The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts
Abstract
1. Introduction
2. Results
2.1. Growth and Weight
2.2. Pigment Content in Broccoli Sprouts
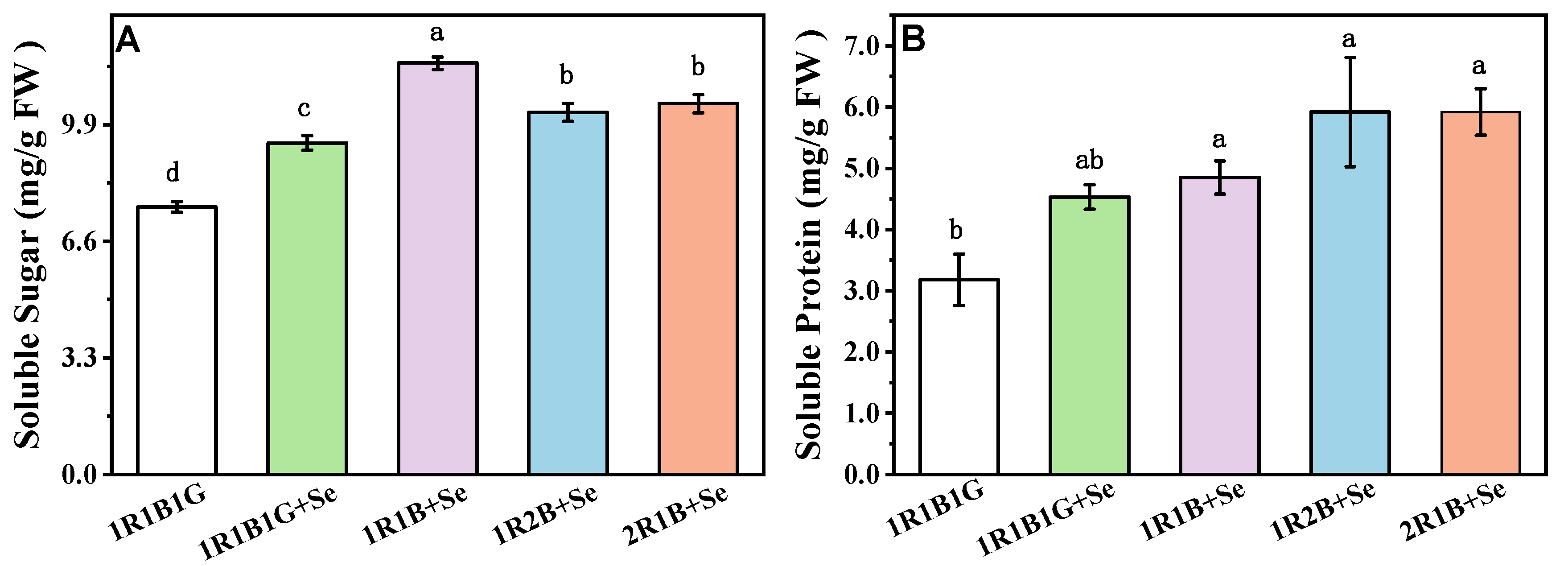
2.3. The Content of Soluble Sugars, Soluble Protein in Broccoli Sprouts
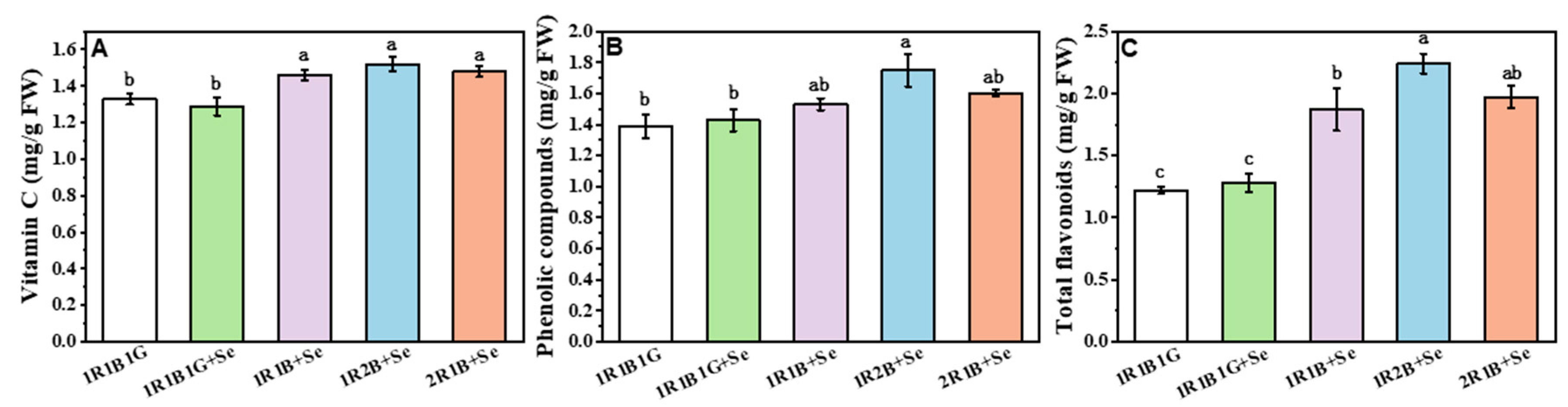
2.4. The Contents of Vitamin C (Vc), Total Phenolic Compounds (TPC) and Total Flavoncids (TF) in Broccoli Sprouts
2.5. The Mineral Element Content in Broccoli Sprouts
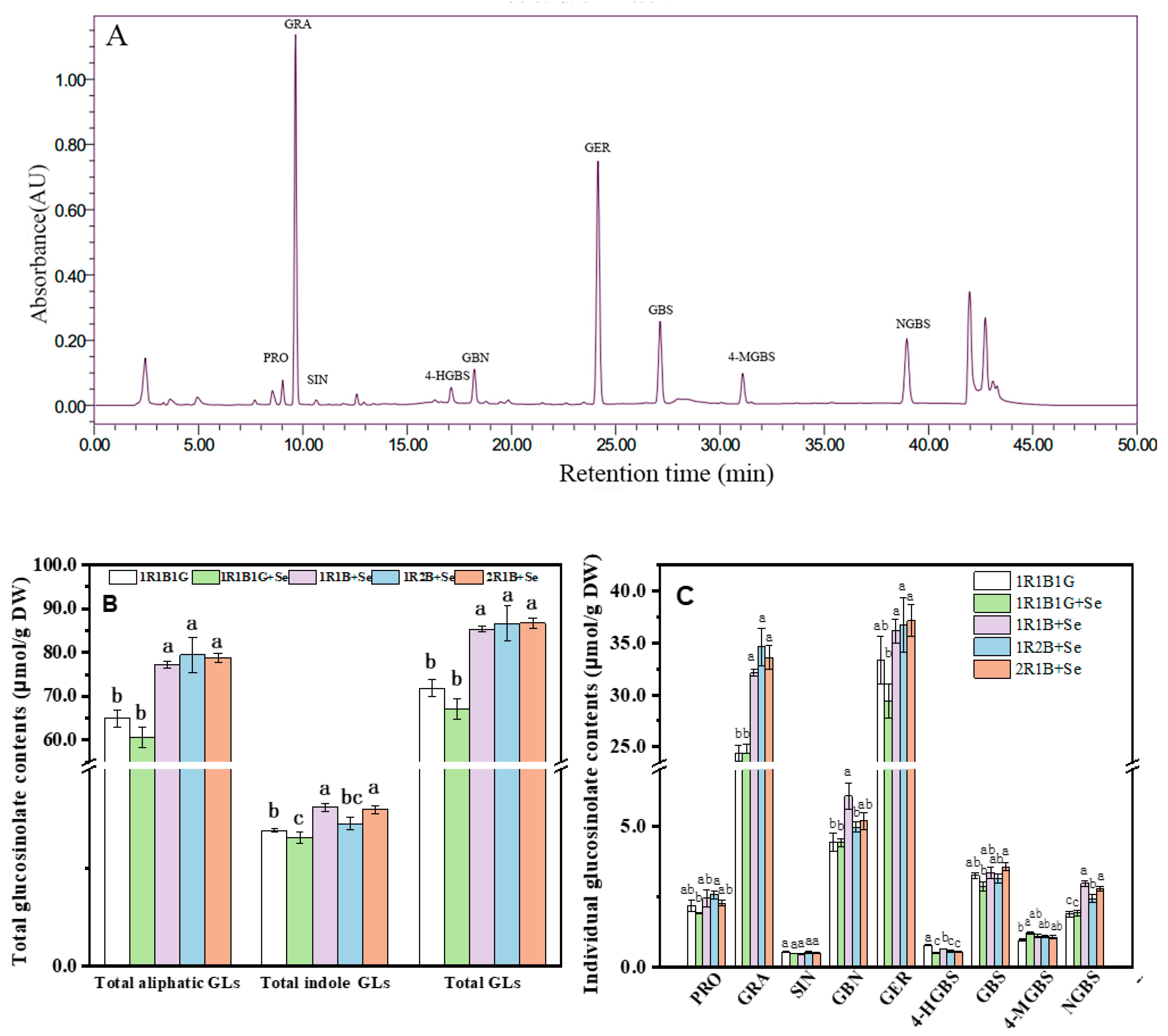
2.6. The Glucosinolate (GL) Contents in Broccoli Sprouts
2.7. Heat Map Analysis
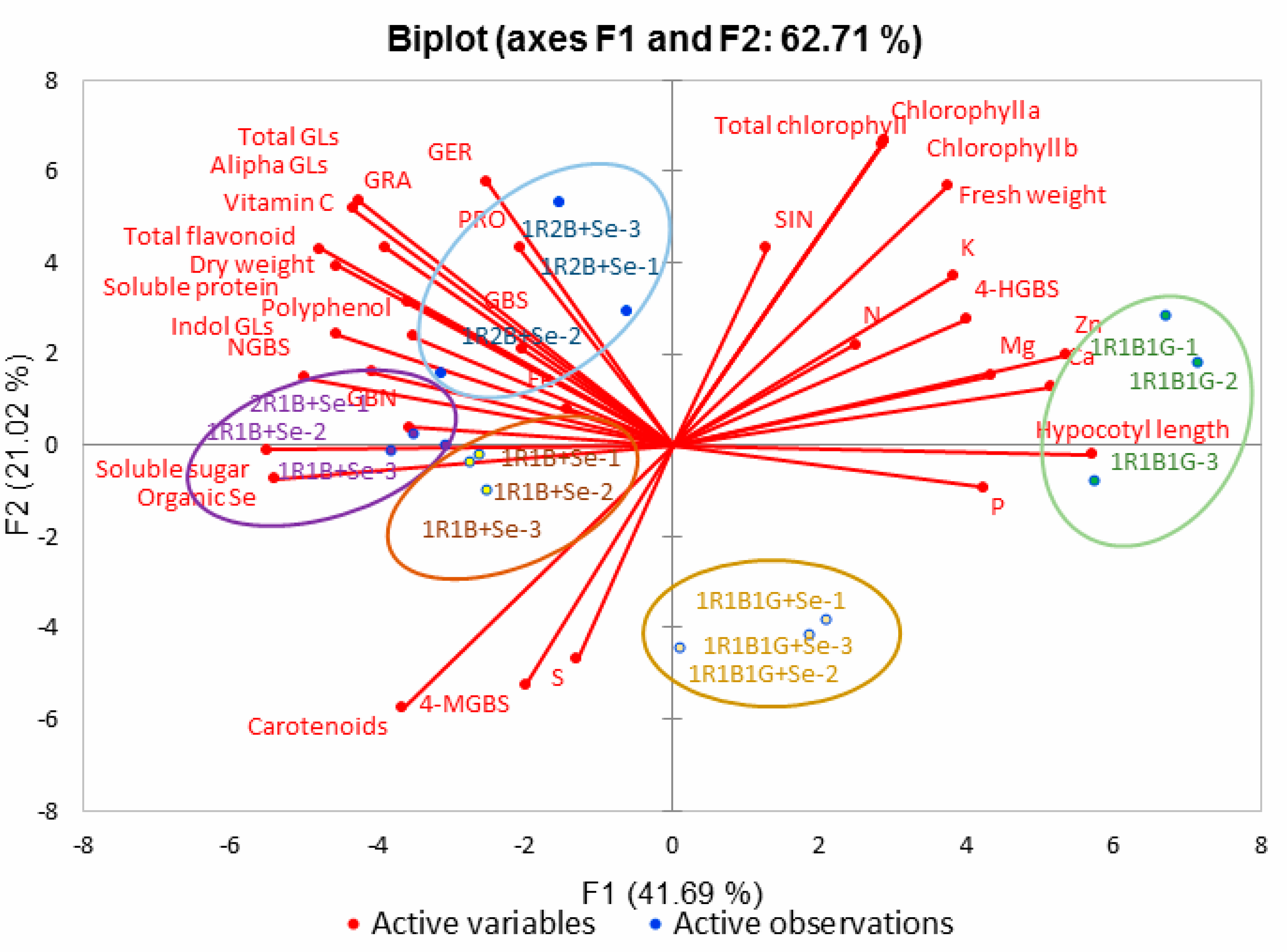
2.8. Principal Component Analysis
3. Discussion
3.1. Effects of Combination of Selenium and LED Light Quality on Broccoli Sprout Growth
3.2. Effects of Combination of Selenium and LED Light Quality on Pigment Content in Broccoli Sprouts
3.3. Effects of Combination of Selenium and LED Light Quality on Phytochemical Content in Broccoli Sprouts
3.4. Effects of Combination of Selenium and LED Light Quality on Mineral Element Content in Broccoli Sprouts
3.5. Effects of Combination of Selenium and LED Light Quality on Glucosinolates Contents in Broccoli Sprouts
4. Materials and Methods
4.1. Plant Materials and Cultivation Conditions
4.2. Biometric Measurements
4.3. Pigment Content Determination
- Chlorophyll a (mg·L−1) = 12.7 ×A663 − 2.69 × A645
- Chlorophyll b (mg·L−1) = 22.9 × A645 − 4.86 × A663
- Total Chlorophyll (mg·L−1) = 8.02 × A663 + 20.20 × A645
- Carotenoids (mg·L−1) = 4.7 × A440 − 0.27 × Total Chlorophyll
- Photosynthetic pigment (mg·g−1) = Photosynthetic pigment (mg·L−1) × 8 mL × 10−3/0.2 g
4.4. Phytochemical Determination
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Baenas, N.; Silván, J.M.; Medina, S.; de Pascual-Teresa, S.; García-Viguera, C.; Moreno, D.A. Metabolism and antiproliferative effects of sulforaphane and broccoli sprouts in human intestinal (Caco-2) and hepatic (HepG2) cells. Phytochem. Rev. 2015, 14, 1035–1044. [Google Scholar] [CrossRef]
- Santhosh Kumar, B.; Priyadarsini, K.I. Selenium nutrition: How important is it? Biomed. Prev. Nutr. 2014, 4, 333–341. [Google Scholar] [CrossRef]
