scholar] [CrossRef]
- Jiang, H.; Pan, X.-J.; Lei, Z.-Y.; Zou, G.; Zhang, Q.-J.; Wang, K.-Y. Control of supramolecular chirality for polydiacetylene LB films with the command azobenzene derivative monolayer. J. Mater. Chem. 2011, 21, 4518–4522. [Google Scholar] [CrossRef]
- Wang, L.; Yin, L.; Zhang, W.; Zhu, X.; Fujiki, M. Circularly Polarized Light with Sense and Wavelengths to Regulate Azobenzene Supramolecular Chirality in Optofluidic Medium. J. Am. Chem. Soc. 2017, 139, 13218–13226. [Google Scholar] [CrossRef]
- Yeom, J.; Yeom, B.; Chan, H.; Smith, K.W.; Dominguez-Medina, S.; Bahng, J.H.; Zhao, G.; Chang, W.S.; Chang, S.J.; Chuvilin, A.; et al. Chiral templating of self-assembling nanostructures by circularly polarized light. Nat. Mater. 2015, 14, 66–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Lee, J.; Kim, W.Y.; Kim, H.; Lee, S.; Lee, H.C.; Lee, Y.S.; Seo, M.; Kim, S.Y. Induction and control of supramolecular chirality by light in self-assembled helical nanostructures. Nat. Commun. 2015, 6, 6959. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Xie, Y.; Zhang, H.; He, C.; Zhang, Q.; Zou, G. Chiral induction, modulation and locking in porphyrin based supramolecular assemblies with circularly polarized light. Chem. Commun. 2019, 55, 4953–4956. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhu, T.; He, C.; Zhang, Y.; Zhang, Q.; Zou, G. Chiral induction, transfer and modulation in C 3-symmetric columnar liquid crystalline assemblies. J. Mater. Chem. C 2017, 5, 5135–5142. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, J.; Yang, G.; Zhang, H.; Zhang, Q.; Wang, F.; Zou, G. Mesomorphism, polymerization, and chirality induction in α-cyanostilbene-functionalized diacetylene-assembled films: Photo-triggered Z/E isomerization. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 2458–2466. [Google Scholar] [CrossRef]
- He, C.; Yang, G.; Kuai, Y.; Shan, S.; Yang, L.; Hu, J.; Zhang, D.; Zhang, Q.; Zou, G. Dissymmetry enhancement in enantioselective synthesis of helical polydiacetylene by application of superchiral light. Nat. Commun. 2018, 9, 5117. [Google Scholar] [CrossRef]
- Ribó, J.M.; Crusats, J.; Sagués, F.; Claret, J.; Rubires, R. Chiral Sign Induction by Vortices During the Formation of Mesophases in Stirred Solutions. Science 2001, 292, 2063–2066. [Google Scholar] [CrossRef]
- Okano, K.; Taguchi, M.; Fujiki, M.; Yamashita, T. Circularly Polarized Luminescence of Rhodamine B in a Supramolecular Chiral Medium Formed by a Vortex Flow. Angew. Chem. Int. Ed. 2011, 50, 12474–12477. [Google Scholar] [CrossRef]
- Micali, N.; Engelkamp, H.; van Rhee, P.G.; Christianen, P.C.M.; Scolaro, L.M.; Maan, J.C. Selection of supramolecular chirality by application of rotational and magnetic forces. Nat. Chem. 2012, 4, 201–207. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, G.; Xia, H.; Zou, G.; Zhang, Q.; Gao, J. Enantioselective synthesis of helical polydiacetylene by application of linearly polarized light and magnetic field. Nat. Commun. 2014, 5, 5050. [Google Scholar] [CrossRef] [Green Version]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular Polarization in Star-Formation Regions: Implications for Biomolecular Homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef] [PubMed]
- Noorduin, W.L.; Bode, A.A.C.; van der Meijden, M.; Meekes, H.; van Etteger, A.F.; van Enckevort, W.J.P.; Christianen, P.C.M.; Kaptein, B.; Kellogg, R.M.; Rasing, T.; et al. Complete chiral symmetry breaking of an amino acid derivative directed by circularly polarized light. Nat. Chem. 2009, 1, 729–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moulin, E.; Armao, J.J.; Giuseppone, N. Triarylamine-Based Supramolecular Polymers: Structures, Dynamics, and Functions. Acc. Chem. Res. 2019, 52, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Moulin, E.; Niess, F.; Maaloum, M.; Buhler, E.; Nyrkova, I.; Giuseppone, N. The hierarchical self-assembly of charge nanocarriers: A highly cooperative process promoted by visible light. Angew. Chem. Int. Ed. 2010, 49, 6974–6978. [Google Scholar] [CrossRef] [PubMed]
