Abstract
Flavonoids are phytochemical compounds present in many plants, fruits, vegetables, and leaves, with potential applications in medicinal chemistry. Flavonoids possess a number of medicinal benefits, including anticancer, antioxidant, anti-inflammatory, and antiviral properties. They also have neuroprotective and cardio-protective effects. These biological activities depend upon the type of flavonoid, its (possible) mode of action, and its bioavailability. These cost-effective medicinal components have significant biological activities, and their effectiveness has been proved for a variety of diseases. The most recent work is focused on their isolation, synthesis of their analogs, and their effects on human health using a variety of techniques and animal models. Thousands of flavonoids have been successfully isolated, and this number increases steadily. We have therefore made an effort to summarize the isolated flavonoids with useful activities in order to gain a better understanding of their effects on human health.
1. Introduction
Flavonoids are secondary metabolites, which mainly consists of a benzopyrone ring bearing a phenolic or poly-phenolic groups at different positions [1]. They are most commonly found in fruits, herbs, stems, cereals, nuts, vegetables, flowers and seeds [2,3]. The presence of bioactive phytochemical constituents present in these different plants parts gives them their medicinal value and biological activities [4]. So far, over 10,000 flavonoid compounds have been isolated and identified [5]. Most of the flavonoids are widely accepted as therapeutic agents [6]. These are naturally synthesized through the phenylpropanoid pathway with bioactivity dependent on its absorption mechanism and bioavailability [7].
Flavonoids have been used in natural dyes [8,9], in cosmetics and skin care products [10,11], and anti-wrinkle skin agents [12]. The most pronounced applications of these polyphenols, however, are in the field of medicine. Flavonoids have been used extensively as anticancer, [13] antimicrobial, antiviral, antiangiogenic [14,15], antimalarial, antioxidant, neuroprotective, antitumor, and anti-proliferative agents [16]. Apple peel extracts rich in flavonoids inhibits acetylcholinesterase (ACE) in vitro and is an effective antihypertensive agent [17,18,19,20]. It also prevents cardio-metabolic disorders [21] and displays better preservation of cognitive performance with aging [22].
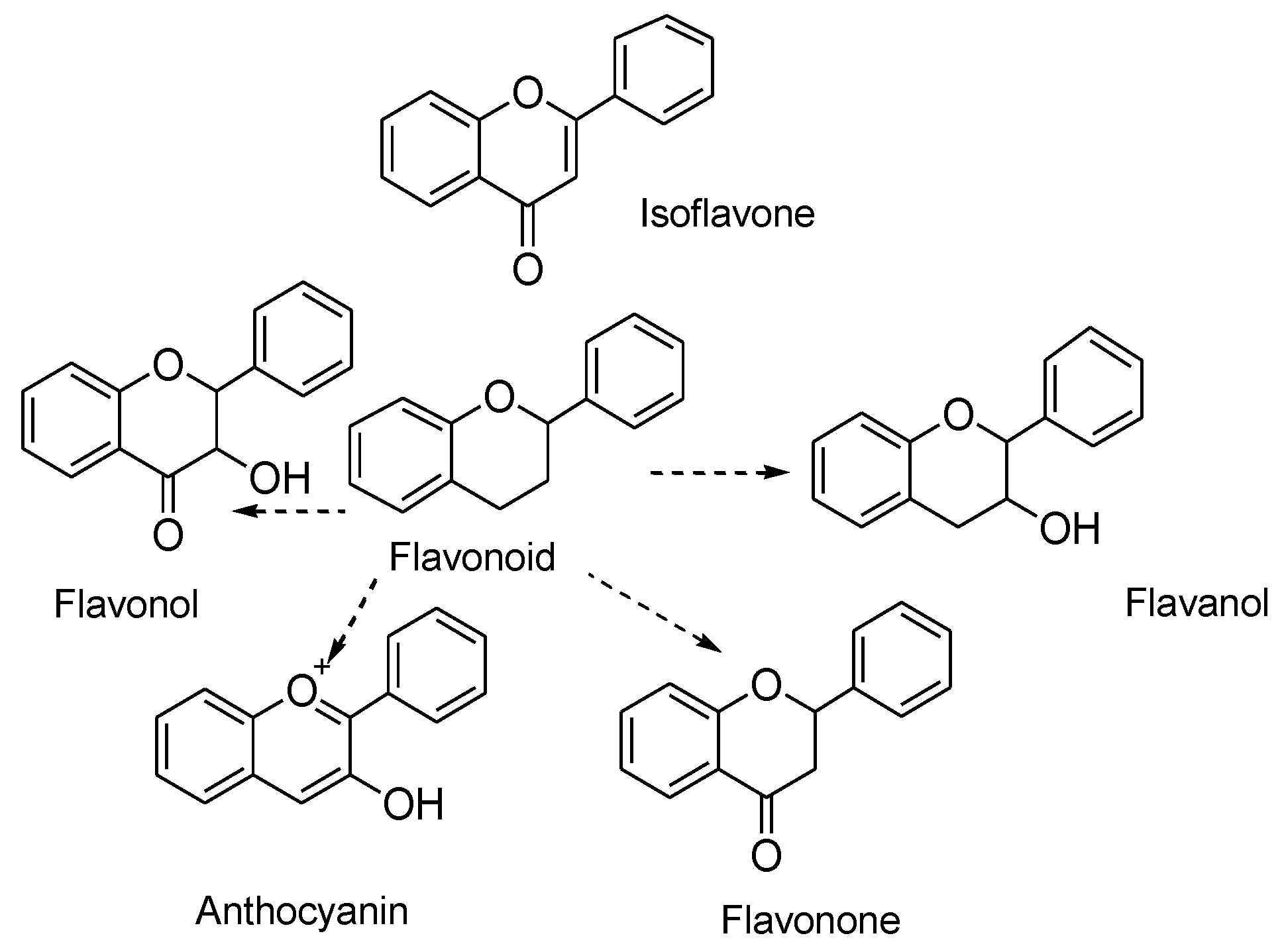
They are classified into various types depending on their chemical structure, degree of unsaturation, and oxidation of carbon ring. Anthoxanthins (flavanone and flavanol), flavanones, flavanonols, flavans, chalchones, anthocyanidins, and isoflavonoids are the different subgroups of flavonoids. Each of these flavonoid is widely distributed in nature (Figure 1) [23]. A higher intake of flavonoid rich foods has a number of health benefits [24]. Since these natural compounds have positive effects on human health, an increasing effort has been made to isolate these compounds from various plants. For instance, citrus fruits are rich sources of flavonoids. Two flavonoids, narigenin and hesperetin, are found in oranges, lemons, and grapes [25]. Anthocyanins and quercetin glycosides flavonoids are present in mulberry [26].
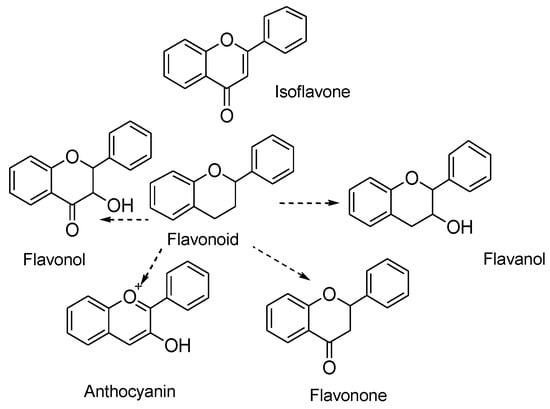
Figure 1.
Chemical structure of flavonoids and its different types.
2. Effects of Flavonoids on Human Health
2.1. Anticancer Action
Cancer is a major health problem caused by abnormal cell growth. There are various anticancer drugs available, and yet only a few display inhibition against oncogenesis, and a majority of them are toxic and have adverse side effects [27]. Natural biomolecules with secondary metabolites have phytomediated content, and display biological activities over a wide range of spectrum, laying the basis for cancer prevention and treatment. Flavonoids are known to inhibit cell growth and act as an anticancer agents [28,29]. Chemoprevention is the use of natural or synthetic substances to inhibit carcinogenesis [30]. Following are copious examples of flavonoids and their use as anticancer agents.
Hesperedin (Hsp) is an important flavonoid which displays efficient anticancer activity [31]. Polylactic-co-glycolic acid (PLGA) nanoparticles were synthesized and loaded with Hsp to form hesperidin nanoparticles (HspNPs) to determine its potential application as an anticancer agent against C6 glioma cells. The encapsulated Hsp exhibited decreased in vitro cell viability against the C6 glioma cell line, and the controlled release of Hsp decreased the cytotoxicity of PLGA [32].
Aurone, a benzo-furanone, is another flavonoid that has been extensively used as an anticancer agent [33]. Various analogues of aurone display different mechanisms against cancer cells because there are many possible targets. These targets include cyclin dependent kinase, histone deacetylase, the adenosine receptor, telomerase, sirtuins, and microtubules [33].
Quercetin is a natural flavonoid present in plants and in commonly consumed foods such as berries, green tea, and grains. It has been used most effectively for colorectal cancer. Cell cycle arrest, increase in apoptosis, antioxidant replication, modulation of estrogen receptors, regulation of signaling pathways, inhibition of metastasis and angiogenesis are among various mechanisms underlying the chemo-preventive effects of quercetin in colorectal cancer [34]. Luteolin, a natural flavonoid with pro-apoptotic activity in hepatocellular carcinoma (HCC) cells, arrests the cancer cell cycle at the G2/M stage. The miR-6809-5p is overexpressed in HCC and it was found to be upregulated by luteolin by directly targeting flotillin-1 [35]. Kaempferol is a natural flavanol, which can reduce the risk of cancer. It stimulates the body’s antioxidants against free radicals that cause cancer [36]. Myricetin is an important flavonoid which has anti-inflammatory and anticancer activities, and in liver cancer it shows antimitotic effects, and it targets different metabolic pathways in mitochondria that result in cancer cell death [37]. Matricaria recutita L. (chamomile) flower has a flavonoid content of 157.9 ± 2.22 mg/g QE of dry extract. It displayed dose dependent enhanced mortality of HepG2 cells in HCC. Angiogenesis is an important process which is hijacked for progression of cancer. Vascular endothelial growth factor (VEGF) facilitates blood vessel formation via angiogenesis through VEGF receptors. This expression of VEGF was dose dependently reduced by synthesized extract, making it an efficient anticancer agent [38].