- Elramady, H.; Abdalla, N.; Taha, H.; Alshaal, T.; Elhenawy, A.; Faizy, S.E.D.A.; Shams, M.S.; Youssef, S.; Shalaby, T.A.; Bayoumi, Y. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016, 14, 123–147. [Google Scholar] [CrossRef]
- Wu, Z.; Bañuelos, G.S.; Lin, Z.-Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant. Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef]
- Ferrarese, M.; Sourestani, M.; Quattrini, E.; Schiavi, M.; Ferrante, A. Biofortification of spinach plants applying selenium in the nutrient solution of floating system. Veg. Crop. Res. Bull. 2012, 76, 127–136. [Google Scholar] [CrossRef]
- Sindelařova, K.; Szakova, J.; Tremlova, J.; Mestek, O.; Praus, L.; Kaňa, A.; Najmanova, J.; Tlustos, P. The response of broccoli (Brassica oleracea Con. var. italica) varieties on foliar application of selenium: Uptake, translocation, and speciation. Food Addit. Contam. Part. A-Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 2027–2038. [Google Scholar]
- Mechora, P.; Stibilj, V.; Kreft, I.; Germ, M. The physiology and biochemical tolerance of cabbage to Se (VI) addition to the soil and by foliar spraying. J. Plant. Nutr. 2014, 37, 2157–2169. [Google Scholar] [CrossRef]
- Schiavon, M.; Berto, C.; Malagoli, M.; Trentin, A.; Sambo, P.; Dall, S.; Pilon-Smits, E.A.H. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front. Plant. Sci. 2016, 7, 1371. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Sams, C.E.; Panthee, D.R.; Charron, C.S.; Kopsell, D.A.; Yuan, J.S. Selenium regulates gene expression for glucosinolate and carotenoid biosynthesis in Arabidopsis. J. Am. Soc. Hortic. Sci. 2011, 136, 23–34. [Google Scholar] [CrossRef]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, Y.; Xie, L.; Pan, S. Effect of Se treatment on glucosinolate metabolism and health-promoting compounds in the broccoli sprouts of three cultivars. Food Chem. 2016, 190, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Cartes, P.; Gianfreda, L.; Mora, M.L. Uptake of selenium and its antioxidant activity in ryegrass when applied as selenate and selenite forms. Plant Soil 2005, 276, 359–367. [Google Scholar] [CrossRef]
- Chory, J. Light signal transduction: An infinite spectrum of possibilities. Plant. J. 2010, 61, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Zhang, G.; Yu, J.; Ma, Y. Effects of different led light qualities on cucumber seedling growth and chlorophyll fluorescence parameters. Entia Agric. Sin. 2013, 46, 323–330. [Google Scholar]
