Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris
Abstract
1. Introduction
2. Results
2.1. Macroscopic Properties and pH Measurement
2.2. Texture Analysis
2.3. In Vitro Diffusion Studies
2.4. MTT Viability Assays on the HaCaT Cell Line
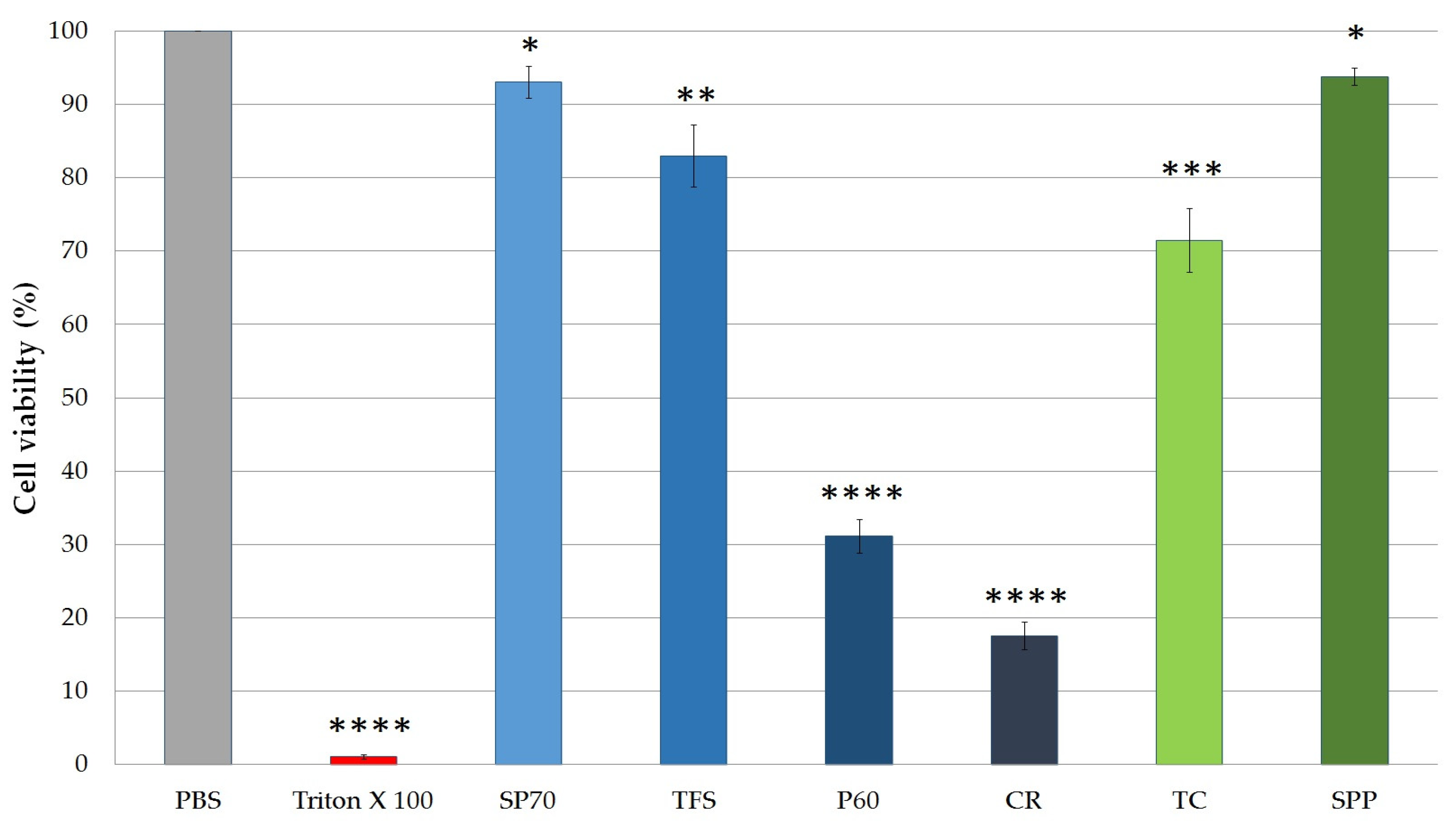
2.4.1. Cytotoxicity of SPP and Excipients
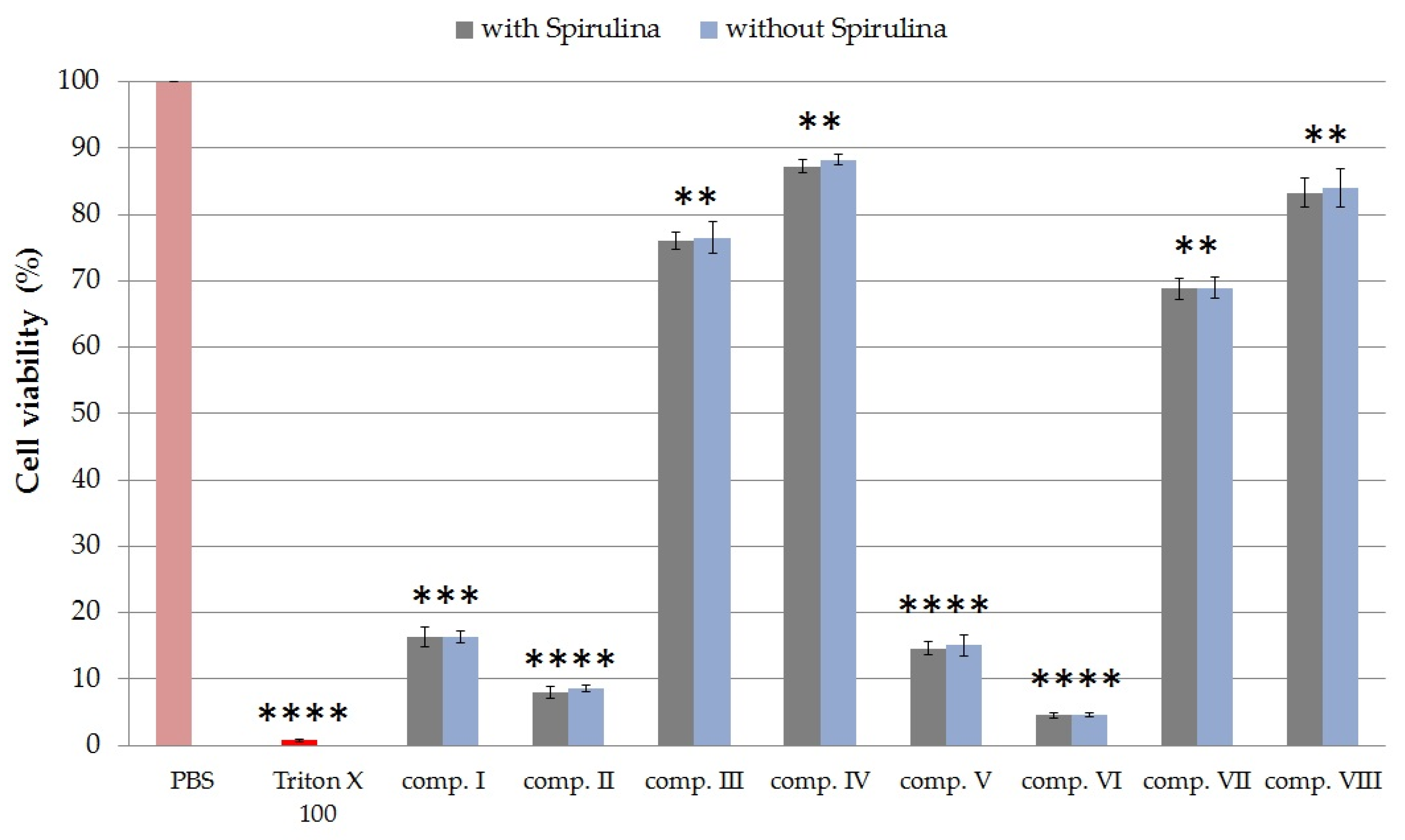
2.4.2. Cytotoxicity of Formulated Compositions
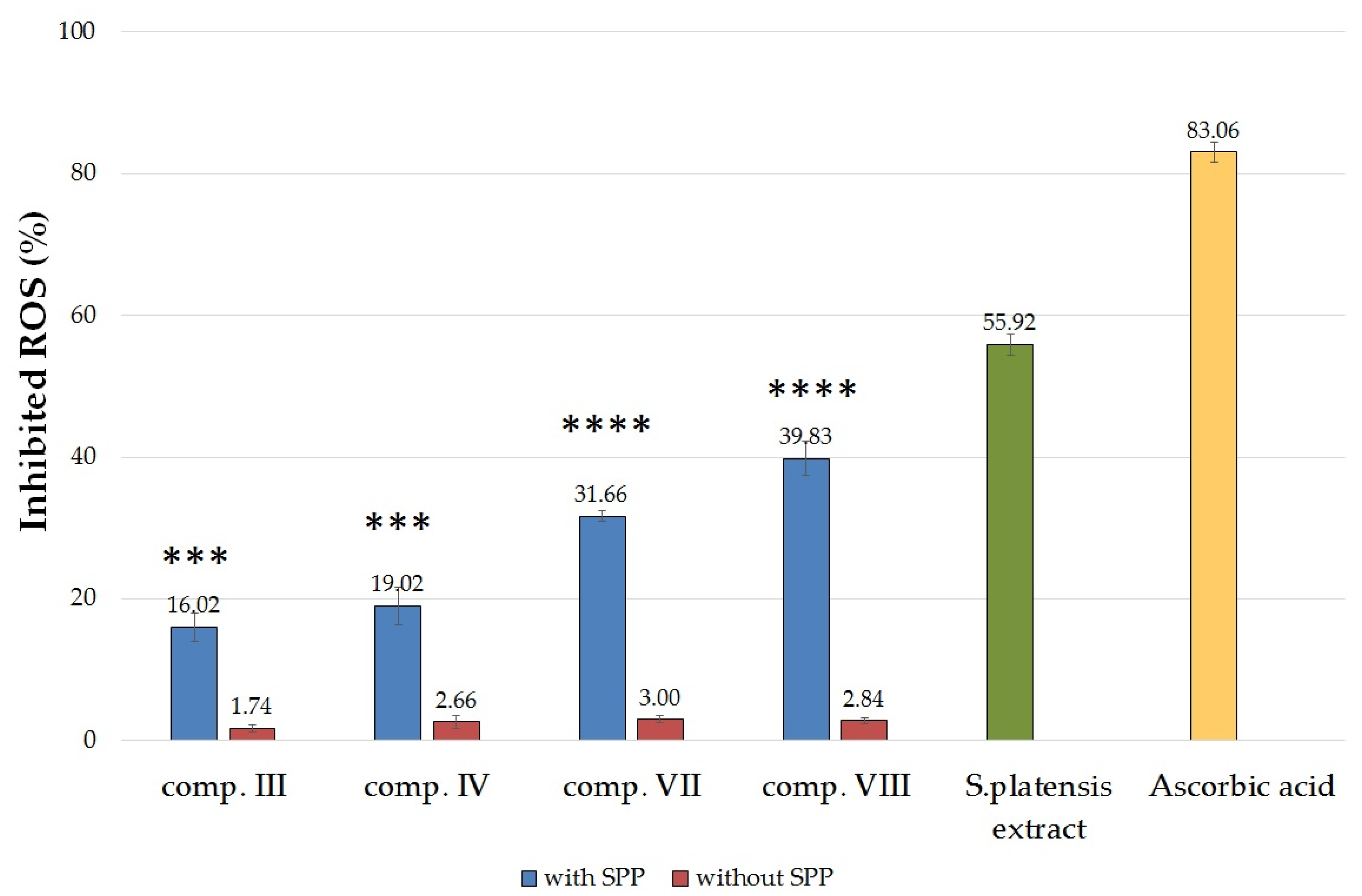
2.5. In Vitro Antioxidant Activity Tests
2.5.1. Determination of Superoxide Dismutase Activity on HaCaT Cell Line
2.5.2. Determination of 2,2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.6. Antibacterial Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation and Characterization of Dry Spirulina platensis Extract
4.3. Formulation of Spirulina platensis-Containing Creams
4.4. Texture Analysis
4.5. In Vitro Diffusion Studies
4.6. Cell Culturing
4.7. In Vitro Cell Viability Assay
4.8. Antioxidant Assay
4.8.1. Superoxide Dismutase (SOD) Assay
4.8.2. DPPH Radical Scavenging Activity
4.9. Antibacterial Test
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Vora, J.; Srivastava, A.; Modi, H. Antibacterial and antioxidant strategies for acne treatment through plant extracts. Inform. Med. Unlocked 2018, 13, 128–132. [Google Scholar] [CrossRef]
- Weber, N.; Biehler, K.; Schwabe, K.; Haarhaus, B.; Quirin, K.-W.; Frank, U.; Schempp, C.M.; Wölfle, U. Hop extract acts as an antioxidant with antimicrobial effects against Propionibacterium Acnes and Staphylococcus Aureus. Molecules 2019, 24, 223. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, M.; Kupperman, E.; Lautenbach, E.; Edelstein, P.H.; Margolis, D.J. Antibiotics, acne, and Staphylococcus aureus colonization. Arch. Dermatol. 2011, 147, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.A.; Dawood, A.A.; Mahmoud, A.A. Oxidants and antioxidants role in Acne vulgaris. Menoufia Med. J. 2014, 27, 465–468. [Google Scholar]
- Arican, O.; Kurutas, E.B.; Sasmaz, S. Oxidative stress in patients with acne vulgaris. Mediat. Inflamm. 2005, 6, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.K.; Bhatia, N.; Zeichner, J.; Kircik, L.H. Topical vehicle formulations in the treatment of acne. J. Drugs Dermatol. 2018, 17, 6–10. [Google Scholar]