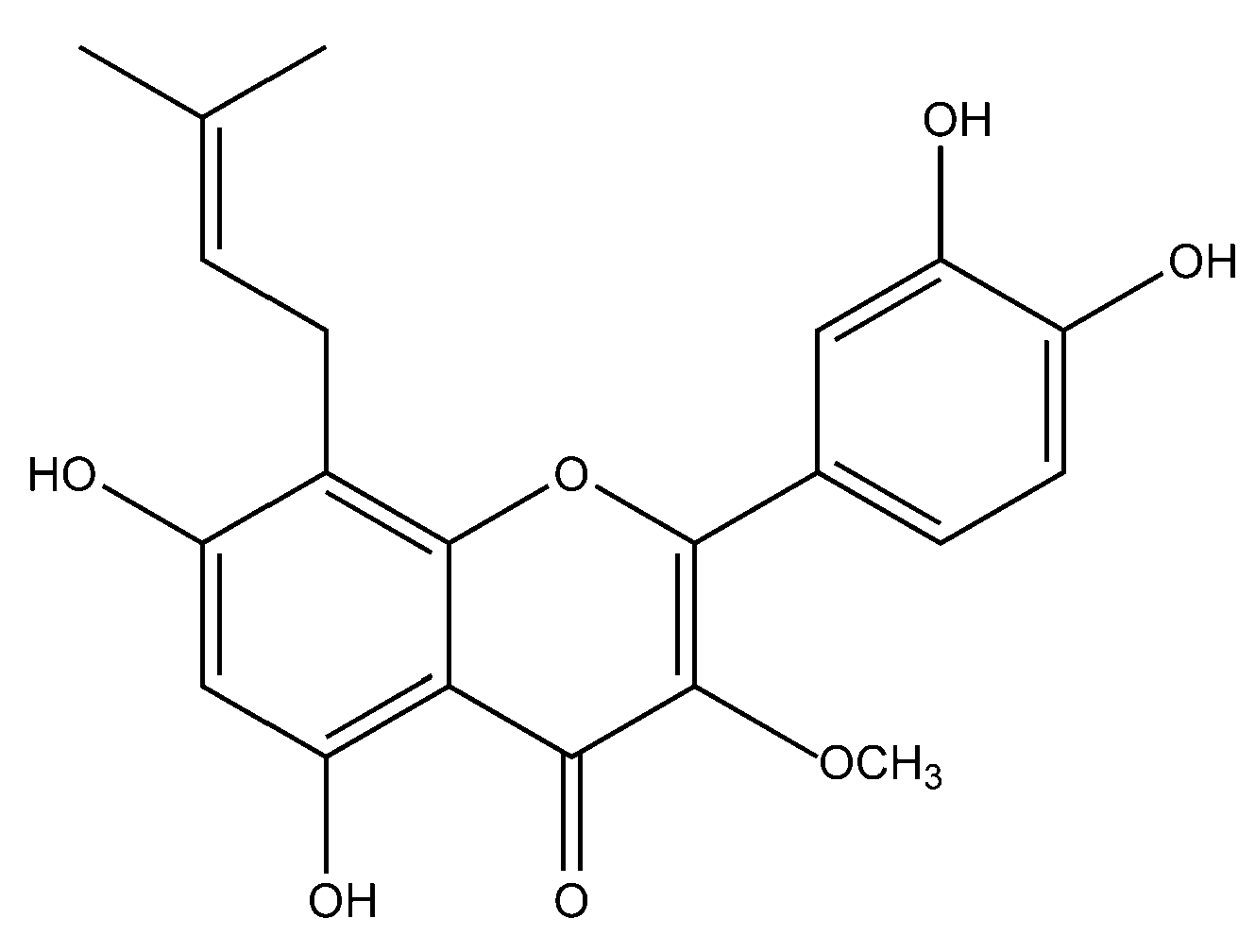
In one of the studies, the aerial parts of Gastrocotyle hispida were air dried and used for extraction. The extract showed a flavonoid content of 178 mg/g QE. The in vitro assay of extract was performed for evaluation of its anticancer activity against kidney, liver and breast cancer cell lines [39]. Some reports suggest the flavonoid anticancer activity is because it inhibits protein kinases, which are responsible for regulating cellular pathways [13]. Prenylated chalcone and flavonoids are structurally related and are often considered analogs of each other. Prenylflavonoids were investigated for their potential anticancer activities in vitro [40]. Recently, four prenylated flavonoids isolated from the fruits of Sinopodophyllum hexandrum were tested for their cytotoxicity against the human breast cancer cell line T47D. The isolated compounds were spectroscopically analyzed, and structures were elucidated (Figure 2). The percent growth inhibition determined showed an IC50 values to be less than 10 μmol·L−1 [41]. Apple is rich in many flavonoids which are directly related to improved cardiovascular health [42] and reduces the risk of asthma and Alzheimer’s disease [43]. Flavonoids present in apple are reported to reduce the risk of colorectal cancer. Studies have shown that consumption of one apple per day reduces the chance of cancer up to 50% [44]. Flavonoids extracted from female hop cones have great pharmaceutical importance including anti-carcinogenic and anti-microbial effects [45].
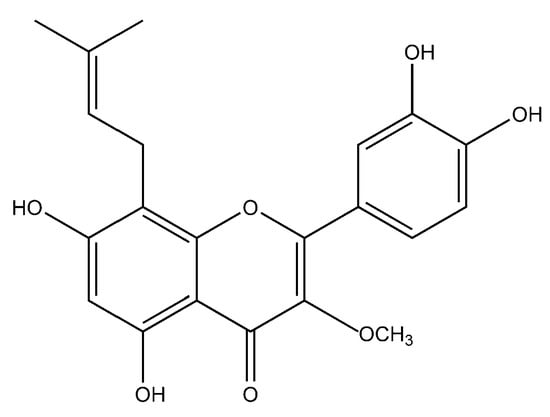
Figure 2.
Flavonoid compound isolated from Sinopodophylli fructus.
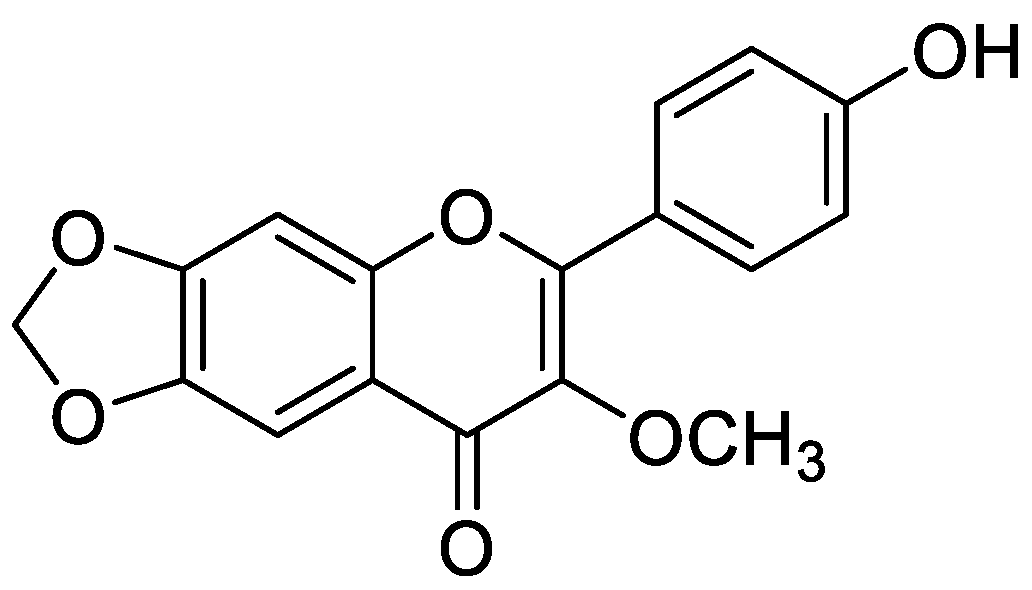
Flavonoids present in Emblica officinalis show certain pharmacological activities such as anticancer, antioxidant, anti-inflammatory and as an immunomodulator [46]. Berry flavonoids have a preventive effect on esophageal cancer [47]. The methoxy-flavone also showed cancer chemopreventive properties [48]. Cocoa polyphenols have anticancer effects along with anti-inflammatory and antioxidant activities [49]. Flavonoids extracted from litchi (Epicatechin, proanthocyanidin B2 and proanthocyanidin B4) have shown anti breast cancer activity [50]. Soy isoflavons are active against prostate cancer [51]. Flavonoids found in tea and other flavonoid rich foods such as apple, onion etc., reduce the risk of lung cancers [52].