- Johkan, M.; Shoji, K.; Goto, F.; Hashida, S.N.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. Hortscience 2010, 45, 414–415. [Google Scholar] [CrossRef]
- Ouzounis, T.; Heuvelink, E.; Ji, Y.; Schouten, H.J.; Visser, R.G.F.; Marcelis, L.F.M. Blue and red LED lighting effects on plant biomass, stomatal conductance, and metabolite content in nine tomato genotypes. Acta Hortic. 2016, 1134, 251–258. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Barickman, T.C.; Morrow, R.C. Sprouting broccoli accumulate higher concentrations of nutritionally important metabolites under narrow-band light-emitting diode lighting. J. Am. Soc. Hortic. Sci. 2014, 139, 469–477. [Google Scholar] [CrossRef]
- Zhang, T.; Folta, K.M. Green light signaling and adaptive response. Plant. Signal. Behav. 2012, 7, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Kelly, N.; Runkle, E.S. Substituting green or far-red radiation for blue radiation induces shade avoidance and promotes growth in lettuce and kale. Environ. Exp. Bot. 2019, 162, 383–391. [Google Scholar] [CrossRef]
- Kim, H.H.; Goins, G.D.; Wheeler, R.M.; Sager, J.C. Green-light supplementation for enhanced lettuce growth under red-and blue-light-emitting diodes. Hortscience 2004, 39, 1617–1622. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant. Soil. 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Carvalho, K.M.; Gallardo-Williams, M.T.; Benson, R.F.; Martin, D.F. Effects of selenium supplementation on four agricultural crops. J. Agric. Food Chem. 2003, 51, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 29, 66–74. [Google Scholar] [CrossRef]
- Alsina, I.; Dubova, L.; Smiltina, Z.; Stroksa, L.; Dūma, M. The effect of selenium on yield quality of lettuce. Acta Hortic. 2012, 939, 269–275. [Google Scholar] [CrossRef]
- Ghasemi, Y.; Ghasemi, K.; Pirdashti, H.; Asgharzadeh, R. Effect of selenium enrichment on the growth, photosynthesis and mineral nutrition of broccoli. Not. Sci. Biol. 2016, 8, 199–203. [Google Scholar] [CrossRef]
- Oraghi Ardebili, Z.; Oraghi Ardebili, N.; Jalili, S.; Safiallah, S. The modified qualities of basil plants by selenium and/or ascorbic acid. Turk. J. Bot. 2015, 39, 401–407. [Google Scholar] [CrossRef]