- Rottboell, L.; De Foenss, S.; Thomsen, K.; Christiansen, H.; Andersen, S.M.; Dam, T.N.; Rosada, C.; Stenderup, K. Exploring valrubicin’s effect on Propionibacterium acnes-induced skin inflammation in vitro and in vivo. Dermatol. Rep. 2015, 7, 39–43. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, J.Y.; Im, A.R.; Chae, S. Phycocyanin protects against UVB-induced apoptosis through the PKC α/βII-Nrf-2/HO-1 dependent pathway in human primary skin cells. Molecules 2018, 23, 478. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.C.; Subhashini, J.; Mahipal, S.V.K.; Bhat, V.B.; Srinivas Reddy, P.; Kiranmai, G.; Madyastha, K.M.; Reddanna, P. C-Phycocyanin, a selective cyclooxygenase-2 inhibitor, induces apoptosis in lipopolysaccharide-stimulated RAW 264.7 macrophages. Biochem. Biophys. Res. Commun. 2003, 304, 385–392. [Google Scholar] [CrossRef]
- Glazer, A.N.; Fang, S.; Brown, D.M. Spectroscopic properties of C phycocyanin and of its α and β subunits. J. Biol. Chem. 1973, 248, 5679–5685. [Google Scholar]
- Karkos, P.D.; Leong, S.C.; Karkos, C.D.; Sivaji, N.; Assimakopoulos, D.A. Spirulina in clinical practice: Evidence-based human applications. Evid. Based Complement. Altern. Med. 2011, 2011, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Delsin, S.D.; Mercurio, D.G.; Fossa, M.M.; Maia Campos, P.M.B.G. Clinical efficacy of dermocosmetic formulations containing spirulina extract on young and mature skin: Effects on the skin hydrolipidic barrier and structural properties. Clin. Pharmacol. Biopharm. 2015, 4, 144. [Google Scholar]
- Wolfe-Simon, F.; Grzebyk, D.; Schofield, O.; Falkowski, P.G. The role and evolution of superoxide dismutases in algae. J. Phycol. 2005, 41, 453–465. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Khosravi-Darani, K.; Mozafari, M.R. Nutritional and medical applications of spirulina microalgae. Mini Rev. Med. Chem. 2013, 13, 1231–1237. [Google Scholar] [CrossRef]
- Zheng, J.-X.; Yin, H.; Shen, C.-C.; Zhang, L.; Ren, D.-F.; Lu, J. Functional and structural properties of spirulina phycocyanin modified by ultra-high-pressure composite glycation. Food Chem. 2020, 306, 125615. [Google Scholar] [CrossRef]
- Lane, M.E. Skin penetration enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Otto, A.; du Plessis, J.; Wiechers, J.W. Formulation effects of topical emulsions on transdermal and dermal delivery. Int. J. Cosmet. Sci. 2009, 31, 1–19. [Google Scholar] [CrossRef]
- Som, I.; Bhatia, K.; Yasir, M. Status of surfactants as penetration enhancers in transdermal drug delivery. J. Pharm. Bioallied Sci. 2012, 4, 2–9. [Google Scholar]
- Fehér, P.; Ujhelyi, Z.; Váradi, J.; Fenyvesi, F.; Róka, E.; Juhász, B.; Varga, B.; Bombicz, M.; Priksz, D.; Bácskay, I.; et al. Efficacy of pre- and post-treatment by topical formulations containing dissolved and suspended silybum marianum against uvb-induced oxidative stress in guinea pig and on Hacat keratinocytes. Molecules 2016, 21, 1269. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Váradi, J.; Fehér, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyés, M.; Bácskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef]