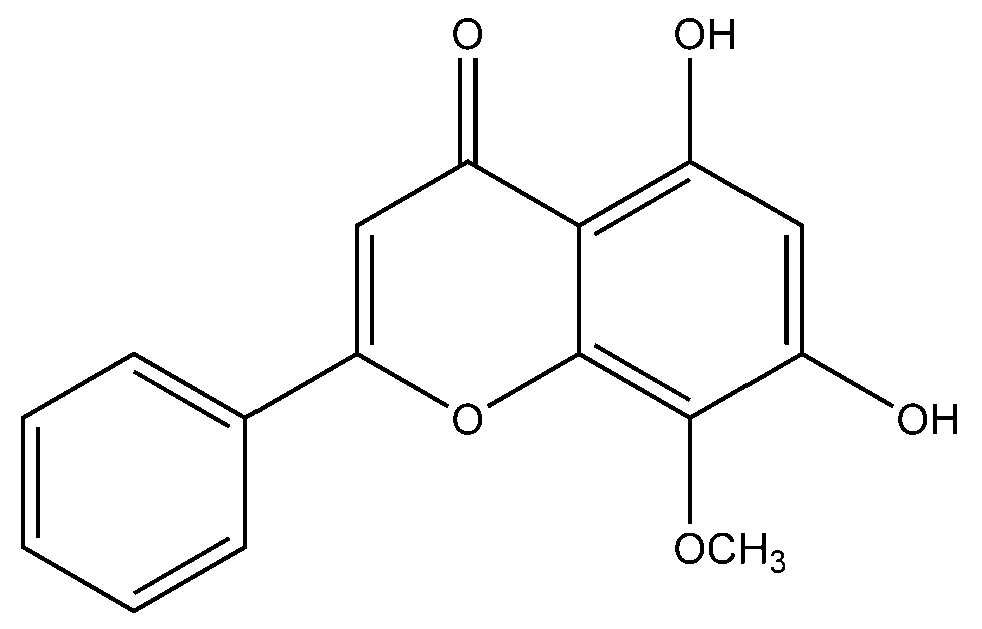
Flavonoid modified drugs (FMD) have much a better effect on lung cancer cell line A549 and L929 [53]. Allium flavum and Allium carinatum are wild, edible onions rich in flavonoids, rutin, quercetin 3-O-glucoside, and kaempferol-3-O-glucoside. These, besides having known antioxidant potential, are effective anticancer agents. When combined with doxorubicin, they upregulate angiogenic factor against human hepatoma (HepG2) and lung carcinoma (A549) cell lines [54]. An isolated flavonoid of Dulacia egleri showed anticancer activity by inhibiting cathepsins B and L. The extract was prepared from the Dulacia plant, and the compound 4′-Hydroxy-6,7-methylenedioxy-3-methoxyflavone were isolated from it (Figure 3). This isolated flavonoids form the leaves of plant were then spectroscopically analyzed for structure determination. The isolated compound showed enzyme inhibition and can be used for cancer therapy [55]. Licorices (the roots of Glycyrrhiza uralensis) contain more than 300 flavonoids, and it is used to alleviate stomach pain, to eliminate phlegm, and to relieve coughing. Recent research proves that it exhibits several pharmacological activities such as antitumor, antiviral and antimicrobial effects [56]. Baicalein and baicalin are phytochemicals which are cytostatic as well as cytotoxic to various human tumor cells, and inhibit tumor growth in vivo [57]. There are a few plants within the genus Peganum which have been used as medicines in China for a long time. This genus contains several alkaloids, flavonoids, etc. It has a wide range of biological activities such as antitumor, anticoagulant, anti-parasitic, anti-hypertension and anti-inflammatory properties [58]. Flavonoids and bioflavonoids extracted from Ouratea and other genera of the Ochnaceae family have important biological activities such as anti-tumor, antimicrobial, antiviral, and DNA topoisomerase inhibition [59]. One of the most popular Chinese medicinal herbs is Scutellaria baicalensis. Wogonin (Figure 4), wogonoside, bicalein, and baicalin are flavonoids extracted from Scutellaria baicalensis. These flavonoids are not only cytostatic, but also show cytotoxic effect on tumor cells both in vitro and in vivo [60]. Besides the naturally isolated flavonoids, these can be synthesized in a laboratory using different techniques. Macgown et al. used microwave assisted approach to synthesize novel bisflavonoids, which exhibited anticancer activity against liver, breast and colorectal carcinoma cell lines [61]. Below is an extensive list of plants whose flavonoid content enable them to be used as anticancer agents (Table 1). Although majority of these studies on the anticancer activities of various flavonoids and their derivatives are in pre-clinical study phase, several flavonoids are under consideration for cancer treatment and are at various phases of clinical trials. For example, the icaritin (ICT) is in the third phase of clinical trial for the cure of hepatocellular carcinoma. This prenylated flavonoids lower the signaling process by affecting various receptors like estrogen receptor splice variant ERα36, STAT3 and NFκB, and CXCR4. The ICT also targets ROS pathways and sphingosine kinase-1. It has been suggested to boost the immune response against the developing tumors. The ICT target a number of metabolic pathways, it can be used in glioblastoma and various types of blood leukemia. Due to its promising antitumor properties, various analogs of ICT synthesis and their clinical trials are in progress [62]. Thoracic radiotherapy for lung cancer treatment results in acute radiation-induced esophagitis (ARIE). It has been noted in phase II clinical trials that the epigallocatechin-3-gallatein reduces the ARIE and other related toxicities caused by thoracic radiotherapy [63]. Protein disulfide isomerase (PDI) is an enzyme present in endoplasmic reticulum and normally makes disulfide bonds in newly formed proteins. This enzyme is also involved in tumor progression and neurodegeneration diseases beside other important infectious ailments. The quercetin flavonoid blocks the function of PDI and is a useful anticancer agent. Phase II clinical trials showed that isoquercetin reduce hypercoagulability in cancer patients having high possibility of thrombosis [64]. Further clinical trials should be conducted on flavonoids and their derivatives for anticancer and other diseases as they have lower toxicity and minor side effects.
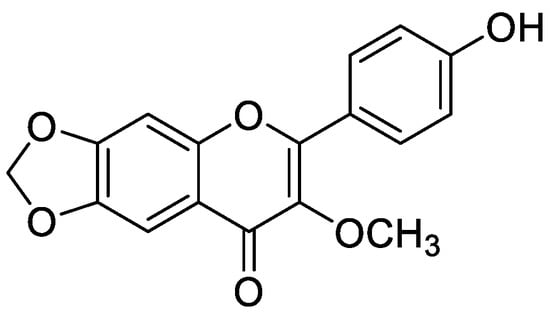
Figure 3.
Chemical structure of 4′-hydroxy-6,7-methylenedioxy-3-methoxyflavone.
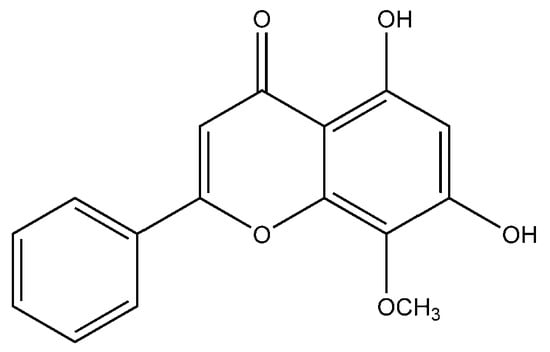
Figure 4.
Chemical structure of dihydroxy-8-methoxyflavone (wogonin).

Table 1.
Flavonoids with anticancer properties.
2.2. Antioxidant Activity
Reactive oxygen species (ROS) are produced in the human body mainly as byproducts of the electron transport chain. They are essential for protein phosphorylation, initiation of numerous transcriptional factors, apoptosis, immunity, and differentiation processes. However, ROS also cause oxidative stress upon reacting with molecules such as lipids, proteins, or nucleic acids. Lipid peroxidation by ROS causes cellular membrane damage. This membrane has a potential, with positive charges on the outside of the cell, and negative charges inside the cell. The damage to membrane alters the cell membrane potential and the cell’sosmotic pressure, eventually causing cell death. The human defense system use different mechanisms and enzymes to battle endogenous elevated ROS [76]. Flavonoids act as exogenous antioxidants and are directly oxidized by radicals to form less reactive species via four mechanisms, namely (1) the inhibition of nitric-oxide synthase activity, (2) inhibition of xanthine oxidase activity, (3) modulation of channel pathways, or by (4) interacting with other enzyme systems [77,78].
The antioxidant potential of flavonoids is associated with the molecular structure, and more precisely, with the location and total number of the –OH groups, the conjugation and resonance effects, the surrounding environment which modifies the thermodynamically favored antioxidant site, and the particular antioxidant mechanism for a compound [79,80]. The most commonly used supplemented antioxidants are vitamins C and E. The antioxidant potential of flavonoids is more robust than vitamin C and vitamin E [81]. It is therefore important to regularly include those fruits and vegetables that are rich in flavonoids in daily food intake. For example, due to enhanced and well-known antioxidant and anti-inflammatory properties, flavonoids improve bone health. The utilization of flavonoids in biomaterials has great prospects for bone tissue engineering [82]. This showed that flavonoids should be supplemented in food of aged people. Quercetin, an antioxidant flavonoid, when present in the blood stream, improves vascular health and reduces risk of cardiovascular disease in its conjugated form [83]. The quercetin and its derivatives prevents thrombosis or blood clotting and prevents chances of stroke [83]. Hesperidin and hesperetin are two flavonoids present in citrus fruits and mushrooms that showed antioxidant, anti-inflammatory, antimicrobial and anticancer effects [84]. Propolis has been used as a folk medicine for many years and is now used in the pharmaceutical industry. It contains many compounds, including flavonoids which are responsible for their pharmacological properties such as antioxidant, antimicrobial, healing, and anti-proliferative activities [85]. Rutin, a flavonol, showed a number of biological activities that includes anticancer, antioxidant and cytoprotective etc [86]. Sorghum has a high antioxidant activity in vitro [87]. Flavonoids and tannins from sorghums are the best antioxidants [88]. These results showed that sorghums grains and the products made from these grains should be promoted for food purposes. The sorghum flavonoids have high level of absorption in the small intestine. Although the presence of tannins makes its grains unsuitable for consumption but alkaline cooking reduces the level of tannins by 73% [89,90,91]. It is therefore important to genetically modify the tannin production pathways in sorghum, so that its grains are free from tannins. This crop is also suitable for arid conditions and can grow in harsh environmental conditions. Through this way a flavonoids rich sorghum grains will be available to the human population across the globe. Carotenoids are a class of polyphenols that possess antioxidant activity [92]. Fisetin (3, 3′, 4′, 7–tetrahydroxy flavone) is a flavonoid which shows antioxidant activity along with anti-inflammatory effects [93]. The aerial parts like bulbs, leaves, and flowers of Eleutherine bulbosa (Mill.) Urb. have high flavonoid content, and showed antioxidant activity [94]. Allium cepa. L (onion) showed antioxidant activity [95]. Dill (Anethum graveolens L.) and parsley (Petroselinum crispum Mill.) are the medicinal aromatic herbs whose phenolic and flavonoid content made them effective antioxidant agents with the ability to reduce ROS species and prevent ROS mediated diseases such as cancer and cardiovascular ailments. The antioxidant potential of these species may be brought about by different mechanisms and that includes shattering peroxides, and chelating metal ions, which catalyze the oxidation process. This antioxidant activity is related to the phenolic (PC) and flavonoid content (FC); in general, the greater the PC and FC, the greater the antioxidant activity shown by the herbal plant [96]. Utilization of these herbs in daily meals will not only provide aroma and taste but it will improve the nutritional value of food.