- Li, G.; Wan, S.; Zhou, J.; Yang, Z.; Qin, P. Leaf chlorophyll fluorescence, hyperspectral reflectance, pigments content, malondialdehyde and proline accumulation responses of castor bean (Ricinus communis L.) seedlings to salt stress levels. Ind. Crop. Prod. 2010, 31, 13–19. [Google Scholar] [CrossRef]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant. Ence. 2016, 7, 250–259. [Google Scholar] [CrossRef]
- Yu, X.; Liu, H.; Klejnot, J.; Lin, C. The cryptochrome blue light receptors. Arab. B 2010, 8, 135–161. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Y.; Zhang, X.; Li, M. Effect of foliar treatment of sodium selenate on postharvest decay and quality of tomato fruits. Entia Hortic. 2016, 198, 304–310. [Google Scholar] [CrossRef]
- Lee, G.J.; Kang, B.K.; Kim, T.I.; Kim, T.J.; Kim, J.H. Effects of different selenium concentrations of the nutrient solution on the growth and quality of tomato fruit in hydroponics. Acta Hortic. 2007, 761, 443–448. [Google Scholar] [CrossRef]
- Chang, T.; Liu, X.; Xu, Z. Effects of light spectral energy distribution on growth and development of tomato seedlings. Sci. Agric. Sin. 2010, 8, 27–35. [Google Scholar]
- Zhang, H.; Xu, Z.G.; Cui, J.; Guo, Y.S.; Gu, A.S. Effects of different spectra on growth and nutritious quality of radish sprouting seedlings. China Veg. 2009, 10, 28–32. [Google Scholar]
- Li, S.; Pan, R.C. Effect of blue light on the metabolism of carbohydrate and protein in rice (Oryza sativa L.) seedlings. Acta Phytophisiol. Sin. 1995, 21, 22–28. [Google Scholar]
- Toledo, M.E.A.; Ueda, Y.; Imahori, Y.; Ayaki, M. L-ascorbic acid metabolism in spinach (Spinacia oleracea L.) during postharvest storage in light and dark. Postharvest Biol. Technol. 2003, 28, 47–57. [Google Scholar] [CrossRef]
- Chen, W.; Xu, Z.; Liu, X.; Yang, Y.; Wang, Z.; Song, F. Effect of LED light source on the growth and quality of different lettuce varieties. Acta Bot. Boreali-Occident. Sin. 2011, 31, 1434–1440. [Google Scholar]
- Ohashi-Kaneko, K.; Tarase, M.; Noya, K.O.N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef]