- Haque, T.; Talukder, M.M.U. Chemical enhancer: A simplistic way to modulate barrier function of the stratum corneum. Adv. Pharm. Bull. 2018, 8, 169–179. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Adibkia, K.; Hamishekar, H. Transcutol® (diethylene glycol monoethyl ether): A potential penetration enhancer. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement, 1st ed.; Dragicevic, N., Maibach, H.I., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; Volume 10, pp. 195–205. [Google Scholar]
- Lambers, H.; Piessens, S.; Bloem, A.; Pronk, H.; Finkel, P. Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int. J. Cosmet. Sci. 2006, 28, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, M.M.S.; El-Ayouty, Y.M.; Piercey-Normore, M. Role of pH on antioxidants production by Spirulina (Arthrospira) platensis. Braz. J. Microbiol. 2016, 47, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Bianchini, R.; Jachowicz, J. Texture analysis of cosmetic/pharmaceutical raw materials and formulations. Int. J. Cosmet. Sci. 2014, 36, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Filipovic, M.; Lukic, M.; Djordjevic, S.; Krstonosic, V.; Pantelic, I.; Vuleta, G.; Savic, S. Towards satisfying performance of an O/W cosmetic emulsion: Screening of reformulation factors on textural and rheological properties using general experimental design. Int. J. Cosmet. Sci. 2017, 39, 486–499. [Google Scholar] [CrossRef]
- Moldovan, M.; Lahmar, A.; Bogdan, C.; Părăuan, S.; Tomuţă, I.; Crişan, M. Formulation and evaluation of a water-in-oil cream containing herbal active ingredients and ferulic acid. Clujul Med. 2017, 90, 212–219. [Google Scholar] [CrossRef]
- Tarko, T.; Duda-Chodak, A.; Kobus, M. Influence of growth medium composition on synthesis of bioactive compounds and antioxidant properties of selected strains of Arthrospira cyanobacteria. Czech J. Food Sci. 2012, 30, 258–267. [Google Scholar] [CrossRef]
- Liebenberg, W.; Engelbrecht, E.; Wessels, A.; Devarakonda, B.; Yang, W.Z.; De Villiers, M. A comparative study of the release of active ingredients from semisolid cosmeceuticals measured with Franz, enhancer or flow-through cell diffusion apparatus. J. Food Drug Anal. 2004, 12, 19–28. [Google Scholar]
- Higuchi, T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J. Soc. Cosmet. Chem. 1960, 11, 85–97. [Google Scholar]