Bryonia alba L. is a medicinal and homeopathic plant of Cucurbitaceae family with a wide range of biological activities. The methanolic extract of air-dried leaves of this plant was used to isolate four flavonoids (lutonarin, saponarin, isoorientin and isovitexin) using HPLC-DAD. The structures of these compounds were elucidated by NMR and UV spectroscopy, and by mass spectroscopy. Their antioxidant and antiradical activity were confirmed by their ability to suppress ROS produced by macrophages, the isolates being more effective in comparison to the crude extract [97]. The flavonoid and phenolic content, along with the antioxidant potential may vary and depends on the method of extraction used [98]. Leaves extract of piper beetle was prepared using sonication, and had a total flavonoid content of 49.79 ± 1.54 mg QE/gm extract, whereas maceration gave 32.10 ± 0.65 mg QE/gm and Soxhlet extraction gave 40.89 ± 0.87 mg QE/gm (Table 2) [99]. The radical scavenging activity of the leaves extract and its role as an antioxidant agent are closely related to the flavonoid and phenolic content. The aerial part of the Salvia aristata was dried and extraction was performed using methanol. The methanolic extract was subjected to the Folin-Ciocalteu and Aluminum colorimetric methods to determine phenolic and flavonoid content, respectively. It turned out that TFC of 50% hydro methanol extract was higher than aqueous and methanolic extract. This was reflected in its potency as an antioxidant agent where IC50 of 50% hydro methanol extract was 3.4 whereas aqueous and methanolic extract gave 4.0 and 5.7 (mg/mL), respectively [100]. Plant derived secondary metabolites possess potent antioxidant activity, and this was evident from the ethanolic extract of leaves of Anisomeles malabarica. The DPPH assay confirmed its free radical quenching ability to be highest at 120 µg/mL. The extract thus showed enhanced antioxidant and antibacterial properties against Staphylococcus aureus, Bacillus subtilis and Proteus vulgaris [101].
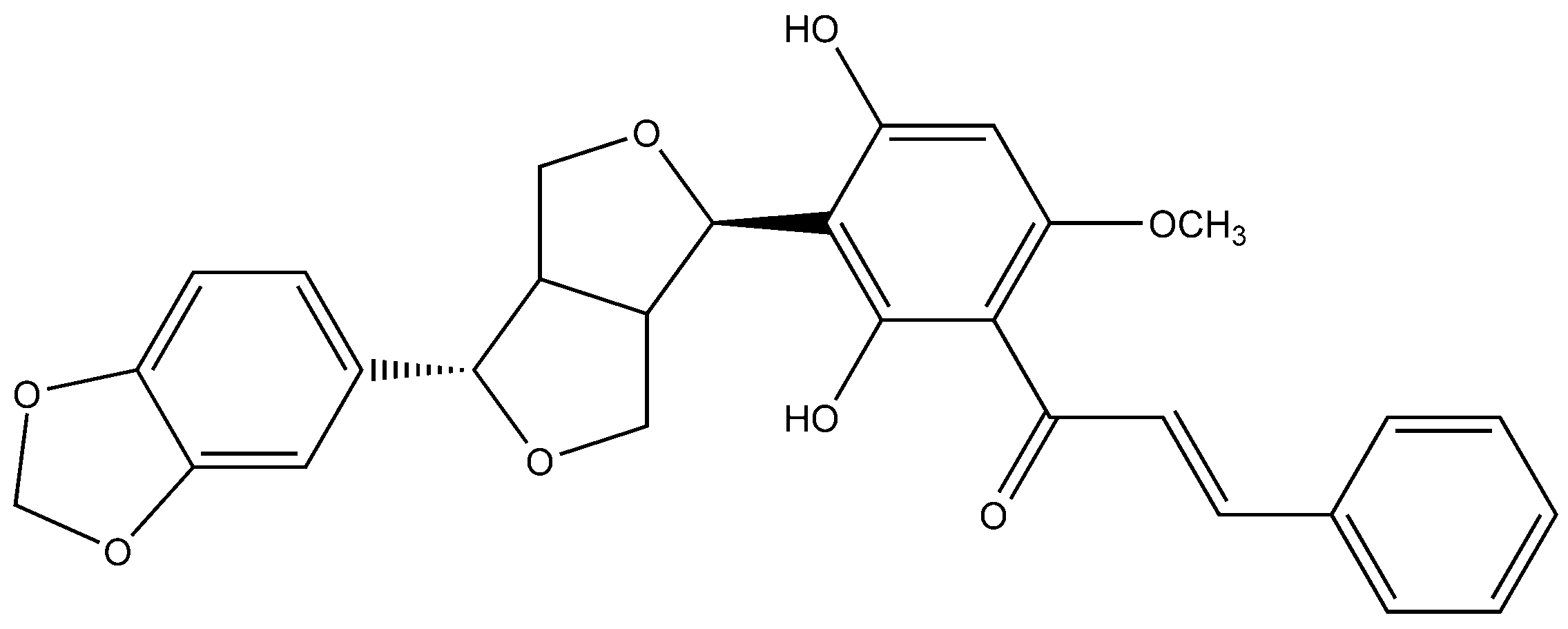
Using natural flavonoids to synthesize its derivatives has immense application and can improve the existing potential of these natural flavonoids. A group of flavonoids derivatives was made through chemically modifying luteolin, apigenin, chrysin, and diosmetin. The synthesis process was carried out by adding different bromo-alkanes with their respective precursors in anhydrous acetone. The derivatives obtained were then assayed for their antioxidant properties using DPPH, FRAP, and ORAC assays. The entire synthesized compounds displayed enhanced antioxidant activity, which may be attributed to their electron or hydrogen radical discharging capacity to DPPH. These derivatives also displayed anti-inflammatory and cytotoxic activity. Luteolin; apigenin; diosmetin; O4′,O7-dihexyl apigenin, and chrysin halt the function of urease as compared to the rest of flavonoids (Figure 5) [102]. Sesame (Sesamum indicum) is used as a starting material and dihydrochalcone, chalcone, flavanone, flavonol, flavone, and flavanol were reacted in a Friedel-Crafts reaction to give rise to a novel flavonolignans series. This one-step process gave twenty different compounds with antioxidant and antidiabetic potential. The α-glucosidase inhibition was investigated using an animal model while the antioxidant activity was determined using DPPH (Figure 6) [103]. The flavonol (−)-(2R,3R)-5,7-dimethoxy-3′,4′-methylenedioxy-flavan-3-ol isolated from cinnamon activates the nuclear factor erythroid 2-related factor 2 (Nrf2) that has been proven to be an efficient chemical against oxidative stress [104]. In most of the studies on antioxidant potential of flavonoids and its derivatives, only few assays were used. In order to detect the true antioxidant potential of the purified compounds and plant extract, several assays should be conducted so that the effects of solvents, other reagents, and temperature are minimized, and conclusive results are obtained.
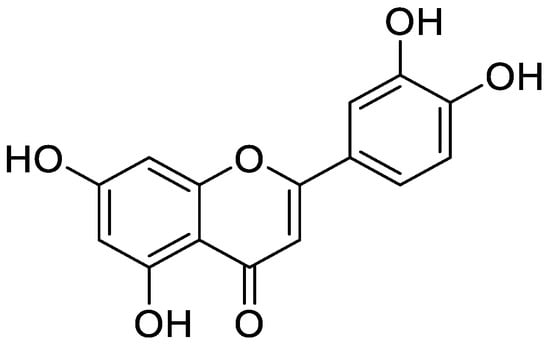
Figure 5.
Chemical structure of luteolin.
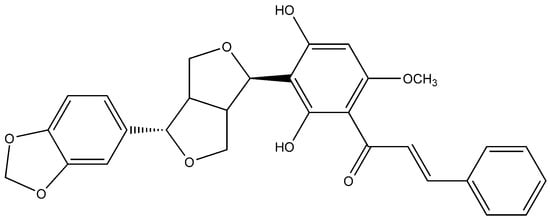
Figure 6.
Lead compound of flavonolignan exhibiting antioxidant and antidiabetic activity.

Table 2.
Flavonoids having antioxidant effects.
Table 2.
Flavonoids having antioxidant effects.