- Costa, D.C.; Costa, H.S.; Albuquerque, T.G.; Ramos, F.; Castilho, M.C.; Sanches-Silva, A. Advances in phenolic compounds analysis of aromatic plants and their potential applications. Trends Food Sci. Technol. 2015, 336–354. [Google Scholar] [CrossRef]
- Mezeyova, I. Impact of biofortification, variety and cutting on chosen qualitative characteristic of basil (Ocimum basilicum L.). Acta Fytotech. Zootech. 2015, 4, 19–27. [Google Scholar]
- Son, K.H.; Oh, M.M. Growth, photosynthetic and antioxidant parameters of two lettuce cultivars as affected by red, green, and blue light-emitting diodes. Hortic. Environ. Biotechnol. 2015, 56, 639–653. [Google Scholar] [CrossRef]
- Son, K.H.; Oh, M.M. Leaf shape, growth, and antioxidant phenolic compounds of two lettuce cultivars grown under various combinations of blue and red light-emitting diodes. Hortscience 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, morphology, yield, and phytochemical accumulation in basil plants influenced by substituting green light for partial red and/or blue light. Hortscience 2019, 54, 1769–1776. [Google Scholar] [CrossRef]
- Son, K.H.; Park, J.H.; Kim, D.; Oh, M.M. Leaf shape, growth, and phytochemicals in two leaf lettuce cultivars grown under monochromatic light-emitting diodes. Korean J. Hortic. Sci. Technol. 2012, 30, 664–672. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Shin, Y.S.; Lee, M.J.; Lee, E.S. Effect of light emitting diodes treatment on growth and mineral contents of lettuce (Lactuca sativa L. Chung Chi Ma). Korean J. Org. Agric. 2013, 21, 659–668. [Google Scholar] [CrossRef]
- Mohammadi, A.; Sabzalian, M.R.; Amoozgar, A. Impact of light-emitting diode irradiation on photosynthesis, phytochemical composition and mineral element content of lettuce cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar]
- Clavijo-Herrera, J.; Van Santen, E.; Gómez, C. Growth, Water-use efficiency, stomatal conductance, and nitrogen uptake of two lettuce cultivars grown under different percentages of blue and red Light. Horticulturae 2018, 4, 16. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Pantanizopoulos, N.I.; Sams, C.E.; Kopsell, D.E. Shoot tissue pigment levels increase in “Florida Broadleaf” mustard (Brassica juncea L.) microgreens following high light treatment. Sci. Hortic. 2012, 140, 96–99. [Google Scholar] [CrossRef]