- Higuchi, T. Rate release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Mura, P.; Faucci, M.T.; Bramanti, G.; Corti, P. Evaluation of transcutol asa clonazepa, transdermal permeation enhancer from hydrophilic gel formulations. Eur. J. Pharm. Sci. 2000, 9, 365–372. [Google Scholar] [CrossRef]
- Srivastava, S.; Mishra, S.; Dewangan, J.; Divakar, A.; Gupta, N.; Kalleti, N.; Mugale, M.N.; Kumar, S.; Sharma, S.; Rath, S.K. Safety assessment of the pharmacological excipient, diethylene glycol monoethyl ether (DEGEE), using in vitro and in vivo systems. DARU J. Pharm. Sci. 2019, 27, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.W.; Gad, S.C.; Julien, M. A review of the nonclinical safety of Transcutol®, a highly purified form of diethylene glycol monoethyl ether (DEGEE) used as a pharmaceutical excipient. Food Chem. Toxicol. 2014, 72, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Benson, H. Transdermal drug delivery: Penetration enhancement techniques. Curr. Drug Deliv. 2005, 2, 23–33. [Google Scholar] [CrossRef] [PubMed]
- El-Leithy, E.S.; Makky, A.M.; Khattab, A.M.; Hussein, D.G. Optimization of nutraceutical coenzyme Q10 nanoemulsion with improved skin permeability and anti-wrinkle efficiency. Drug Dev. Ind. Pharm. 2018, 44, 316–328. [Google Scholar] [CrossRef]
- Szűts, A.; Szabó-Révész, P. Sucrose esters as natural surfactants in drug delivery systems—A mini-review. Int. J. Pharm. 2012, 433, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Csizmazia, E.; Erős, G.; Berkesi, O.; Berkó, S.; Szabó-Révész, P.; Csányi, E. Pénétration enhancer effect of sucrose laurate and Transcutol on ibuprofen. J. Drug Deliv. Sci. Technol. 2011, 21, 411–415. [Google Scholar] [CrossRef]
- Youan, B.B.C.; Hussain, A.; Nguyen, N.T. Evaluation of sucrose esters as alternative surfactants in microencapsulation of proteins by the solvent evaporation method. AAPS Pharmsci. 2003, 5, 123–131. [Google Scholar] [CrossRef]
- Cázares-Delgadillo, J.; Naik, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Skin permeation enhancement by sucrose esters: A pH-dependent phenomenon. Int. J. Pharm. 2005, 297, 204–212. [Google Scholar] [CrossRef]
- Jang, H.J.; Shin, C.Y.; Kim, K.B. Safety evaluation of polyethylene glycol (PEG) compounds for cosmetic use. Toxicol. Res. 2015, 31, 105–136. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. The potential of Raman spectroscopy as a process analytical technique during formulations of topical gels and emulsions. Pharm. Res. 2004, 21, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramata-Stunda, A.; Boroduskis, M.; Vorobjeva, V.; Ancans, J. Cell and tissue culture-based in vitro test systems for evaluation of natural skin care product ingredients. Environ. Exp. Biol. 2013, 11, 159–177. [Google Scholar]
