Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix dactylifera L.)
Abstract
:1. Introduction
2. Results
2.1. Influence of Elicitors on Biomass Accumulation
2.2. The Effect of Elicitors on Total Phenolic Content (TPC), Total Flavonoid Content (TFC), and Percentage of Radical Scavenging Activity (RSA)
2.3. Impact of Elicitors on Phenolic Compounds Production
3. Discussion
3.1. Biomass Accumulation
3.2. Total Phenolic, Flavonoid Content, and %RSA
3.3. Production of Bioactive Compounds
4. Materials and Methods
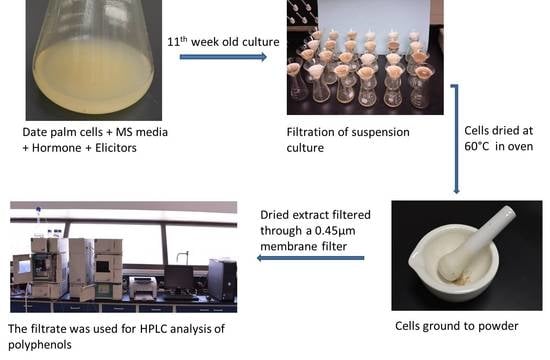
4.1. Plant Material
4.2. Cell Suspension Culture
4.3. Biomass
4.4. Extraction of Cell Culture
4.5. Total Phenolic and Flavonoid Content
4.6. Determination of Radical Scavenging Activity
4.7. Chromatographic Analysis of Phenolic Compounds
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al-Khayri, J.M.; Naik, P.M. Date palm micropropagation: Advances and applications. Cienc. Agrotec. 2017, 41, 347–358. [Google Scholar] [CrossRef] [Green Version]
- Vayalil, P.K. Date fruits (Phoenix dactylifera Linn): An emerging medicinal food. Crit. Rev. Food Sci. Nutr. 2012, 52, 249–271. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Handoussa, H.; Fekry, M.I.; Wessjohann, L.A. Metabolite profiling in 18 Saudi date palm fruit cultivars and their antioxidant potential via UPLC-qTOF-MS and multivariate data analyses. Food Funct. 2016, 7, 1077–1086. [Google Scholar] [CrossRef]
- Calderon-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A review on the dietary flavonoid kaempferol. MiniRev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef] [PubMed]
- Naik, P.M.; Al-Khayri, J.M. Cell suspension culture as a means to produce polyphenols from date palm (Phoenix dactylifera L.). Cienc. Agrotec. 2018, 42, 464–473. [Google Scholar] [CrossRef] [Green Version]
- Naik, P.M.; Al-Khayri, J.M. Abiotic and biotic elicitors-role in secondary metabolites production through in vitro culture of medicinal plants. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; Shanker, A.K., Shankar, C., Eds.; InTech: Rijeka, Croatia, 2016; pp. 247–277. [Google Scholar]
- Alsoufi, A.S.M.; Pa˛czkowski, C.; Długosz, M.; Szakiel, A. Influence of selected abiotic factors on triterpenoid biosynthesis and saponin secretion in marigold (Calendula officinalis L.) in vitro hairy root cultures. Molecules 2019, 24, 2907. [Google Scholar] [CrossRef] [Green Version]
- Makowski, W.; Tokarz, K.M.; Tokarz, B.; Banasiuk, R.; Witek, K.; Królicka, A. Elicitation-based method for increasing the production of antioxidant and bactericidal phenolic compounds in Dionaea muscipula J. Ellis tissue. Molecules 2020, 25, 1794. [Google Scholar] [CrossRef]
- Sonja, G.S.; Oliver, T.; Stéphane, M.; Alain, D.; Eric, L.; Claude, J.; Daniel, H. Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell Tissue Organ Cult. 2015, 122, 649–663. [Google Scholar] [CrossRef]