- Faramarzi, V.; Niess, F.; Moulin, E.; Maaloum, M.; Dayen, J.F.; Beaufrand, J.B.; Zanettini, S.; Doudin, B.; Giuseppone, N. Light-triggered self-construction of supramolecular organic nanowires as metallic interconnects. Nat. Chem. 2012, 4, 485–490. [Google Scholar] [CrossRef]
- Armao, J.J.; Maaloum, M.; Ellis, T.; Fuks, G.; Rawiso, M.; Moulin, E.; Giuseppone, N. Healable supramolecular polymers as organic metals. J. Am. Chem. Soc. 2014, 136, 11382–11388. [Google Scholar] [CrossRef]
- Yasuda, Y.; Takebe, Y.; Fukumoto, M.; Inada, H.; Shirota, Y. 4,4′,4”-Tris (stearoylamino) triphenylamine as a novel material for functional molecular gels. Adv. Mater. 1996, 8, 740–741. [Google Scholar] [CrossRef]
- Kamiyama, T.; Yasuda, Y.; Shirota, Y. A Novel Family of Low Molecular-Weight Organic Gels. 1,3,5-Tris (N-phenyl-N-4-stearoyl-aminophenylamino) benzene and 4,4′,4”-Tris (N-phenyl-N-4-stearoylaminophenyl-amino) triphenylamine/Organic Solvent Systems. Polym. J. 1999, 31, 1165–1170. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, Y.; Kamiyama, T.; Shirota, Y. Ionic conductivities of low molecular-weight organic gels and their application as electrochromic materials. Electrochim. Acta 2000, 45, 1537–1541. [Google Scholar] [CrossRef]
- Kim, T.; Mori, T.; Aida, T.; Miyajima, D. Dynamic propeller conformation for the unprecedentedly high degree of chiral amplification of supramolecular helices. Chem. Sci. 2016, 7, 6689–6694. [Google Scholar] [CrossRef] [Green Version]
- Mtangi, W.; Tassinari, F.; Vankayala, K.; Vargas Jentzsch, A.; Adelizzi, B.; Palmans, A.R.A.; Fontanesi, C.; Meijer, E.W.; Naaman, R. Control of Electrons’ Spin Eliminates Hydrogen Peroxide Formation during Water Splitting. J. Am. Chem. Soc. 2017, 139, 2794–2798. [Google Scholar] [CrossRef] [PubMed]
- Adelizzi, B.; Aloi, A.; Markvoort, A.J.; Ten Eikelder, H.M.M.; Voets, I.K.; Palmans, A.R.A.; Meijer, E.W. Supramolecular Block Copolymers under Thermodynamic Control. J. Am. Chem. Soc. 2018, 140, 7168–7175. [Google Scholar] [CrossRef] [PubMed]
- Van Gorp, J.J.; Vekemans, J.A.J.M.; Meijer, E.W. C3-Symmetrical Supramolecular Architectures: Fibers and Organic Gels from Discotic Trisamides and Trisureas. J. Am. Chem. Soc. 2002, 124, 14759–14769. [Google Scholar] [CrossRef] [PubMed]
- Haedler, A.T.; Meskers, S.C.J.; Zha, R.H.; Kivala, M.; Schmidt, H.W.; Meijer, E.W. Pathway Complexity in the Enantioselective Self-Assembly of Functional Carbonyl-Bridged Triarylamine Trisamides. J. Am. Chem. Soc. 2016, 138, 10539–10545. [Google Scholar] [CrossRef] [PubMed]
- Adelizzi, B.; Filot, I.A.W.; Palmans, A.R.A.; Meijer, E.W. Unravelling the Pathway Complexity in Conformationally Flexible N-Centered Triarylamine Trisamides. Chem. Eur. J. 2017, 23, 6103–6110. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Chen, T.; Huang, S.; Li, L.; Peng, H. Chromatic polydiacetylene with novel sensitivity. Chem. Soc. Rev. 2010, 39, 4244–4257. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, G.; Peng, X.; Yoon, J. Biosensors and chemosensors based on the optical responses of polydiacetylenes. Chem. Soc. Rev. 2012, 41, 4610–4630. [Google Scholar] [CrossRef]
- Karunakaran, S.C.; Cafferty, B.J.; Weigert-Muñoz, A.; Schuster, G.B.; Hud, N.V. Spontaneous Symmetry Breaking in the Formation of Supramolecular Polymers: Implications for the Origin of Biological Homochirality. Angew. Chem. Int. Ed. 2019, 58, 1453–1457. [Google Scholar] [CrossRef]