| Plant (Family)—Local Name | Part of Plant | Phytochemical Screening | Total FC | Methods Used Antioxidant Assay | Values of Antioxidant Assay | Bioactivity | Ref. |
|---|---|---|---|---|---|---|---|
| Tamarix aphylla L. (Tamaricaceae)—Athel tamarisk | Leaves | Flavonoid glycosides, carboxylic acid steroids, cardiac glycosides, terpenoids, steroidal compounds, alkaloids, saponins | N/A | DPPH | N/A | Antidiabetic, Hypolipidemic, Antifungal, Antibacterial, Anti-inflammatory, Antioxidant, Wound Healing | [105] |
| Oryza sativa (Poaceae)—Bramo, Serang and Menthi | Caryopsis | Phytosterols, vitamin B group and polyphenols, and polyphenols | N/A | DPPH | (Bramo) 15.25 ± 0.07, (Serang) 25.37 ± 0.07, Menthi (28.15 ± 0.19) | Antioxidant | [106] |
| Diospyros kaki | peel | Vitamins, and flavonoids including catechin, epicatechin, and gallocatechin | N/A | DPPH, FRAP | DPPH (165.75 ± 1.57) FRAP (1609.56 ± 90.88) | Antioxidant | [107] |
| Melastoma malabathricum (Melastomataceae)—karamunting | Leaves and fruits | Terpenoids, phenolic compound, tannin, flavonoids, triterpenes and saponin | N/A | DAPPH | (Leaves) 82% at 50 ppm (Fruit ) 77% at 25 ppm | Antioxidant | [108] |
| Rosa damascena (Rosaceae)—Damask rose | Rose water | Saponins, triterpenoids, tannins, fixed oil flavonoids | Reducing Power Ability (RPA) | 3.612 | Antioxidant, Skin protecting effect | [109] | |
| Bauhinia variegate (Fabaceae)—orchid tree, mountain ebony | Leaves | Anthraquinone, and saponins, erpenoids and alkaloids | 11–222.67 mg QE/g | beta carotene bleaching assay. | 56.79% inhibition of Beta carotene at 200 ug/mL | Antibacterial, Anticancer, Antioxidant | [110] |
| Calotropis procera (Apocynaceae)—dead sea apple | Roots | N/A | 1.62 ± 0.05 mg QE/g | DPPH | 42–90% | Antioxidant, metal ion chelating ability | [111] |
| Tinospora cordifolia (Menispermaceae)—heart-leaved moonseed, giloy | Whole plant | Tinocordioside, cordifolide A, palmatine, quercetin, heptacosanol, and syringin | 18.91 ± 0.21 mg QE/g | DPPH, MC, FRAP, SA, NO | 60–80% | Antibacterial, antifungal, antioxidant, anti-inflammatory activity | [112] |
| Vernonia oligocephala (Asteraceae)—bicoloured-leaved vernonia, groenamarabossie | Roots | flavonoids, saponins, terpenoids, and phenolics | flavonoid 35 (97.35 mg QE/g) contents | DPPH | (% RSC) 90.93 ± 0.66 | Antioxidant and inhibitor of AChE, BChE | [113] |
2.3. Effects on the Cardiovascular System
Dietary flavonoids shows a favorable relationship between their consumption and reduction of cardiovascular diseases [3,114]. Several studies demonstrated that those who consume a large number of flavonoids have 18% lower mortality risk of cardiovascular diseases. Various studies have shown that flavonoids have cardioprotective and neuroprotective [115,116] actions and chemoprotective abilities [117]. Tea is a rich source of flavonoids, and its intake reduces the risk of cardiovascular diseases [118]. Anthocyanidin and proanthocyanidin are flavonoids, that have proven to be effective against cardiac diseases [119]. Isoflavone, anthocyanins [120], and cocoa [121] flavan-3-ols improve vascular health [122]. High consumption of these flavonoids decrease arterial stiffness which reduces the risk of cardiovascular diseases [123]. Oils from leaves and fruits of Hippophae rhamnoides (sea buckthorn) have many compounds including flavonoids that showed positive effects on the cardiovascular system [124]. Morin hydrate showed high biological activity such as anti-inflammatory, anticancer, and protection against cardiovascular diseases [125]. Brazil nuts are rich in flavonoids which help to prevent heart disease and cancer [126]. Chrysin is a flavone and has beneficial effects on epilepsy and depression, also suppresses neuro-inflammation, and has neuro-protective effects [127].
For the purpose of pre-clinical studies on animal models, Primula veris L. solid herbal extract (PVSHE) effect on myocontractile function was studied [128]. The extract composition of phenolic compounds was investigated by purifying the compounds using column chromatography. The differential UV spectrophotometry showed the total flavonoids concentration. The obtained compounds were then characterized by NMR. An experiment on adult Wister rats was performed in which they were divided into control group, intact group, and experimental group. They were fed with herbal extract of Primula veris L. and second experimental group was administered with comparative drug. Cardiodynamic changes were determined using surgical procedures, while myocardial contraction rate was determined computationally. Enzyme linked immune-sorption assay (ELISA) was used for determination of CHF markers. Polymethoxylated flavonoids acted as Primula L. biological markers. The extract was further processed to obtain polymethoxylated flavonoids, flavonoid aglycons and its glycosides along with its constituents. The presence of flavonoids is the most likely reason for decreased ROS production and suppressed peroxide formation, which lead to its cardio-protective effect. A lower number of animal deaths, low CHF markers, and a higher rate of myocardial contraction and relaxation were evident in the results [128]. Several flavonoids have been characterized through NMR spectroscopy. For example, saturation transfer difference (STD)-NMR showed the binding of luteolin and its glycosylated form to the ATP binding domain of multidrug resistant transporter [129]. Thus, these flavonoids have anticancer properties. The antioxidant properties of flavonoids can be accessed through 13C-NMR and QSAR methods [130,131].
Morin, a bioflavonoid, has proven to be a cardio-protective agent via animal modeling. Rats were divided into groups, and morin was orally and dose dependently administered to the experimental group. Myocardial necrosis was induced, and the result suggested the improved antioxidant effects and apoptosis. The mechanism for this cardio protection was reported due to alteration of MAPK/NF-kappa B/TNF-alpha pathway (Table 3) [132].
The high flavonoid content of dark chocolate cocoa has proven to be an effective cardioprotective agent [133] by acting as an effective anti-inflammatory agent, by inhibiting NF-kb, by acting as an antihypertensive agent by increasing the bioactivity of nitric oxide, displaying antiatherogenic activity by decreasing triglyceride concentration, and by decreasing insulin resistance and increasing platelet reactivity [134]. Dihydro-quercetin (DHQ), a dihydroxyflavone used in animal models, proved to be effective against cardiac dysfunction by decreasing the generation of ROS and lipid peroxidation and increasing the biological function of antioxidant enzyme. The PI3K/Akt pathway activation is found to have protective effects [135]. Clinical trials on oxerutin showed that it is quite active in treatment of chronic venous hypertension [136]. Interestingly, there are no detected toxicities or side effects of oxerutin clinical trilas results [136]. Different studies and clinical trials showed that a mixture of flavonoids like diosmin, troxerutin, RutinS hesperidin, quercetin, etc., enhance the veins function, soothe the capillary permeability, and increase the lymphatic and hemorrhoidal drainage [137,138]. Thus, flavonoids are useful in control of piles and hemorrhoid diseases. Diosmin that is the flavone glycoside of diosmetin effectively control various types of blood vessel disease like hemorrhoids, bleeding from gums and eyes, etc. [139]. It enhances blood flow inside the body.

Table 3.
Flavonoids having cardio protective effects.
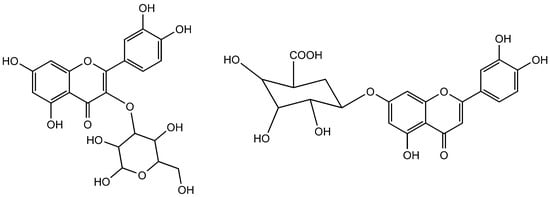
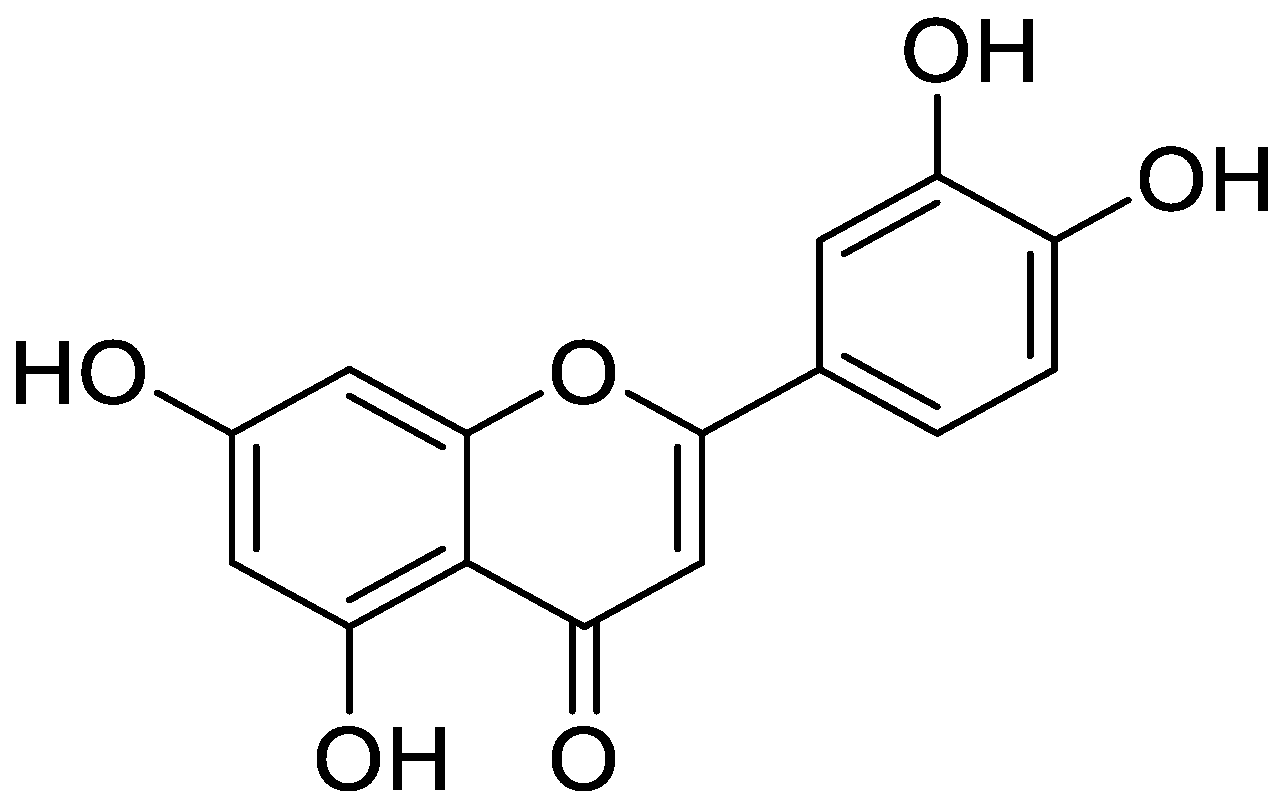
Figure 7.
Quercetin-3-O-glucoside (Left side) and luteolin-7-O-β-d-glucuronide (Right side).
Table 3.