- Song, X.; Wu, H.; Yin, Z.; Lian, M.; Yin, C. Endophytic bacteria isolated from Panax ginseng improves ginsenoside accumulation in adventitious ginseng root culture. Molecules 2017, 22, 837. [Google Scholar] [CrossRef] [Green Version]
- Shakya, P.; Marslin, G.; Siram, K.; Beerhues, L.; Franklin, G. Elicitation as a tool to improve the profiles of high-value secondary metabolites and pharmacological properties of Hypericum perforatum. J. Pharm. Pharmacol. 2019, 71, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.M.A.; El-Nour, M.E.M.; Yagi, S.M. Total phenolic and flavonoid contents and antioxidant activity of ginger (Zingiber officinale Rosc.) rhizome, callus and callus treated with some elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ma, Y.; Hu, P.; Zhang, Y.; Chen, J.; Li, X. Elicitation of furanocoumarins in Changium smyrnioides suspension cells. Plant Cell Tissue Organ Cult. 2017, 130, 1–12. [Google Scholar] [CrossRef]
- Açıkgöz, M.A. Establishment of cell suspension cultures of Ocimum basilicum L. and enhanced production of pharmaceutical active ingredients. Ind. Crops Prod. 2020, 148, 112278. [Google Scholar] [CrossRef]
- Silja, P.K.; Gisha, G.P.; Satheeshkumar, K. Enhanced plumbagin accumulation in embryogenic cell suspension cultures of Plumbago rosea L. following elicitation. Plant Cell Tissue Organ Cult. 2014, 119, 469–477. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Czubacka, A.P.; Lukasz, P.; Marcin, D.; Teresa, S.; Anna, W.O. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha piperita cell suspension cultures. Plant Cell Tissue Organ Cult. 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Park, Y.G.; Jeong, B.R. Chemical elicitor-induced modulation of antioxidant metabolism and enhancement of secondary metabolite accumulation in cell suspension cultures of Scrophularia kakudensis Franch. Int. J. Mol. Sci. 2016, 17, 399. [Google Scholar] [CrossRef]
- Ali, M.; Abbasi, B.H.; Ali, G.S. Elicitation of antioxidant secondary metabolites with jasmonates and gibberellic acid in cell suspension cultures of Artemisia absinthium L. Plant Cell Tissue Organ Cult. 2015, 120, 1099–1106. [Google Scholar] [CrossRef]
- Wen, T.; Hao, Y.J.; An, X.L.; Sun, H.D.; Li, Y.R.; Chen, X.; Piao, X.C.; Lian, M.L. Improvement of bioactive compound accumulation in cell cultures of Orostachys cartilaginous A. Bor. through elicitation with salicylic acid and effect of cell extract on bioactive activity. Ind. Crops Prod. 2019, 139, 111570. [Google Scholar] [CrossRef]
- El-Nabarawy, M.A.; El-Kafafi, S.H.; Hamza, M.A.; Omar, M.A. The effect of some factors on stimulating the growth and production of active substances in Zingiber officinale callus cultures. Ann. Agric. Sci. 2015, 60, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dowom, S.A.; Abrishamchia, P.; Radjabianb, T.; Salami, S.A. Enhanced phenolic acids production in regenerated shoot cultures of Salvia virgate Jacq. after elicitation with Ag+ ions, methyl jasmonate and yeast extract. Ind. Crops Prod. 2017, 103, 81–88. [Google Scholar] [CrossRef]
