Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production
Abstract
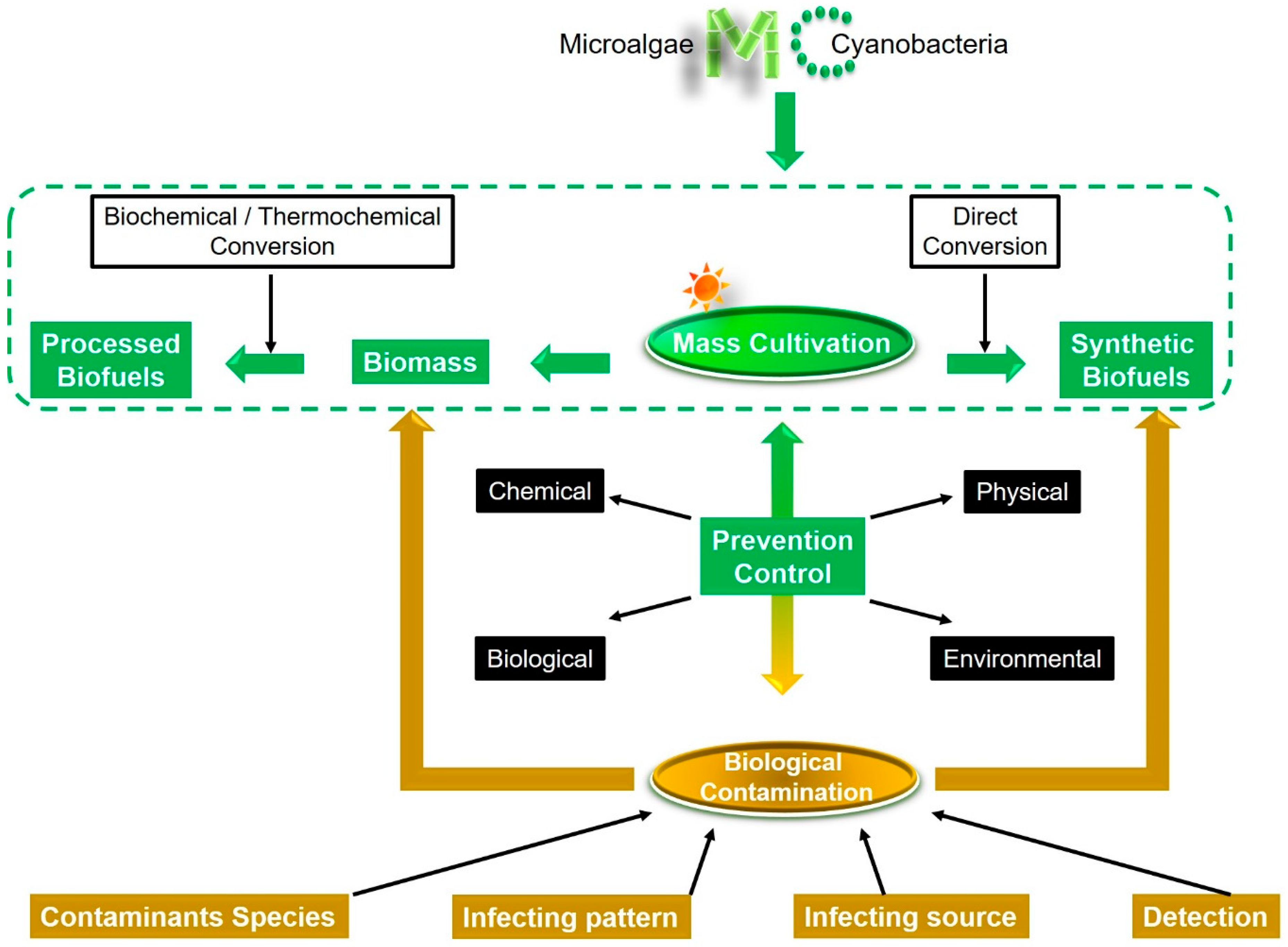
:1. Introduction
2. Major Biological Contaminations and the Infection Patterns
2.1. Cell Growth-Affecting Contaminations
2.2. Products Accumulation-Affecting Contaminations
| Categories of Biological Contaminants | Species of Biological Contaminants | Microalgae/Cyanobacteria Host | Culture System | Impact of Contamination | Reference |
|---|---|---|---|---|---|
| Zooplankton | Rotifer Brachionus calyciflorus | Chlorella kessleri | Laboratory condition | Pond crash within days of infection | [44,46] |
| Amoeboaphelidium protococcarum | Scenedesmus dimorphus | 400 L outdoor ponds | Rapid event with devastating consequences for the algal population | [49] | |
| Ciliates | Dunaliella salina | Laboratory condition | Clarified the algal culture within 2 days | [50] | |
| Fungi | Chytrid (phylum Blastocladiomycota) | Haematococcus pluvialis | Laboratory condition | Caused epidemics resulting in damage to the cultures | [51] |
| Other Algae | Golden algae (Poterioochromonas sp.) | Synechocystis sp. PCC 6803 | 1 L Pyrex Roux-type photobioreactor | Killing effect on the culture | [52] |
| Virus | Heterosigma akashiwo virus (HaV) | Heterosigma akashiwo | Laboratory condition | Algal culture became transparent within 33 h after inoculation | [53] |
| Products-Consuming Bacteria | Pannonibacter phragmitetus | Synechocystis sp. PCC 6803 | 6 L hanging membrane photobioreactor | Accumulation of the target product ethanol was exhausted | [37] |
3. Infecting Sources of Biological Contaminants
3.1. Aquatic Pollution
3.2. Air Pollution
3.3. Blind Angles of Photobioreactor
4. Detection of Biological Contaminants
5. Strategies for Controlling Biological Contamination
5.1. Chemical Control
5.2. Biological Control
5.3. Physical Control
5.4. Environmental Control
6. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Hellingwerf, K.J.; de Mattos, M.J.T. Alternative routes to biofuels: Light-driven biofuel formation from CO2 and water based on the ‘photanol’ approach. J. Biotechnol. 2009, 142, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Keasling, J.D.; Chou, H. Metabolic engineering delivers next-generation biofuels. Nat. Biotechnol. 2008, 26, 298–299. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Patra, P.; Ghosh, A. Metabolic engineering for enhancing microbial biosynthesis of advanced biofuels. Renew. Sustain. Energy Rev. 2020, 119, 109562. [Google Scholar] [CrossRef]
- Liu, X.; Miao, R.; Lindberg, P.; Lindblad, P. Modular engineering for efficient photosynthetic biosynthesis of 1-butanol from CO2 in cyanobacteria. Energy Environ. Sci. 2019, 12, 2765–2777. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Nielsen, J. Recent trends in metabolic engineering of microbial chemical factories. Curr. Opin. Biotechnol. 2019, 60, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Brennan, L.; Owende, P. Biofuels from microalgae-A review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Singh, S.P.; Pathak, J.; Sinha, R.P. Cyanobacterial factories for the production of green energy and value-added products: An integrated approach for economic viability. Renew. Sustain. Energy Rev. 2017, 69, 578–595. [Google Scholar]