Flavonoids having cardio protective effects.
| Plants Whose FCC Have Cardio Protective Effect | Myocardial Injury | Animal/Cell Line Used for Experiment | In-Vivo/Ex-Vivo | Mechanism | p Value | Ref. |
|---|---|---|---|---|---|---|
| Euphorbia humifusa, Agrimomia pilosa, Juglans regia | Isoproterenol (ISO) | Male Wistar Rats | In-vivo | activation of PI3K/Akt signaling pathway | p < 0.01 | [140] |
| Dracocephalum moldavica L (Figure 7, Right side) | Ischemia Reperfusion-induced | Male Sprague-Dawley rats | In-vivo | TFDM halted myocardial apoptosis as mediated by the PI3K/Akt/GSK-3β and ERK1/2 signaling pathways. | p >0.05 | [141] |
| Rutin | ischemia-reperfusion (MI/R) | Male Sprague-Dawley rats | In-vivo | SIRT1/Nrf2 signaling pathway is a possible therapeutic target for the treatment of oxidative stress and apoptosis related myocardial diseases | p < 0.01 | [142] |
| Dalbergia stipulacea and Hymendictyon excelsum | N/A | Blood | E-vivo | the extracts produced anti-inflammatory effect due to surface area/volume ratio of cells, and this can be obtained through an extension of membrane or the reduction of the cells volume and an interaction with membrane proteins | p < 0.0001 | [143] |
| Ulva lactuca (Figure 7, Left side) | cervical decapitation | Hypercholesterolemic mice | In vitro | TNF-a, IL-1b and IL-6 significantly decreased | p < 0.05 | [144] |
| Clinopodium chinense | Intragastric ISO | Male Sprague-Dawley (SD) | In vivo and In vitro | TFCC safeguard in myocardial injury and increases the cellular antioxidant defense power by stimulating the phosphorylation of AKT, which subsequently triggered the Nrf2/HO-1 signaling pathway | p < 0.05 | [145] |
| Carya cathayensis | Hypoxia/Reoxygenation | H9c2 cell line | In vitro | Halt the cell apoptosis, which is possibly mediated by changes in the expression of miR-21, PTEN/Akt, and Bcl/Bax. | p < 0.01 | [146] |
| Panax notoginseng, safflower, Carthamus tinctorius | isoproterenol (ISO)-induced MI | Sprague-Dawley rats | In vivo | attenuate the NF-κB signaling pathway, depress the expressions of TNF-α, IL-6, IL-1β, and PLA2 | p < 0.05 | [147] |
| Rhododendron simsii | Myocardial Ischemia/Reperfusion | Sprague-Dawley rat | In vivo | Inhibition of UTR and the further blocking of RhoA/ROCK signaling pathway. | p < 0.01 | [148] |
| Potentilla reptans roots | ischemia/reperfusion | Male Wistar rats | In vitro | NO release, Nrf2 pathway, and antioxidant activity resulted into lowering of apoptotic index | N/A | [149] |
| Gymnema sylvestre leaves | doxorubicin induced cardiac damage | Male Wistar rats | In vitro | pathological biochemical markers like creatine kinase-MB (CK-MB), lactate dehydrogenase (LDH), serum glutamic oxaloacetic transaminase (SGOT), total cholesterol, triglycerides, uric acid, calcium, nitric oxide and melanoldehyde, and significantly raises the levels of endogenous protective antioxidant proteins | uric acid (p < 0.05) total cholesterol <0.05), triglycerides (p < 0.05) | [150] |
2.4. Effects on Nervous System
Flavonoids prevent age related neurodegenerative diseases, and in particular, dementia [151,152], Parkinson’s [153,154,155] and Alzheimer’s disease [156,157,158]. The ROS and nitrogen species (NOCs) have roles in many neurodegenerative diseases. Tangeretin, a flavonoid found in citrus fruits, acts as an antioxidant against ROS and NOCs species and provides protection in neurodegeneration disorders such as Parkinson’s disease [159].
Foods which have abundant amounts of flavonoids lower the hazard of neurodegenerative diseases and also counteract age related cognitive disorders [160]. It is beneficial in two ways; first, it regulates neuronal signal cascade caused by cell apoptosis, and second, shows beneficial effects on the peripheral and central nervous system. Hesperidin (Hsd) and hesperetin (Hst) are two flavonoids known for their neuro-pharmacological effects, including neuroprotective, antidepressant, and effects on memory [161]. Berries contain several natural flavonoids, such as polyphenolic compounds like stilbene, anthocyanins etc. These flavonoids are reported to be effective as anti-neurodegenerative agents, anti-mutagenic and antimicrobial agents [162]. Epicatechin, an antioxidant flavonoid abundantly found in wood plants, has an analog 3-O-methyl epicatechin which inhibits neurotoxicity in vitro [163]. The polyphenolic luteolin flavonoid has neuroprotective effects and also as protective effect against age related neuro-disorders [164]. Forsythia suspensa is a dried fruit and a Chinese medicinal herb with activities determined against infectious diseases, showing antioxidant activity as well as acting as a neuroprotective agent [165].
Excessive drinking of alcohol causes various health disorders and negatively affects the brain. The acetylpectolinarin (ACP) flavonoid obtained from the Linaria vulgaris Mill. has been reported to treat hangover by increasing the spontaneous network function of the cultured hippocampal neurons when treated with low concentration of ethanol. It does so by agonistic action on GABAergic synapses mediated by SK potassium channel [166].
Hyperalgesia is an enhanced pain sensation due to peripheral nerve damage, and is associated with diabetic patients. Quercetin and sodium, when used together, can act as antinociceptives, decreasing diabetes complications [167]. Cisplatin induced hyperalgesia can also be reduced by 6-methoxyflavone [168].
2.5. Prevention of Alzheimer’s Disease (AD)
Cyanidin-3-O-glucoside, or kuromanin is a flavonoid, a subgroup of anthocyanins that is found in a variety of vegetables and fruits. The ROS are involved in DNA damage, lipid peroxidation, protein and nucleic acid, which resulted into different diseases including Alzheimer’s disease (AD). This can be controlled using natural products, which may slow down its progression by acting as cholinesterase inhibitor, reducing oxidative stress, and preventing neuron damage. This neurodegenerative disorder is identified by excess of amyloid beta (Aβ) fibrils, which accumulates, in the extracellular spaces of brain and tau protein [169]. The neuro decline is thought to be associated with these two proteins. Anthocyanins contain a pseudo aromatic ring C that increases their structural planarity and promotes amyloid fibril disruption due to effective incorporation of anthocyanins inside the amyloid beta fibril groove. They can also cross the membrane separating the blood from the cerebrospinal fluid and prevent neuron degeneration. The compounds of anthocyanin subcategory can therefore be potentially employed as a therapeutic agent in those diseases that are mediated by oxidative stress. Cyanidin-3-O-glucoside, an anthocyanin, can therefore act as neuroprotective agent [170]. Similarly, another flavonoid rich plant named gangobilobia may be used in the treatment of age-related dementia and Alzheimer’s disease. Butyrylcholinesterase is an enzyme, found in the blood plasma of humans. Two types of neurotoxic inhibitors of cholinesterase are usually used for AD that are active against acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Increasing acetylcholine results in better neuron transport and in turn, better cognitive function [171]. Flavonoids have been extensively used as BChE inhibitors (Table 4). The Hypericum lydium plant show AChE and BChE inhibition with total FC varying from 4.97 ± 4.56 to 156.44 ± 5.51 mg [172]. The cholinesterase inhibition potential could be linked with a higher number of polyphenolics and flavonoids. Procyanidins, a class of flavonoids, improve cognitive function by means of CREB-SIRT1 [173]. The Stachys cretica extracts showed relief of oxidative stress, decrease in Alzheimer’s disease, hyperglycemia, and melisma. A number of phenolic compounds have been identified in the extract of Stachys cretica (Table 4 and Figure 8) [174]. The Actinidia arguta fruits have six flavonols, seven flavanols, seven phenolic acids, and one anthocyanin with the ability to inhibit AChE and BChE [175]. Most of these extracts and purified flavonoids are active against AD in preclinical studies in murine models that provided very favorable results. It is therefore important to explore these flavonoids in clinical trials so that their usefulness can be shared with the AD patients.

Table 4.
Flavonoids with Anti-Alzheimer’s effect.
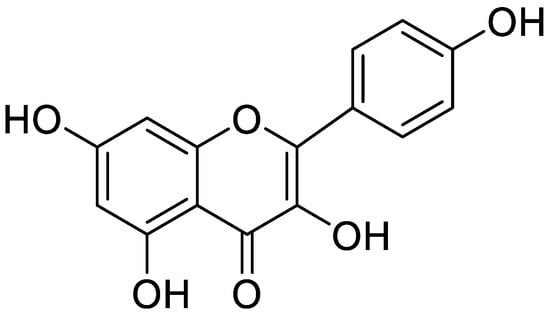
Figure 8.
Kaempferol identified in Stachys cretica.
2.6. Inhibition of Neuropathy
Nerve malfunction is called neuropathy. Peripheral neuropathy is one of the four types of neuropathy. It refers to the conditions that result when nerves that carry messages to and from the brain and spinal cord and to the rest of the body are damaged or diseased. High levels of glucose destroy the blood vessel that goes to the nerve, and thus, affects the nerves of hand and feet which progress with age. Natural compounds containing flavonoid have been used for to relieve neuropathic pain [184]. A mixture of water and alcoholic solvent has been used for the preparation of root extract of Cichorium intybus [185]. Pyridoxine or vitamin B6 is a coenzyme in biological reactions whose high dose intake causes damage to peripheral neurons [185]. This process is known as pyridoxine-induced neuropathy. Cichorium intybus is a medicinal plant that contains a variety of beneficial biochemical constituents present, including flavonoid, saponin, and tannin. The presence of these compounds enables them to be used to suppress oxidative stress, and its possible interference of the two amino acid systems, namely GABAergic and glutamatergic systems in nerve injury and neuropathy [186].