- Biglari, F.; Alkarkhi, A.F.M.; Easa, A.M. Cluster analysis of antioxidant compounds in dates (Phoenix dactylifera): Effect of long-term cold storage. Food Chem. 2009, 112, 998–1001. [Google Scholar] [CrossRef]
- Namdeo, A.G. Plant cell elicitation for production of secondary metabolites: A review. Pharmacogn. Rev. 2007, 1, 69–79. [Google Scholar]
- Baldi, A.; Singh, D.; Dixit, V.K. Dual elicitation for improved production of withaferin A by cell suspension cultures of Withania somnifera. Appl. Biochem. Biotechnol. 2008, 151, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, T.; Wu, Y.; Zhou, Y.; Jiang, Y.; Zhang, L. Effect of elicitors on the metabolites in the suspension cell culture of Salvia miltiorrhiza Bunge. Physiol. Mol. Biol. Plants 2019, 25, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Mallubhotla, S. Elicitation and enhancement of bacoside production using suspension cultures of Bacopa monnieri (L.) Wettst. 3 Biotech 2020, 10, 256. [Google Scholar] [CrossRef]
- Al-Khayri, J.M. Determination of the date palm cell suspension growth curve, optimum plating efficiency, and influence of liquid medium on somatic embryogenesis. Emir. J. Food Agric. 2012, 24, 444–455. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Somatic embryogenesis of date palm (Phoenix dactylifera L.) through cell suspension culture. In Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants, 2nd ed.; Methods in Molecular Biology; Jain, S.M., Ed.; Springer: New York, NY, USA, 2016; Volume 1391, pp. 357–366. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 37, 144–158. [Google Scholar]
- Naik, P.M.; Al-Khayri, J.M. Influence of culture parameters on phenolics, flavonoids and antioxidant activity in cell culture extracts of date palm (Phoenix dactylifera L.). Erwerbs Obstbau 2020, 62, 181–188. [Google Scholar] [CrossRef]
- Thiruvengadam, M.; Praveen, N.; John, K.M.M.; Yang, Y.S.; Kim, S.H.; Chung, I.M. Establishment of Momordica charantia hairy root cultures for the production of phenolic compounds and determination of their biological activities. Plant Cell Tissue Organ Cult. 2014, 118, 545–557. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Elicitors | Total Phenolic Content (mg GAE/100 g DW) | Total Flavonoid Content (mg QE/100 g DW) | Radical Scavenging Activity (%) |
|---|---|---|---|
| Control | 266.750 ± 8.746 ab | 68.660 ± 12.222 bc | 89.86 ± 1.57 a |
| 50 mg/L Pectin | 256.910 ± 23.733 ab | 74.650 ± 9.530 b | 79.75 ± 6.03 a |
| 100 mg/L Pectin | 124.656 ± 3.085 def | 44.300 ± 1.322 cde | 20.85 ± 1.09 ef |
| 200 mg/L Pectin | 127.120 ± 17.031 def | 33.760 ± 2.592 def | 9.700 ± 0.22 f |
| 50 mg/L YE | 209.226 ± 1.024 bcd | 67.453 ± 2.572 bc | 46.66 ± 7.83 cd |
| 100 mg/L YE | 215.883 ± 67.611 bc | 65.676 ± 13.691 bc | 59.59 ± 2.56 b |
| 200 mg/L YE | 234.696 ± 36.761 b | 72.403 ± 6.671 b | 58.49 ± 10.40 bc |