- Kumar, M.; Sun, Y.Q.; Rathour, R.; Pandey, A.; Thakur, I.S.; Tsang, D.C.W. Algae as potential feedstock for the production of biofuels and value-added products: Opportunities and challenges. Sci. Total Environ. 2020, 716, 137116. [Google Scholar] [CrossRef]
- Klanchui, A.; Raethong, N.; Prommeenate, P.; Vongsangnak, W.; Meechai, A. Cyanobacterial Biofuels: Strategies and Developments on Network and Modeling. Adv. Biochem. Eng. Biot. 2017, 160, 75–102. [Google Scholar]
- Sarsekeyeva, F.; Zayadan, B.K.; Usserbaeva, A.; Bedbenov, V.S.; Sinetova, M.A.; Los, D.A. Cyanofuels: Biofuels from cyanobacteria. Reality and perspectives. Photosynth. Res. 2015, 125, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Machado, I.M.P.; Atsumi, S. Cyanobacterial biofuel production. J. Biotechnol. 2012, 162, 50–56. [Google Scholar] [CrossRef]
- Ho, S.H.; Ye, X.; Hasunuma, T.; Chang, J.S.; Kondo, A. Perspectives on engineering strategies for improving biofuel production from microalgae—A critical review. Biotechnol. Adv. 2014, 32, 1448–1459. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sofia; Sharma, V.K.; Lu, P.M.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Parmar, A.; Singh, N.K.; Pandey, A.; Gnansounou, E.; Madamwar, D. Cyanobacteria and microalgae: A positive prospect for biofuels. Bioresour. Technol. 2011, 102, 10163–10172. [Google Scholar] [CrossRef] [PubMed]
- Gudmundsson, S.; Nogales, J. Cyanobacteria as photosynthetic biocatalysts: A systems biology perspective. Mol. Biosyst. 2015, 11, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.Y.; Sun, T.; Pei, G.S.; Chen, L.; Zhang, W.W. Cyanobacterial chassis engineering for enhancing production of biofuels and chemicals. Appl. Microbiol. Biotechnol. 2016, 100, 3401–3413. [Google Scholar] [CrossRef]
- Gao, X.; Gao, F.; Liu, D.; Zhang, H.; Nie, X.Q.; Yang, C. Engineering the methylerythritol phosphate pathway in cyanobacteria for photosynthetic isoprene production from CO2. Energy Environ. Sci. 2016, 9, 1400–1411. [Google Scholar] [CrossRef]
- Oliver, J.W.K.; Atsumi, S. Metabolic design for cyanobacterial chemical synthesis. Photosynth. Res. 2014, 120, 249–261. [Google Scholar] [CrossRef]
- Wang, W.; Liu, X.; Lu, X. Engineering cyanobacteria to improve photosynthetic production of alka(e)nes. Biotechnol. Biofuels 2013, 6, 69. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.D.; Coleman, J.R. Ethanol synthesis by genetic engineering in cyanobacteria. Appl. Environ. Microbiol. 1999, 65, 523–528. [Google Scholar] [CrossRef] [Green Version]
- Dexter, J.; Fu, P.C. Metabolic engineering of cyanobacteria for ethanol production. Energy Environ. Sci. 2009, 2, 857–864. [Google Scholar] [CrossRef]
- Gao, Z.X.; Zhao, H.; Li, Z.M.; Tan, X.M.; Lu, X.F. Photosynthetic production of ethanol from carbon dioxide in genetically engineered cyanobacteria. Energy Environ. Sci. 2012, 5, 9857–9865. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar] [CrossRef]
- Gomaa, M.A.; Al-Haj, L.; Abed, R.M. Metabolic engineering of Cyanobacteria and microalgae for enhanced production of biofuels and high-value products. J. Appl. Microbiol. 2016, 121, 919–931. [Google Scholar] [CrossRef]