Diabetes is associated with a number of complications that includes nephropathy, neuropathy, and cardiovascular disease [187]. A number of studies demonstrate that diabetes neuropathy (DN) and T2DM may be related to increased risk of Alzheimer’s disease [188]. Diabetic neuropathy is a complication that is most commonly faced by 50% diabetes patients, with development of burning sensation to complete loss of sensation of heat and cold in legs and feet, and the loss of peripheral nerve fibers [189]. Adult Sprague-Dawley rats have been used to evaluate the effect of flavanoglycone, hesperidin on DN pain induced by streptozotocin. The animals were divided largely into two groups of diabetic and non-diabetic. After the end of the experiment, the blood serum of used animals were assayed for cholesterol, insulin, etc., determination, while the sacrificed animals were studied for biochemical and molecular investigation immediately after removing the sciatic nerve. Dose dependent reduction in body weight of treated rats were observed at the end of week 8 with p < 0.05. The plasma glucose level was observed to be elevated as to that of control group. The combination of insulin and hesperidin decreased the neuropathic pain to a significant amount inducing neuroprotective effect [190]. Nobiletin is a non-polar methoxy flavone found in citrus fruits showing diverse biological activity including anticancer, reverse learning impairment by regulating ERK signal [191], improves memory impairment by reducing AChE expression [192]. Nobiletin has been proven by animal models to dose dependently increase nerve conduction velocity in diabetic ulcer group (DU) [193].
A fat rich diet with a combination of streptozotocin induces glucose intolerance in an animal model; this is then followed by beta cell toxins that are engineered to respond to beta cells. The combination results in sciatic nerve injury, oxidative stress and diabetic neuropathy. The proposed model was used to the effect of flavonoid rich fraction of Helicteres isora fruits in diabetic neuropathy in male Sprague-Dawley rats. Thirty rats were divided into two groups, the control and the diabetic neuropathy group. The experiment was performed for three weeks where the diabetic neuropathy group was fed with high fat diet and control group was fed with the pellet diet. After the end of experimentation, the glucose intolerance test confirmed the insulin resistance. This was then followed by dose dependent injection of streptozotocin. Ten days later, the diabetic rats were again grouped into control and FRFHI feed rats. The oral dosage of the extract for further three weeks was continued and then the diabetic neuropathy was assessed by body weight, biochemical parameters and behavioral parameters such as cold, heat, locomotion, and walking test. The FRFHI feed rats restored their body weight, and had reduced blood glucose and cholesterol levels. The FRFHI fed rats also had improved paw and tail withdrawal to heat and cold sensation with improved walking and locomotor activity. The nitric oxide (NO) induced oxidative stress was also noted to be reduced by the extract [194]. Oxaliplatin is a drug that is used in chemotherapy with an adverse side effect of causing painful neuropathy. The compound was introduced into the mice which induced peripheral neuropathy; the flavonoids rutin and quercetin reduced the production of ROS species by acting as an antioxidant agents and reduced the side effect induced by oxaliplatin [195]. The flavonoid quercetin reduced the neuropathic pain by inhibition of p-ERK induced in Sprague-Dawley rats by sciatic nerve injury [196].
2.7. Stroke Prevention
Chalcones are natural precursor compounds of flavonoids and iso-flavonoids. They are present in various plants and vegetables with a wide range of biological activities. Chalcone is an aromatic ketone and an enone with an ability to activate nuclear factor erythroid 2-related factor (2NRF2) pathway. Several novel dihydroxy chalcones were synthesized and evaluated for their ability to suppress ROS species and oxidative stress acting as an anti-ischemic stroke through KEAP1/NRF2/ARE pathway activation. Cerebral ischemia-reperfusion injury (CIRI) in stroke was studied using rat models. These compounds had a potent protection of H2O2-induced oxidative damage in the neuron-like PC12 cells, but also played a neuroprotective role against ischemia/reperfusion-related brain injury in animals [197].
Ischemic stroke most common therapeutic drug is the tissue plasminogen activator, which due to complications can only be administered to 5% of the patients; therefore, the need to have an effective drug for ischemic stroke is necessary. Different systems such as vascular and inflammatory system interact with each other to form a stable homeostasis of CNS and the semipermeable membrane that separates the circulating blood from the brain and extracellular fluid in the CNS known as the blood brain barrier (BBB). The brain recovers itself from injury using CNS and BBB. The endothelium cells of BBB secretes molecules that adjust the after effect of ischemic stroke (stroke vasculome) when its genes are upregulated [198]. Inflammation after stroke is associated with the upregulation of these inflammation genes. Stem cells can release anti-inflammatory agents. Endothelial progenitor cells (EPCs) directly modulate the inflammation-associated stroke vasculome after ischemic stroke. Fisetin, a flavonoid inhibited LPS-induced TNFα production and suppressing nuclear factor jB activation thus acting as a neuroprotective and anti-inflammatory agent after post ischemia injury in vitro [199]. The cortical development, where neurons in brain failed to migrate in the proper formation in utero, results in malfunctioning known as Focal cortical dysplasia (FCD). The naturally occurring flavonoid rutin has been used on animal models to treat FCD. At a dose of 50 mg/kg, a pronounced recovery was observed in motor neurons. This may act as a clinical drug in the future [200].
2.8. Recovery of Injured Nerves and Anti-inflmmatory Properties
Injury to the spinal cord or central nervous system (CNS) causes paralysis of the body from the waist down, and this condition is known as Paraplegia. Experimentation of animal model using rats revealed that using isoflurane increased the number of motor neurons. The delayed preconditioning with a neuroprotective effect was observed, which may be associated with the expression of protein complex NF-κB [201]. The Hypericum perforatum L. is a medicinal plant with flavonoids as an active phytoconstituent. It has a flavonoid content of 6%. It protects the neuron of adrenal phaeochromo cytoma from oxidative stress caused by ROS species such as H2O2. Sciatic nerve injury (SNI) was induced in order to determine the effect of plant extract on oxidative stress, cell signaling molecules, cytokine production, and caspase expression in brain muscle. Wistar albino female rats were used for the study. The result suggested a delay in the progression of SNI caused by the plant extract [202]. Flavonoid rich food derived from natural compounds such as mulberry [203], genistein [204], Acanthus syriacus [205] etc., display protective effect against sciatic nerve injury.
Angiogenesis is the development of new blood vessels from existing vessels which is important for normal development. Uncontrolled angiogenesis causes serious diseases such as inflammatory disorders, obesity, multiple sclerosis, asthma, endometrioses, and cirrhosis. Plant polyphenols such as flavonoids and chalcones inhibit angiogenesis by regulating multiple signaling pathways [206]. Viscosine is a flavonoid isolated from Dodonea viscosa, which has anti-inflammatory, antipyretic and antioxidant properties [207]. Baicalin is a flavonoid present in medicinal plants named Scutellaria baicalensis Georgi and Oroxylum indicum (Table 5). This flavonoid exhibits antioxidant and anti-inflammatory activity and is used to treat certain diseases such as asthma, liver and kidney diseases, inflammatory bowel diseases, carcinogenesis, and cardiovascular diseases [208,209]. Kaempferol is a flavonoid that possesses anti-inflammatory effects [210]. Rutin is a common dietary flavonoid, having various pharmacological properties such as anti-inflammatory, antimicrobial, and anticancer properties [211]. Chrysin, a type of flavonoid, also has anti-inflammatory and antioxidant effect [212]. It has been observed that fruits which are rich in cyanidin and peonidin have higher anti-inflammatory effects [213]. Barringtonia racemosa (L.) leaves and branches water soluble extracts have been used to isolate three acylated flavonoid glycosides which showed moderate anti-inflammatory activity by inhibiting LPS-induced NO production in RAW-2647 cells [214]. The Eucalyptus globulus and Arum palaestinum herbal extract inhibits interleukin 1 alpha with high phenolic and flavonoid content with potential anti-inflammatory and anti-acne agent for Acne vulgaris (Table 5) [215]. Several clinical trials showed that flavonoids have anti-inflammatory properties and block several enzymes involved in inflammation pathways.

Table 5.
Anti-inflammatory activity of Flavonoid’s.
2.9. Antimalarial Properties
Malaria is caused by the parasitic species of Plasmodium. Plasmodium falciparum and other species are now increasingly resistant to the common antimalarial drugs like chloroquine [226], and this resistance is spreading toward artemisinin and its analogues [227,228]. New drugs are required to treat drug resistant strains of Plasmodium [227,228]. In the continuing effort of synthesizing antimalarial drug it is reported that plant extracts that are rich in compounds, such as flavonoids, chalcones, terpenes, quinones, and xanthones, are antimalarial in nature [229]. Prosopis is plant genus which has been used for medicinal purposes since ancient times. Its components include flavonoids, tannins, alkaloids etc. These bioactive compounds are antimalarial, antiulcer and antibiotic in nature [230]. Species of the genus Psiadia extract contain flavonoids, coumarins, phenylpropanoids, and terpenoids. It shows pharmacological activities such as antimicrobial, antimalarial, antiviral, and anti-inflammatory etc [231]. Extracts of some plants such as Psiadia dentata and Psiadia arguta inhibit the growth of Plasmodium falciparum [231]. Waltheria indica (syn. Waltheria americana) extract have flavonoids, (−) epicatechin, kaempferol, quercetin, sterols etc. It is used for the treatment of malaria and other infectious diseases (e.g diarrhea due to Escherichia coli, lungs infection due to Klebsiella pneumoniae), inflammation, and prevention of oxidative stress [232]. Prenylated flavonoids obtained from the bark of Artocarpus styracifolius show antiplasmodial and antitrypanosomal effects [233]. Silymarin from Silybum marianum has been isolated and purified. Anti-plasmodial activity of silymarin, a polyphenolic flavonoid, has been reported and it forms the silymarin-heme complex, and inhibits the conversion of toxic free heme into crystalline non-toxic heamozoid [234]. Indigofera oblongifolia leaf extract has significant effects against malaria and protects the liver from injury caused by P. chabaudi via antioxidant and anti-inflammatory ways [235].