- Ludwig-Müller, J.; Schubert, B.; Pieper, K.; Ihmig, S.; Hilgenberg, W. Glucosinolate content in susceptible and resistant Chinese cabbage varieties during development of clubroot disease. Phytochemistry 1997, 44, 407–414. [Google Scholar] [CrossRef]
- Tian, M.; Xu, X.; Liu, F.; Fan, X.; Pan, S. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018, 111, 205–211. [Google Scholar] [CrossRef]
- Tian, M.; Yang, Y.; Ávila, F.W.; Fish, T.; Yuan, H.; Hui, M.; Pan, S.; Thannhauser, T.W.; Li, L. Effects of selenium supplementation on glucosinolate biosynthesis in broccoli. J. Agric. Food Chem. 2018, 66, 8036–8044. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, Y.; Liu, H.; LI, Y.; Liu, Y.; Hao, Y.; Lei, B. Supplemental blue light increases growth and quality of greenhouse pak choi depending on cultivar and supplemental light intensity. J. Integr. Agric. 2018, 17, 2245–2256. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of light quality on main health-promoting compounds and antioxidant capacity of Chinese kale sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of light-emitting diodes on the accumulation of glucosinolates and phenolic compounds in sprouting canola (Brassica napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef]
- Gratani, L. A non-destructive method to determine chlorophyll content of leaves. Photosynthetica 1992, 26, 469–473. [Google Scholar]
- Xu, J.; Zhang, M.; Liu, X.; Lu, G.; Chi, J.; Sun, L. Extraction and antioxidation of anthocyanin of black soybean seed coat. Trans. Chin. Soc. Agric. Eng. 2005, 21, 161–164. [Google Scholar]
- Kohyama, K.; Nishinari, K. Effect of soluble sugars on gelatinization and retrogradation of sweet potato starch. J. Agric. Food Chem. 1991, 39, 1406–1410. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilising the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chanwitheesuk, A.; Teerawutgulrag, A.; Rakariyatham, N. Screening of antioxidant activity and antioxidant compounds of some edible plants of Thailand. Food Chem. 2005, 92, 491–497. [Google Scholar] [CrossRef]