- Green, M.M.; Reidy, M.P.; Johnson, R.J.; Darling, G.; O’Leary, D.J.; Willson, G. Macromolecular Stereochemistry: The Out-of-Proportion Influence of Optically Active Comonomers on the Conformational Characteristics of Polyisocyanates. The Sergeants and Soldiers Experiment. J. Am. Chem. Soc. 1989, 111, 6452–6454. [Google Scholar] [CrossRef]
- Palmans, A.R.A.; Vekemans, J.A.J.M.; Havinga, E.E.; Meijer, E.W. Sergeants-and-soldiers principle in chiral columnar stacks of disc-shaped molecules with C3 symmetry. Angew. Chem. Int. Ed. Engl. 1997, 36, 2648–2651. [Google Scholar] [CrossRef] [Green Version]
- Ohira, A.; Okoshi, K.; Fujiki, M.; Kunitake, M.; Naito, M.; Hagihara, T. Versatile helical polymer films: Chiroptical inversion switching and memory with re-writable (RW) and write-once read-many (WORM) modes. Adv. Mater. 2004, 16, 1645–1650. [Google Scholar] [CrossRef]
- Narushima, T.; Okamoto, H. Circular Dichroism Microscopy Free from Commingling Linear Dichroism via Discretely Modulated Circular Polarization. Sci. Rep. 2016, 6, 35731. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-H.; Hsu, H.-S.; Sun, S.-J.; Chang, Y.-Y.; Misiuna, P.; Baczewski, L.T. Extraction of magnetic circular dichroism effects from blended mixture of magnetic linear dichroism signals in the cobalt/Scotch tape system. Sci. Rep. 2019, 9, 17192. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Han, L.; Jiang, H.; Zou, G.; Zhang, Q.; Zhang, D.; Wang, P.; Ming, H. Enantioselective synthesis of helical polydiacetylenes in the visible light region. Chem. Commun. 2014, 50, 2338–2340. [Google Scholar] [CrossRef] [Green Version]
- Körsten, S.; Mohr, G.J. Star-shaped tripodal chemosensors for the detection of aliphatic amines. Chem. Eur. J. 2011, 17, 969–975. [Google Scholar] [CrossRef]
- Gaus, M.; Cui, Q.; Elstner, M. DFTB3: Extension of the Self-Consistent-Charge Density-Functional Tight-Binding Method (SCC-DFTB). J. Chem. Theory Comput. 2011, 7, 931–948. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Korth, M.; Pitoňák, M.; Řezáč, J.; Hobza, P. A Transferable H-Bonding Correction for Semiempirical Quantum-Chemical Methods. J. Chem. Theory Comput. 2010, 6, 344–352. [Google Scholar] [CrossRef]
- Řezáč, J. Empirical Self-Consistent Correction for the Description of Hydrogen Bonds in DFTB3. J. Chem. Theory Comput. 2017, 13, 4804–4817. [Google Scholar] [CrossRef]
- Aradi, B.; Hourahine, B.; Frauenheim, T. DFTB+, a Sparse Matrix-Based Implementation of the DFTB Method. J. Phys. Chem. A 2007, 111, 5678–5684. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compound TSADA is available from the authors. |




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, C.; Lee, J.; Kim, T.; Lim, J.; Park, J.; Kim, W.Y.; Kim, S.Y. Homochiral Supramolecular Thin Film from Self-Assembly of Achiral Triarylamine Molecules by Circularly Polarized Light. Molecules 2020, 25, 402. https://doi.org/10.3390/molecules25020402
Park C, Lee J, Kim T, Lim J, Park J, Kim WY, Kim SY. Homochiral Supramolecular Thin Film from Self-Assembly of Achiral Triarylamine Molecules by Circularly Polarized Light. Molecules. 2020; 25(2):402. https://doi.org/10.3390/molecules25020402
Chicago/Turabian StylePark, Changjun, Jinhee Lee, Taehyoung Kim, Jaechang Lim, Jeyoung Park, Woo Youn Kim, and Sang Youl Kim. 2020. "Homochiral Supramolecular Thin Film from Self-Assembly of Achiral Triarylamine Molecules by Circularly Polarized Light" Molecules 25, no. 2: 402. https://doi.org/10.3390/molecules25020402
APA StylePark, C., Lee, J., Kim, T., Lim, J., Park, J., Kim, W. Y., & Kim, S. Y. (2020). Homochiral Supramolecular Thin Film from Self-Assembly of Achiral Triarylamine Molecules by Circularly Polarized Light. Molecules, 25(2), 402. https://doi.org/10.3390/molecules25020402