- Choi, S.Y.; Seop, S.Y.; Hyun, M.Y.; Yoo, K.H.; Kim, B.J.; Kim, M.N.; Cho, J.W. Safety evaluation of topical valproate application. Toxicol. Res. 2013, 29, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Bácskay, I.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vasvári, G.; Fehér, P.; Vecsernyés, M.; Ujhelyi, Z. Role of cytotoxicity experiments in pharmaceutical development. In Cytotoxicity; Tulay, A.C., Ed.; IntechOpen: London, UK, 2018; pp. 132–146. [Google Scholar]
- Sibi, G. Inhibition of lipase and inflammatory mediators by Chlorella lipid extracts for antiacne treatment. J. Adv. Pharm. Technol. Res. 2015, 6, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Xu, Q.-Y.; Guo, C.-Y.; Huang, J.-W.; Wang, S.-M.; Li, Y.-M.; Tu, Y.; He, L.; Bi, Z.-G.; Ji, C.; et al. MHY1485 ameliorates UV-induced skin cell damages via activating mTOR-Nrf2 signaling. Oncotarget 2017, 8, 12775–12783. [Google Scholar] [CrossRef][Green Version]
- Jadoon, S.; Karim, S.; Bin Asad, M.H.; Akram, M.R.; Khan, A.K.; Malik, A.; Chen, C.; Murtaza, G. Anti-aging potential of phytoextract loaded-pharmaceutical creams for human skin cell longetivity. Oxidative Med. Cell Longev. 2015, 2015, 709628. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- Sailou, C.; Kitazawa, M.; McLaughin, L.; Yang, J.P.; Lodge, J.K.; Tetsuka, T.; Iwasaki, K.; Cillard, J.; Okamoto, T.; Packer, L. Antioxidants modulate acute solar ultraviolet radiation-induced NF-kappa-B activation in a human keratinocyte cell line. Free Radic. Biol. Med. 1999, 26, 174–183. [Google Scholar] [CrossRef]
- Asghari, A.; Fazilati, M.; Latifi, A.M.; Salavati, H.; Choopani, A. A review on antioxidant properties of Spirulina. J. Appl. Biotechnol. Rep. 2016, 3, 345–351. [Google Scholar]
- Gabr, G.A.; El-Sayed, S.M.; Hikal, M.S. Antioxidant activities of phycocyanin: A bioactive compound from spirulina platensis. J. Pharm. Res. Int. 2020, 32, 73–85. [Google Scholar] [CrossRef]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Gurhan, I.D. In vitro evaluation of spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, K.H.M.; Guaratini, T.; Barros, M.P.; Falcão, V.R.; Tonon, A.P.; Lopes, N.P.; Campos, S.; Torres, M.A.; Souza, A.O.; Colepicolo, P.; et al. Metabolites from algae with economical impact. Comp. Biochem. Physiol. C Toxicol. Pharm. 2007, 146, 60–78. [Google Scholar] [CrossRef]