- Vernon, L.S.; Rudolf, O.; Rosa, M.L.R. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Xie, Y.; Zheng, Y.; Dai, X.; Wang, Q.; Cao, J.; Xiao, J. Seasonal dynamics of total flavonoid contents and antioxidant activity of Dryopteris erythrosora. Food Chem. 2015, 186, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Ojeda, G.; Alcaniz, J.M.; Le Bissonnais, Y. Differences in aggregate stability due to various sewage sludge treatments on a Mediterranean calcareous soil. Agric. Ecosyst. Environ. 2008, 125, 48–56. [Google Scholar] [CrossRef]
- Qin, W.; Wang, D.; Guo, X.; Yang, T.; Oenema, O. Productivity and sustainability of rainfed wheat-soybean system in the North China Plain: Results from a long-term experiment and crop modelling. Sci. Rep. 2015, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Gerchikova, T.N. Determination of the content of sodium and potassium in erythrocytes by the method of flame photometry. Lab. Delo 1963, 9, 5–9. [Google Scholar] [PubMed]
- Sanui, H. Activated oxygen ashing of biological specimens for the microdetermination of Na, K, Mg, and Ca by atomic absorption spectrophotometry. Anal. Biochem. 1971, 42, 21–28. [Google Scholar] [CrossRef]
- Sun, M.; Liu, G.; Wu, Q. Speciation of organic and inorganic selenium in selenium-enriched rice by graphite furnace atomic absorption spectrometry after cloud point extraction. Food Chem. 2013, 141, 66–71. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not are available from the authors. |

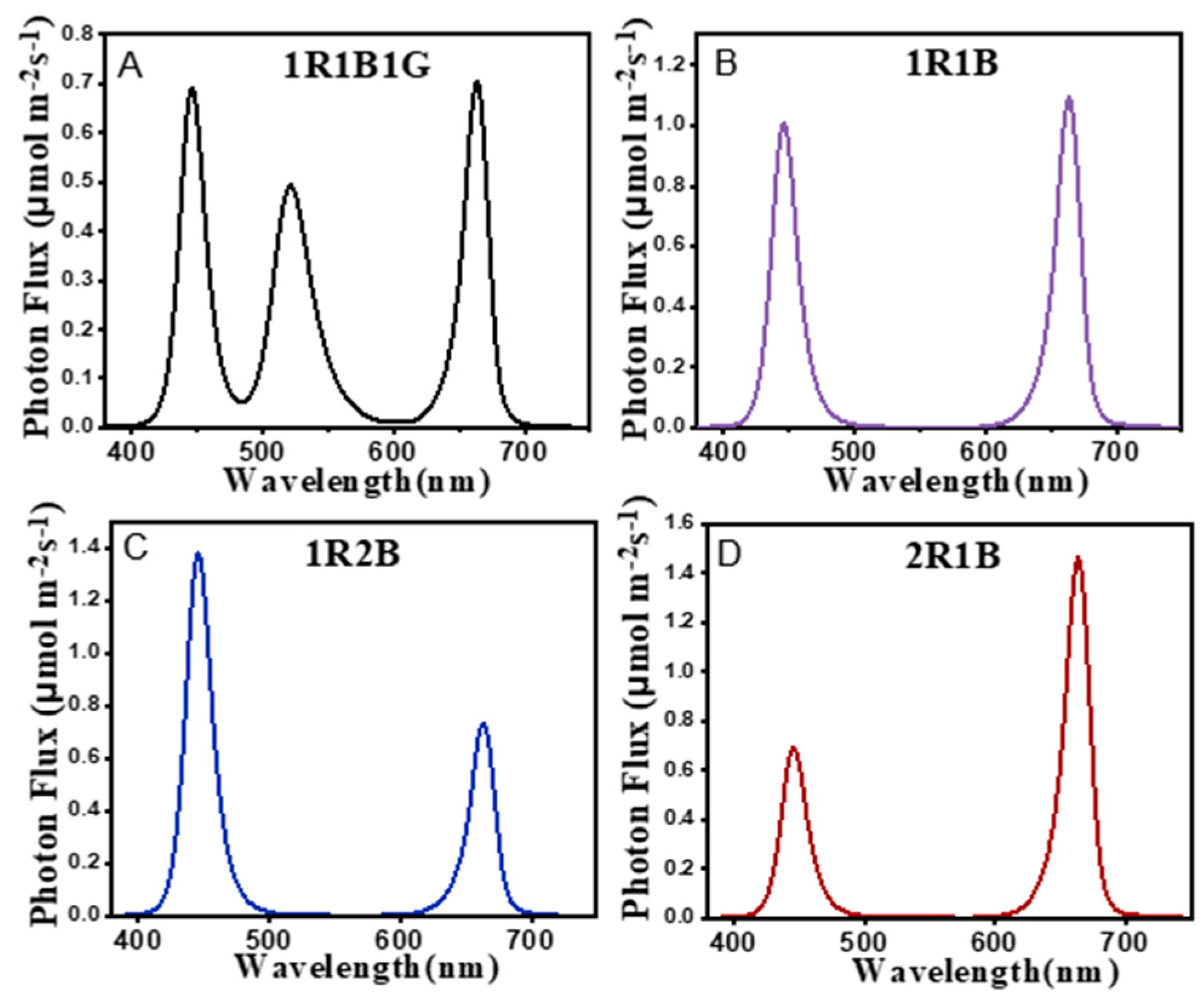
| Treatments | Hypocotyl Length (cm) | Fresh Weight (mg/plant) | Dry Weight (mg/plant) | Moisture Content (%) |
|---|---|---|---|---|
| 1R1B1G | 4.27 ± 0.06 a | 51.44 ± 1.17 a | 3.43 ± 0.07 b | 93.39% ± 0.55 a |
| 1R1B1G+Se | 3.76 ± 0.05 b | 49.22 ± 1.05 a | 3.63 ± 0.09 ab | 92.62% ± 0.40 b |
| 1R1B+Se | 3.54 ± 0.04 c | 49.22 ± 0.80 a | 3.61 ± 0.04 ab | 92.26% ± 0.61 b |
| 1R2B+Se | 3.32 ± 0.06 d | 50.78 ± 0.85 a | 3.87 ± 0.17 a | 92.65% ± 1.12 b |
| 2R1B+Se | 3.37 ± 0.06 d | 49.22 ± 1.21 a | 3.64 ± 0.07 ab | 92.52% ± 0.63 b |
| Treatments | 1R1B1G | 1R1B1G+Se | 1R1B+Se | 1R2B+Se | 2R1B+Se |
|---|---|---|---|---|---|
| Chl a (mg/g FW) | 1.45 ± 0.10 a | 1.23 ± 0.03 c | 1.24 ± 0.03 c | 1.43 ± 0.06 ab | 1.26 ± 0.02 bc |
| Chl b (mg/g FW) | 0.53 ± 0.05 a | 0.43 ± 0.01 c | 0.44 ± 0.02 bc | 0.52 ± 0.02 ab | 0.44 ± 0.01 bc |
| Chl (mg/g FW) | 2.00 ± 0.14 a | 1.68 ± 0.04 c | 1.70 ± 0.05 c | 1.96 ± 0.08 ab | 1.72 ± 0.03 bc |