- Savakis, P.; Hellingwerf, K.J. Engineering cyanobacteria for direct biofuel production from CO2. Curr. Opin. Biotechnol. 2015, 33, 8–14. [Google Scholar] [CrossRef]
- Khan, A.Z.; Bilal, M.; Mehmood, S.; Sharma, A.; Iqbal, H.M.N. State-of-the-Art Genetic Modalities to Engineer Cyanobacteria for Sustainable Biosynthesis of Biofuel and Fine-Chemicals to Meet Bio-Economy Challenges. Life 2019, 9, 54. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, C.; Dubey, K.K.; Shukla, P. Metabolic engineering of microalgal based biofuel production: Prospects and challenges. Front. Microbiol. 2016, 7, 432. [Google Scholar] [CrossRef] [Green Version]
- de Andrade, G.A.; Berenguel, M.; Guzman, J.L.; Pagano, D.J.; Acien, F.G. Optimization of biomass production in outdoor tubular photobioreactors. J. Process Control 2016, 37, 58–69. [Google Scholar] [CrossRef]
- Zemke, P.E.; Sommerfeld, M.R.; Hu, Q. Assessment of key biological and engineering design parameters for production of Chlorella zofingiensis (Chlorophyceae) in outdoor photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 5645–5655. [Google Scholar] [CrossRef]
- Soman, A.; Shastri, Y. Optimization of novel photobioreactor design using computational fluid dynamics. Appl. Energy 2015, 140, 246–255. [Google Scholar] [CrossRef]
- Pulz, O. Photobioreactors: Production systems for phototrophic microorganisms. Appl. Microbiol. Biotechnol. 2001, 57, 287–293. [Google Scholar]
- Heimann, K. Novel approaches to microalgal and cyanobacterial cultivation for bioenergy and biofuel production. Curr. Opin. Biotechnol. 2016, 38, 183–189. [Google Scholar] [CrossRef]
- Khoo, C.G.; Lam, M.K.; Lee, K.T. Pilot-scale semi-continuous cultivation of microalgae Chlorella vulgaris in bubble column photobioreactor (BC-PBR): Hydrodynamics and gas-liquid mass transfer study. Algal Res. 2016, 15, 65–76. [Google Scholar] [CrossRef]
- Han, S.F.; Jin, W.B.; Tu, R.J.; Wu, W.M. Biofuel production from microalgae as feedstock: Current status and potential. Crit. Rev. Biotechnol. 2014, 35, 255–268. [Google Scholar] [CrossRef]
- Zhu, Z.; Luan, G.; Tan, X.; Zhang, H.; Lu, X. Rescuing ethanol photosynthetic production of cyanobacteria in non-sterilized outdoor cultivations with a bicarbonate-based pH-rising strategy. Biotechnol. Biofuels 2017, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Magdouli, S.; Brar, S.K.; Blais, J.F. Co-culture for lipid production: Advances and challenges. Biomass Bioenergy 2016, 92, 20–30. [Google Scholar] [CrossRef]
- Lam, T.P.; Lee, T.M.; Chen, C.Y.; Chang, J.S. Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour. Technol. 2018, 252, 180–187. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Chen, L.; Wang, J.; Liu, T. The contamination and control of biological pollutants in mass cultivation of microalgae. Bioresour. Technol. 2013, 128, 745–750. [Google Scholar] [CrossRef]
- Molina, D.; Carvalho, J.C.; Magalhaes, A.I.; Faulds, C.; Bertrand, E.; Soccol, C.R. Biological contamination and its chemical control in microalgal mass cultures. Appl. Microbiol. Biotechnol. 2019, 103, 9345–9358. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Liu, J.G.; Lin, W. Botanical pesticides as potential rotifer-control agents in microalgal mass culture. Algal Res. 2014, 4, 62–69. [Google Scholar] [CrossRef]