| 50 mg/L SA | 317.986 ± 28.743 a | 47.340 ± 3.412 cd | 86.09 ± 4.54 a |
| 100 mg/L SA | 138.566 ± 22.231 cdef | 4.056 ± 1.423 g | 27.42 ± 1.74 e |
| 200 mg/L SA | 129.000 ± 19.555 def | 13.120 ± 1.016 fg | 22.07 ± 2.97 ef |
| 50 mg/L CdCl2 | 242.620 ± 24.835 ab | 157.286 ± 20.775 a | 85.25 ± 2.27 a |
| 100 mg/L CdCl2 | 140.613 ± 34.443 cde | 0.163 ± 0.026 g | 40.83 ± 3.42 d |
| 200 mg/L CdCl2 | 53.766 ± 11.804 f | 4.053 ± 0.718 g | 21.10 ± 3.24 ef |
| 50 mg/L AgNO3 | 135.716 ± 3.975 cdef | 20.940 ± 5.328 efg | 63.21 ± 3.01 b |
| 100 mg/L AgNO3 | 55.336 ± 13.768 ef | 13.896 ± 2.376 fg | 19.59 ± 1.49 ef |
| 200 mg/L AgNO3 | 59.956 ± 7.156 ef | 14.260 ± 0.508 fg | 19.66 ± 2.09 ef |
| Elicitors | Catechin µg/g DW | Caffeic Acid µg/g DW | Kaempferol µg/g DW | Apigenin µg/g DW |
|---|---|---|---|---|
| Control | 21.8 ± 0.5 b | 23.2 ± 2.4 b | 13.2 ± 0.7 a | 30.4 ± 5.7 a |
| 50 mg/L Pectin | 17.9 ± 0.7 cd | 15.2 ± 0.9 cd | 6.1 ± 0.6 cde | 25.4 ± 1.4 abc |
| 100 mg/L Pectin | 6.3 ± 1.3 f | 6.4 ± 2.1 efgh | 0.0 ± 0.0 f | 23.8 ± 2.0 abc |
| 200 mg/L Pectin | 5.5 ± 1.4 f | 5.6 ± 1.6 fgh | 0.0 ± 0.0 f | 26.8 ± 3.8 ab |
| 50 mg/L YE | 11.2 ± 1.4 e | 10.6 ± 0.7 def | 7.1 ± 0.3 cd | 22.7 ± 2.9 abc |
| 100 mg/L YE | 16.2 ± 1.0 cd | 14.4 ± 2.2 cd | 7.5 ± 0.7 bc | 19.3 ± 0.7 abc |
| 200 mg/L YE | 18.7 ± 1.7 bc | 18.6 ± 1.5 bc | 9.7 ± 1.2 b | 21.2 ± 2.6 abc |
| 50 mg/L SA | 26.6 ± 1.3 a | 31.4 ± 3.8 a | 13.6 ± 1.6 a | 28.3 ± 6.1 ab |
| 100 mg/L SA | 17.6 ± 0.6 cd | 7.5 ± 1.4 efgh | 6.9 ± 1.2 cde | 22.7 ± 3.8 abc |
| 200 mg/L SA | 10.9 ± 0.5 e | 3.6 ± 0.3 gh | 4.4 ± 0.2 e | 18.1 ± 3.9 bc |
| 50 mg/L CdCl2 | 14.7 ± 1.5 d | 16.5 ± 0.6 c | 6.1 ± 1.1 cde | 22.2 ± 3.3 abc |
| 100 mg/L CdCl2 | 6.3 ± 1.6 f | 8.3 ± 1.1 efg | 0.0 ± 0.0 f | 22.3 ± 1.4 abc |
| 200 mg/L CdCl2 | 1.9 ± 0.2 g | 2.5 ± 0.4 h | 0.0 ± 0.0 f | 18.5 ± 3.2 bc |
| 50 mg/L AgNO3 | 7.1 ± 1.5 f | 11.1 ± 1.0 de | 5.3 ± 1.1 cde | 21.6 ± 4.9 abc |
| 100 mg/L AgNO3 | 2.1 ± 0.2 g | 7.1 ± 1.0 efgh | 4.5 ± 0.9 de | 14.2 ± 0.9 c |
| 200 mg/L AgNO3 | 2.0 ± 0.4 g | 5.1 ± 0.7 gh | 0.0 ± 0.0 f | 14.4 ± 1.8 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khayri, J.M.; Naik, P.M. Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix dactylifera L.). Molecules 2020, 25, 4669. https://doi.org/10.3390/molecules25204669
Al-Khayri JM, Naik PM. Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix dactylifera L.). Molecules. 2020; 25(20):4669. https://doi.org/10.3390/molecules25204669
Chicago/Turabian StyleAl-Khayri, Jameel Mohammed, and Poornananda Madhava Naik. 2020. "Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix dactylifera L.)" Molecules 25, no. 20: 4669. https://doi.org/10.3390/molecules25204669
APA StyleAl-Khayri, J. M., & Naik, P. M. (2020). Elicitor-Induced Production of Biomass and Pharmaceutical Phenolic Compounds in Cell Suspension Culture of Date Palm (Phoenix dactylifera L.). Molecules, 25(20), 4669. https://doi.org/10.3390/molecules25204669