- Chu, W.L.; Lim, Y.W.; Radhakrishnan, A.K.; Lim, P.E. Protective effect of aqueous extract from Spirulina platensis against cell death induced by free radicals. BMC Complement. Altern. Med. 2010, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Dreno, B. Topical antibacterial therapy for acne vulgaris. Drugs 2004, 64, 2389–2397. [Google Scholar] [CrossRef] [PubMed]
- Al-Hammadi, A.; Al-Ismaily, A.; Al-Ali, S.; Ramadurai, R.; Jain, R.; McKinley-Grant, L.; Mughal, T.I. Topical, biological and clinical challenges in the management of patients with Acne vulgaris. Sultan Qaboos Univ. Med. J. 2016, 16, e152–e160. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Bahmani, M.; Mehrabani, D. Bacterial resistance to antibiotics in acne vulgaris: An in vitro study. Indian J. Dermatol. 2008, 53, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, A.; Maisonneuve, J.-F.; Persing, D.H. Propionibacterium acnes and chronic diseases. In The Infectious Etiology of Chronic Diseases, 1st ed.; Knobler, S.L., O’Connor, S., Lemon, S.M., Najafi, M., Eds.; National Academies Press: Washington, DC, USA, 2004; pp. 74–80. [Google Scholar]
- Moon, S.H.; Roh, H.S.; Kim, Y.H.; Kim, J.E.; Ko, J.Y.; Ro, Y.S. Antibiotic resistance of microbial strains isolated from Korean acne patients. J. Dermatol. 2012, 39, 833–837. [Google Scholar] [CrossRef]
- El-Sheekh, M.M.; Daboo, S.; Swelim, M.A.; Mohamed, S. Production and characterization of antimicrobial active substance from Spirulina platensis. Iran. J. Microbiol. 2014, 6, 112–119. [Google Scholar]
- Mala, R.; Saravanababu, S.A.; Umadevi, G. Screening for antimicrobial activity of crude extracts of Spirulina platensis. J. Cell Tissue Res. 2009, 9, 1951–1955. [Google Scholar]
- Nihal, B.; Vishal Gupta, N.; Gowda, D.V.; Manohar, M. Formulation and development of topical anti acne formulation of spirulina extract. Int. J. Appl. Pharm. 2018, 10, 229–233. [Google Scholar] [CrossRef]
- Parsons, J.B.; Yao, J.; Frank, M.W.; Jackson, P.; Rock, C.O. Membrane disruption by antimicrobial fatty acids releases low-molecular-weight proteins from staphylococcus aureus. J. Bacteriol. 2012, 194, 5294–5304. [Google Scholar] [CrossRef] [PubMed]
- Toyoda, M.; Morohashi, M. Pathogenesis of acne. Med. Electron Microsc. 2001, 34, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, N.; Hernandez, P.O.; Tyring, S.K.; Haitz, K.A.; Motta, A. Antimicrobial susceptibility of Propionibacterium acnes isolates from acne patients in Colombia. Int. J. Dermatol. 2013, 52, 688–692. [Google Scholar] [CrossRef]