- Fischer, B.B.; Roffler, S.; Eggen, R.I. Multiple stressor effects of predation by rotifers and herbicide pollution on different Chlamydomonas strains and potential impacts on population dynamics. Environ. Toxicol. Chem. 2012, 31, 2832–2840. [Google Scholar] [CrossRef]
- Park, S.; Van Ginkel, S.W.; Pradeep, P.; Igou, T.; Yi, C.; Snell, T.; Chen, Y. The Selective Use of Hypochlorite to Prevent Pond Crashes for Algae-Biofuel Production. Water Environ. Res 2016, 88, 70–78. [Google Scholar] [CrossRef]
- Lubzens, E. Raising rotifers for use in aquaculture. Hydrobiologia 1987, 147, 245–255. [Google Scholar] [CrossRef]
- Pradeep, V.; Van Ginkel, S.W.; Park, S.; Igou, T.; Yi, C.; Fu, H.; Johnston, R.; Snell, T.; Chen, Y.S. Use of Copper to Selectively Inhibit Brachionus calyciflorus (Predator) Growth in Chlorella kessleri (Prey) Mass Cultures for Algae Biodiesel Production. Int. J. Mol. Sci. 2015, 16, 20674–20684. [Google Scholar] [CrossRef] [Green Version]
- Luan, G.; Qi, Y.; Wang, M.; Li, Z.; Duan, Y.; Tan, X.; Lu, X. Combinatory strategy for characterizing and understanding the ethanol synthesis pathway in cyanobacteria cell factories. Biotechnol. Biofuels 2015, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Medipally, S.R.; Yusoff, F.M.; Banerjee, S.; Shariff, M. Microalgae as sustainable renewable energy feedstock for biofuel production. BioMed Res. Int. 2015, 2015, 519513. [Google Scholar] [CrossRef]
- Letcher, P.M.; Lopez, S.; Schmieder, R.; Lee, P.A.; Behnke, C.; Powell, M.J.; McBride, R.C. Characterization of Amoeboaphelidium protococcarum, an Algal Parasite New to the Cryptomycota Isolated from an Outdoor Algal Pond Used for the Production of Biofuel. PLoS ONE 2013, 8, e56232. [Google Scholar] [CrossRef]
- Moreno-Garrido, I.; Canavate, J.P. Assessing chemical compounds for controlling predator ciliates in outdoor mass cultures of the green algae Dunaliella salina. Aquacult. Eng. 2001, 24, 107–114. [Google Scholar] [CrossRef]
- Hoffman, Y.; Aflalo, C.; Zarka, A.; Gutman, J.; James, T.Y.; Boussiba, S. Isolation and characterization of a novel chytrid species (phylum Blastocladiomycota), parasitic on the green alga Haermatococcus. Mycol. Res. 2008, 112, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 3, 1333–1341. [Google Scholar] [CrossRef] [Green Version]
- Nagasaki, K.; Tarutani, K.; Yamaguchi, M. Growth characteristics of Heterosigma akashiwo virus and its possible use as a microbiological agent for red tide control. Appl. Environ. Microbiol. 1999, 65, 898–902. [Google Scholar] [CrossRef] [Green Version]
- Borowitzka, M.A. Commercial production of microalgae: Ponds, tanks, tubes and fermenters. J. Biotechnol. 1999, 70, 313–321. [Google Scholar] [CrossRef]
- Anto, S.; Mukherjee, S.S.; Muthappa, R.; Mathimani, T.; Deviram, G.; Kumar, S.S.; Verma, T.N.; Pugazhendhi, A. Algae as green energy reserve: Technological outlook on biofuel production. Chemosphere 2020, 242, 125079. [Google Scholar] [CrossRef]