| Car (mg/g FW) | 0.19 ± 0.01 b | 0.22 ± 0.00 a | 0.23 ± 0.00 a | 0.20 ± 0.01 b | 0.23 ± 0.00 a |
| TA (μg/g FW) | 7.29 ± 0.17 b | 9.23 ± 0.39 a | 9.13 ± 0.24 a | 9.85 ± 0.90 a | 9.88 ± 0.46 a |
| Chl a/b | 2.72 ± 0.07 a | 2.87 ± 0.03 a | 2.84 ± 0.03 a | 2.75 ± 0.03 a | 2.84 ± 0.05 a |
| Chl/Car | 10.64 ± 1.39 a | 7.47 ± 0.12 b | 7.57 ± 0.29 b | 9.80 ± 0.71 ab | 7.53 ± 0.19 b |
| Treatment | 1R1B1G | 1R1B1G+Se | 1R1B+Se | 1R2B+Se | 2R1B+Se |
|---|---|---|---|---|---|
| Macro-nutrients (g/kg DW) | |||||
| N | 63.81 ± 0.32 a | 62.42 ± 0.85 a | 62.98 ± 0.90 a | 63.17 ± 0.96 a | 61.83 ± 0.95 a |
| P | 9.55 ± 0.05 a | 9.34 ± 0.21 a | 9.08 ± 0.20 a | 9.05 ± 0.16 a | 9.05 ± 0.12 a |
| K | 8.22 ± 0.09 a | 7.38 ± 0.31 b | 7.33 ± 0.06 b | 7.98 ± 0.04 a | 6.94 ± 0.14 b |
| Ca | 11.70 ± 0.45 a | 10.39 ± 0.06 b | 10.32 ± 0.24 b | 10.08 ± 0.26 b | 9.8 ± 0.07 b |
| Mg | 3.54 ± 0.07 a | 3.30 ± 0.05 b | 3.35 ± 0.10 ab | 3.31 ± 0.06 b | 3.16 ± 0.01 b |
| S | 21.84 ± 0.58 bc | 22.98 ± 0.41 ab | 23.62 ± 0.23 a | 21.30 ± 0.16 c | 22.20 ± 0.17 bc |
| Micro-nutrients (mg/kg DW) | |||||
| Zn | 130.09 ± 0.46 a | 86.15 ± 1.70 b | 86.85 ± 0.75 b | 85.90 ± 1.29 b | 84.04 ± 1.39 b |
| Fe | 107.94 ± 4.96 a | 108.30 ± 7.12 a | 109.88 ± 5.53 a | 113.38 ± 3.60 a | 114.34 ± 4.68 a |
| Organic Se | 4.48 ± 0.69 c | 88.82 ± 0.30 b | 107.10 ± 5.48 a | 110.33 ± 4.40 a | 95.86 ± 0.72 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Gao, M.; Shi, R.; Song, S.; Zhang, Y.; Su, W.; Liu, H. The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts. Molecules 2020, 25, 4788. https://doi.org/10.3390/molecules25204788
He R, Gao M, Shi R, Song S, Zhang Y, Su W, Liu H. The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts. Molecules. 2020; 25(20):4788. https://doi.org/10.3390/molecules25204788
Chicago/Turabian StyleHe, Rui, Meifang Gao, Rui Shi, Shiwei Song, Yiting Zhang, Wei Su, and Houcheng Liu. 2020. "The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts" Molecules 25, no. 20: 4788. https://doi.org/10.3390/molecules25204788
APA StyleHe, R., Gao, M., Shi, R., Song, S., Zhang, Y., Su, W., & Liu, H. (2020). The Combination of Selenium and LED Light Quality Affects Growth and Nutritional Properties of Broccoli Sprouts. Molecules, 25(20), 4788. https://doi.org/10.3390/molecules25204788