- Zarrouk, C. Contribution a L’etude D’une Cyanophycee. Influence de Divers Facteurs Physiques et Chimiques Sur la Croissance et Photosynthese de Spirulina maxima (Setch et Gardner) Geitler. Ph.D. Thesis, University of Paris, Paris, France, 1966. [Google Scholar]
- Franz, T.J. Percutaneous absorption. On the relevance of in vitro data. J. Investig. Dermatol. 1975, 64, 190–195. [Google Scholar] [CrossRef]
- Chen, H.W.; Yang, T.S.; Chen, M.J.; Chang, Y.C.; Wang, E.I.C.; Ho, C.L.; Lai, Y.J.; Yu, C.C.; Chou, J.C.; Chao, L.K.P.; et al. Purification and immunomodulating activity of C-phycocyanin from Spirulina platensis cultured using power plant flue gas. Process Biochem. 2014, 49, 1337–1344. [Google Scholar] [CrossRef]
- Vasvári, G.; Haimhoffer, Á.; Horváth, L.; Budai, I.; Trencsényi, G.; Béresová, M.; Dobó-Nagy, C.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Development and characterisation of gastroretentive solid dosage form based on melt foaming. AAPS Pharmscitech 2019, 20, 290. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay to cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Bahuguna, A.; Khan, I.; Bajpai, V.K.; Kang, S.C. MTT assay to evaluate the cytotoxic potential of a drug. Bangladesh J. Pharmacol. 2017, 12, 115–118. [Google Scholar] [CrossRef]
- Liu, Y.; Peterson, D.A.; Kimura, H.; Schubert, D. Mechanism of cellular 3-(4.5-dimethylthiazol 2-yl)-2.5-diphenyltetrazolium bromide (MTT) reduction. J. Neurochem. 1997, 69, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Romanet, R.; Coelho, C.; Liu, Y.; Bahut, F.; Ballester, J.; Nikolantonaki, M.; Gougeon, R.D. The antioxidant potential of white wines relies on the chemistry of sulfur-containing compounds: An optimized DPPH assay. Molecules 2019, 24, 1353. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yeap Foo, L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitao, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef] [PubMed]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID). Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2000, 6, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Tille, P. Traditional cultivation and identification. In Bailey & Scott’s Diagnostic Microbiology, 13th ed.; Elsevier, Mosby: St. Louis, MO, USA, 2013; pp. 81–89. [Google Scholar]
- Lukic, M.; Jaksic, I.; Krstonosic, V.; Cekic, N.; Savic, S. A combined approach in characterization of an effective w/o hand cream: The influence of emollient on textural, sensorial and in vivo skin performance. Int. J. Cosmet. Sci. 2012, 34, 140–149. [Google Scholar] [CrossRef]