- Tu, Q.; Lu, M.; Thiansathit, W.; Keener, T.C. Review of Water Consumption and Water Conservation Technologies in the Algal Biofuel Process. Water Environ. Res 2016, 88, 21–28. [Google Scholar] [CrossRef]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.F.; Liu, J.L.; Liu, T.Z. The difference in effective light penetration may explain the superiority in photosynthetic efficiency of attached cultivation over the conventional open pond for microalgae. Biotechnol. Biofuels 2015, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Carney, L.T.; Lane, T.W. Parasites in algae mass culture. Front. Microbiol. 2014, 5, 278. [Google Scholar] [CrossRef] [Green Version]
- Gerphagnon, M.; Latour, D.; Colombet, J.; Sime-Ngando, T. A double staining method using SYTOX green and calcofluor white for studying fungal parasites of phytoplankton. Appl. Environ. Microbiol. 2013, 79, 3943–3951. [Google Scholar] [CrossRef] [Green Version]
- Sieracki, C.K.; Sieracki, M.E.; Yentsch, C.S. An imaging-in-flow system for automated analysis of marine microplankton. Mar. Ecol. Prog. Ser. 1998, 168, 285–296. [Google Scholar] [CrossRef] [Green Version]
- Havlik, I.; Reardon, K.F.; Unal, M.; Lindner, P.; Prediger, A.; Babitzky, A.; Beutel, S.; Scheper, T. Monitoring of microalgal cultivations with on-line, flow-through microscopy. Algal Res. 2013, 2, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Sarma, S.S.S.; Nandini, S.; Flores, J.L.G. Effect of methyl parathion on the population growth of the rotifer Brachionus patulus (O. F. Muller) under different algal food (Chlorella vulgaris) densities. Ecotoxicol. Environ. Saf. 2001, 48, 190–195. [Google Scholar] [CrossRef]
- Marcial, H.S.; Hagiwara, A. Effect of diazinon on life stages and resting egg hatchability of rotifer Brachionus plicatilis. Hydrobiologia 2007, 593, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Jiang, J.G. Toxic effects of chemical pesticides (trichlorfon and dimehypo) on Dunaliella salina. Chemosphere 2011, 84, 664–670. [Google Scholar] [CrossRef]
- Benderliev, K.M.; Pouneva, I.D.; Ivanova, N.I. Fungicide Effect of Triton-N on Phlyctidium. Biotechnol. Tech. 1993, 7, 335–338. [Google Scholar] [CrossRef]
- Fott, B. Phlyctidium scenedesmi spec. nova, a new chytrid destroying mass cultures of algae. Z. Allg. Mikrobiol. 1967, 7, 97–102. [Google Scholar] [CrossRef]
- Hanazato, T. Pesticide effects on freshwater zooplankton: An ecological perspective. Environ. Pollut. 2001, 112, 1–10. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.G.; Wang, H.Y.; Gao, Z.G. Treatment potential of a synergistic botanical pesticide combination for rotifer extermination during outdoor mass cultivation of Spirulina platensis. Algal Res. 2014, 6, 139–144. [Google Scholar] [CrossRef]
- Zhang, L.T.; Xu, R.; Liu, J.G. Efficacy of botanical pesticide for rotifer extermination during the cultivation of Nannochloropsis oculata probed by chlorophyll a fluorescence transient. Photosynthetica 2020, 58, 341–347. [Google Scholar] [CrossRef]
- Holm, E.R.; Stamper, D.M.; Brizzolara, R.A.; Barnes, L.; Deamer, N.; Burkholder, J.M. Sonication of bacteria, phytoplankton and zooplankton: Application to treatment of ballast water. Mar. Pollut. Bull. 2008, 56, 1201–1208. [Google Scholar] [CrossRef]
- Rego, D.; Redondo, L.M.; Geraldes, V.; Costa, L.; Navalho, J.; Pereira, M.T. Control of predators in industrial scale microalgae cultures with Pulsed Electric Fields. Bioelectrochemistry 2015, 103, 60–64. [Google Scholar] [CrossRef]
- Kosta, S.; Jain, R.; Tiwari, A. Marine blue green algae: Microorganism of bioactive potential. Indian J. Microbiol. 2010, 50, 142–143. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, C.S.; Durandchastel, H. Utilization of Spirulina Algae for Industrial Photosynthesis. J. Phycol. 1977, 13, 60. [Google Scholar]
- Vonshak, A.; Boussiba, S.; Abeliovich, A.; Richmond, A. Production of Spirulina biomass: Maintenance of monoalgal culture outdoors. Biotechnol. Bioeng. 1983, 25, 341–349. [Google Scholar] [CrossRef]
- Borowitzka, L.J.; Borowitzka, M.A. Commercial Production of β-Carotene by Dunaliella-Salina in Open Ponds. Bull. Mar. Sci. 1990, 47, 244–252. [Google Scholar]
- Bader, F.G.; Tsuchiya, H.M.; Fredrickson, A.G. Grazing of Ciliates on Blue-Green-Algae—Effects of Light Shock on Grazing Relation and on Algal Population. Biotechnol. Bioeng. 1976, 18, 333–348. [Google Scholar] [CrossRef]
- Post, F.J.; Borowitzka, L.J.; Borowitzka, M.A.; Mackay, B.; Moulton, T. The protozoa of a western australian hypersaline lagoon. Hydrobiologia 1983, 105, 95–113. [Google Scholar] [CrossRef]
- Huntley, M.E.; Lopez, M.D. Temperature-dependent production of marine copepods: A global synthesis. Am. Nat. 1992, 140, 201–242. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, J.; Li, L.; Pang, T.; Zhang, L. Efficacy of binary combinations of botanical pesticides for rotifer elimination in microalgal cultivation. Bioresour. Technol. 2014, 154, 67–73. [Google Scholar] [CrossRef]
- Moulton, T.P.; Borowitzka, L.J.; Vincent, D.J. The Mass-Culture of Dunaliella-Salina for Beta-Carotene—From Pilot-Plant to Production Plant. Hydrobiologia 1987, 151, 99–105. [Google Scholar] [CrossRef]
| Solution Type | Controlling Treatment | Reference |
|---|---|---|
| Chemical Control | Copper Salts Surfactant Triton-N Trichlorphon | [67] [66] [65] |
| Biological Control | Specific pathogen | [59] |
| Celangulin/toosendanin(1:9) | [69,70,80] | |
| Physical Control | Filtration Pulsed Electric Fields Sonication | [40,59] [72] [59] |
| Environmental Control | High pH High salinity Light shock High temperature | [52,74] [81] [77] [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Jiang, J.; Fa, Y. Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules 2020, 25, 5220. https://doi.org/10.3390/molecules25225220
Zhu Z, Jiang J, Fa Y. Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules. 2020; 25(22):5220. https://doi.org/10.3390/molecules25225220
Chicago/Turabian StyleZhu, Zhi, Jihong Jiang, and Yun Fa. 2020. "Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production" Molecules 25, no. 22: 5220. https://doi.org/10.3390/molecules25225220
APA StyleZhu, Z., Jiang, J., & Fa, Y. (2020). Overcoming the Biological Contamination in Microalgae and Cyanobacteria Mass Cultivations for Photosynthetic Biofuel Production. Molecules, 25(22), 5220. https://doi.org/10.3390/molecules25225220