| Composition | SPP (5 g) | TC (14.2 g) | Nonionic Emulgents | Cetostearyl Alcohol (4.6 g) Stearic Acid (10 g) Glycerol (5 g) IPM (5 g) Propylene Glycol (5 g) Purified Water (ad 100 g) | |||
|---|---|---|---|---|---|---|---|
| P60 (3 g) | CR (3 g) | TFS (3 g) | SP70 (3 g) | ||||
| I | + | + | + | ||||
| II | + | + | + | ||||
| III | + | + | + | ||||
| IV | + | + | + | ||||
| V | + | + | + | + | |||
| VI | + | + | + | + | |||
| VII | + | + | + | + | |||
| VIII | + | + | + | + | |||
| Composition | pH Value ± SD |
|---|---|
| I | 6.77 ± 0.04 |
| II | 6.83 ± 0.04 |
| III | 6.95 ± 0.05 |
| IV | 6.92 ± 0.02 |
| V | 6.77 ± 0.04 |
| VI | 6.82 ± 0.02 |
| VII | 6.90 ± 0.03 |
| VIII | 6.91 ± 0.03 |
| Pairwise Comparison | f2 |
|---|---|
| comp. VII vs. comp. III | 45.42 |
| comp. VIII vs. comp. IV | 43.59 |
| comp. VI vs. comp. II | 53.91 |
| comp. V vs. comp. I | 97.74 |
| Composition | Release Rate | Diffusion Coefficient |
|---|---|---|
| k 102 (µg/cm2 min1/2) ± S.D. | D 105 (cm2/min) ± S.D. | |
| I | 59.12 ± 2.08 | 0.0178 ± 0.002 |
| II | 144.4 ± 6.21 | 0.1777 ± 0.015 |
| III | 313.01 ± 11.3 | 0.8227 ± 0.041 |
| IV | 258.09 ± 5.96 | 0.5699 ± 0.032 |
| V | 60.72 ± 4.51 | 0.0191 ± 0.002 |
| VI | 263.34 ± 11.63 | 0.5470 ± 0.028 * |
| VII | 455.41 ± 22.62 | 1.6601 ± 0.091 * |
| VIII | 490.42 ± 27.87 | 1.6472 ± 0.083 * |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Józsa, L.; Ujhelyi, Z.; Vasvári, G.; Sinka, D.; Nemes, D.; Fenyvesi, F.; Váradi, J.; Vecsernyés, M.; Szabó, J.; Kalló, G.; et al. Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris. Molecules 2020, 25, 4856. https://doi.org/10.3390/molecules25204856
Józsa L, Ujhelyi Z, Vasvári G, Sinka D, Nemes D, Fenyvesi F, Váradi J, Vecsernyés M, Szabó J, Kalló G, et al. Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris. Molecules. 2020; 25(20):4856. https://doi.org/10.3390/molecules25204856
Chicago/Turabian StyleJózsa, Liza, Zoltán Ujhelyi, Gábor Vasvári, Dávid Sinka, Dániel Nemes, Ferenc Fenyvesi, Judit Váradi, Miklós Vecsernyés, Judit Szabó, Gergő Kalló, and et al. 2020. "Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris" Molecules 25, no. 20: 4856. https://doi.org/10.3390/molecules25204856
APA StyleJózsa, L., Ujhelyi, Z., Vasvári, G., Sinka, D., Nemes, D., Fenyvesi, F., Váradi, J., Vecsernyés, M., Szabó, J., Kalló, G., Vasas, G., Bácskay, I., & Fehér, P. (2020). Formulation of Creams Containing Spirulina Platensis Powder with Different Nonionic Surfactants for the Treatment of Acne Vulgaris. Molecules, 25(20), 4856. https://doi.org/10.3390/molecules25204856
