2.10. Antiviral Activities
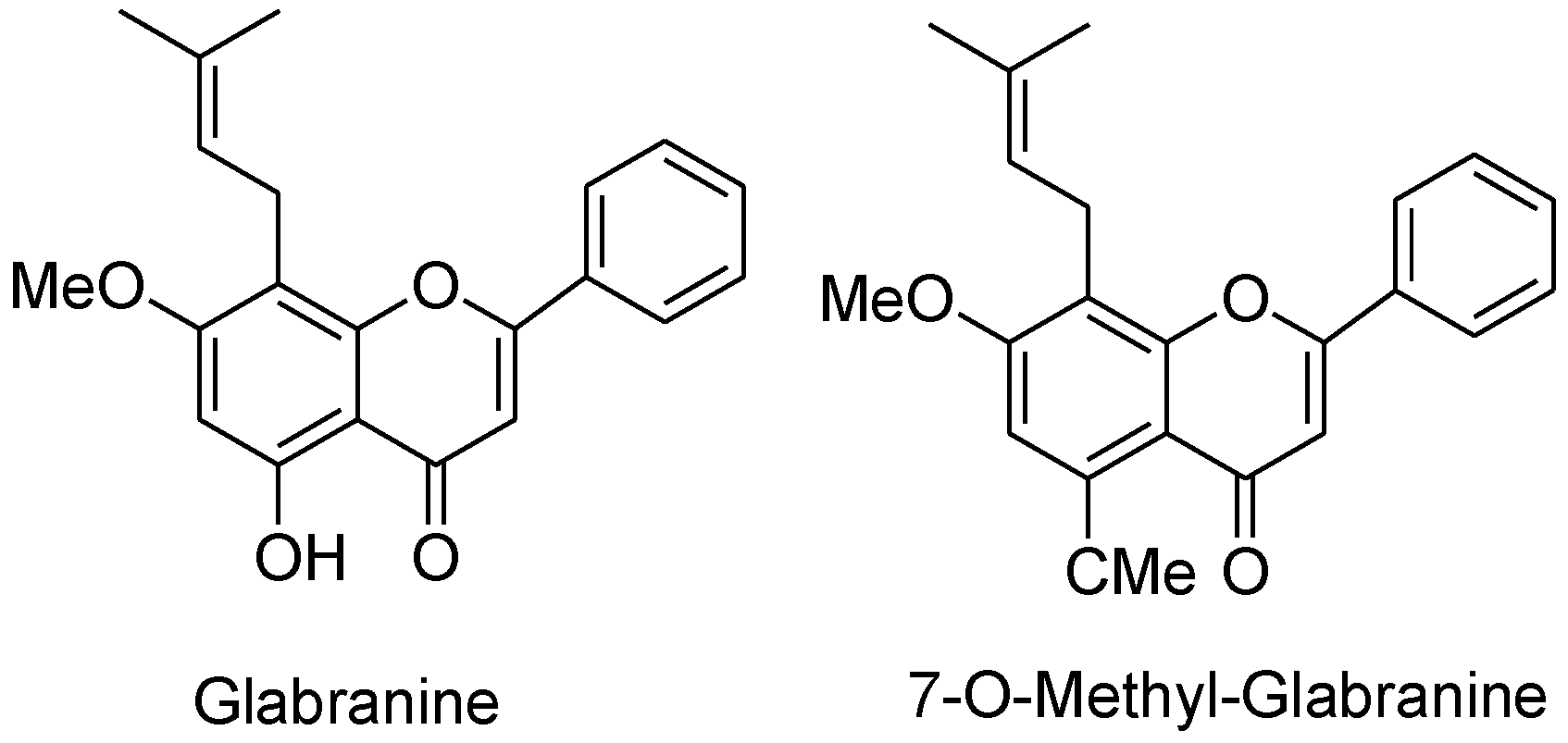
Patients with weak immunity when infected with a contagious viral infection can be deadly and the recent novel coronavirus pandemic showed that immunocompromised people are especially vulnerable to the COVID-19 [236]. Serious efforts have been made to synthesize antiviral agents with efficient activity. Natural bioactive flavonoids present in medicinal plants and herbs have been widely reported to have antiviral activity and are concentrated and modified for its better action [237]. Houttuynia cordata Thunb is a plant found in Eastern Asia, having promising antiviral activities against enveloped viruses such as influenza virus, herpes simplex virus-1 and human immunodeficiency virus-1 in vitro [238]. Both natural and synthetic flavonoids are potential medicines for many diseases, including HIV [239]. Glabranine and 7-O-methyl-glabranine are the two flavonoids extracted from Mexican plant Tephrosia madrensis. The plaque assay confirmed that these isolates exhibit antiviral activities, and inhibit dengue virus replication. The structures of the isolates were determined spectroscopically and then the stoichiometry was analyzed alongside cytotoxicity using [3H]-thymidine assay. At a concentration of 25 μM, glabranine inhibited the dengue virus by 76.9% while 7-O-methyl-glabranine inhibited the replication of virus at the same concentration by 75%. Both of these compounds have a prenyl side-chain in C-8 which is considered to have a role in inhibition of virus (Figure 9) [240], and catechins against the influenza virus [241]. Anthocyanins found in berry fruits have antiviral activity against influenza virus [242]. Baicalein, quercetin, and fisetin are active inhibitors of chikungunya virus [243]. The epicatechin 3-gallate, fisetin, quercetin reduces murine norovirus and daidzein, and kaempferol reduce feline calicivirus [244]. Four flavonoids (5-hydroxy-7,8-dimethoxyflavone, 5-hydroxy-6,7-dimethoxyflavone, acacetin, apigenin) extracted from Mosla scabra display antiviral activity against influenza viruses [245]. Burs (involucre) of Castanea crenata, have a wide range of biological activities attributed to the bioactive phtyo-constituents present in it that includes flavonoid, tannins, phenolic acids, coumarins, phenylpropanoids, and steroids. The antiviral activity of the extract was determined by SRB method using cytopathic effect (CPE) reduction assay in HeLa or Vero cells. The isolated flavonoid, kaempferol, showed enhanced antiviral properties against HRV1B, CVB3, and PR8 [246]. Using molecular docking and simulation studies, flavonoid analogues are also found to be active against H1N1 virus neuraminidase [247]. Of the various types of influenza virus, IAV is a single stranded RNA virus that is dangerous and most responsible for an annual deaths of around 500,000 globally. The antigenic shift give rise to new a subtype of viruses, this occurs when two or more strains of same virus or of different viruses combine or mutate to form a new subtype that is resistance to available, common antiviral agents. Purified guava flavonoid glycosides inhibit IAV replication by early regulation of interleukin-1 beta and interleukin-8 via protein gene expression [248].
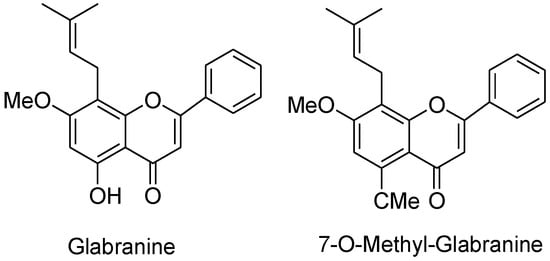
Figure 9.
Chemical structures of glabranine and 7-O-methyl-glabranine.
Scutellaria baicalensis of Lamiaceae family has a beneficial health impacts as its roots contain flavonoid as bioactive component, and has been used for centuries as medicinal herb. Using an animal model, influenza virus was introduced through the nose, and after two hours, the root extract was administered. The histopathology indicated the reduction of oxidative stress by inhibiting NO production as well as significant inhibition of IAV-infected mice with an increased survival rate [249].
2.11. Antibacterial Action
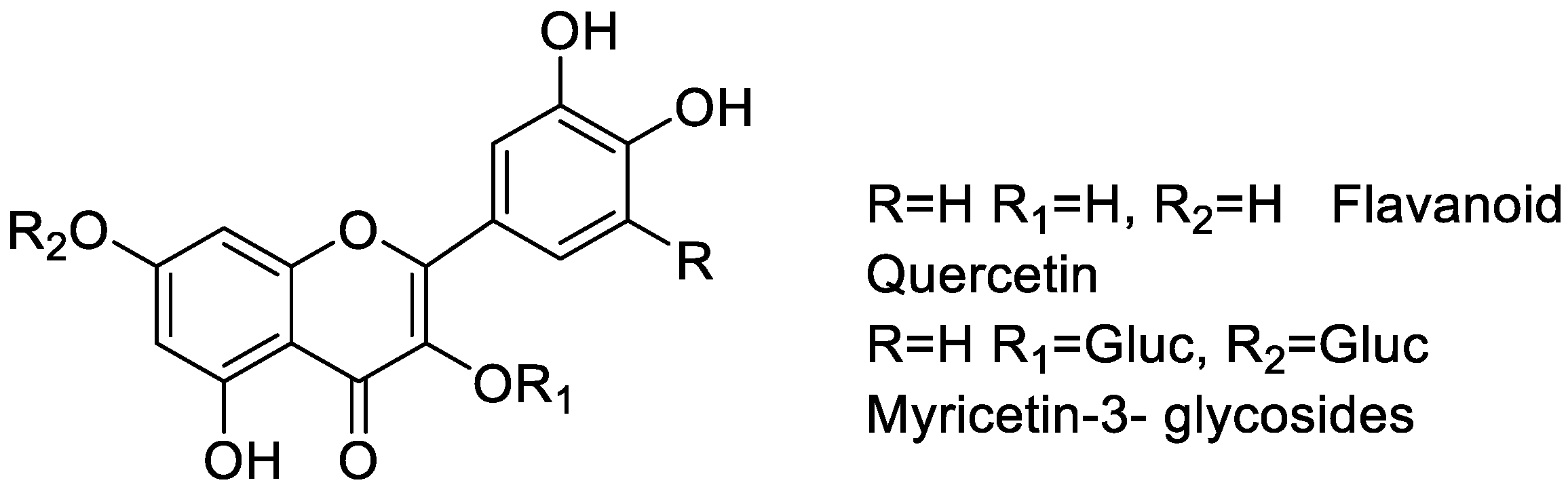
The increase and spread of multi-drug resistance in pathogenic bacteria resulted into a number of antibiotics that have become ineffective for the treatment of a number of bacterial infections [250,251]. Flavonoids have the ability to enhance the protective immune systems of humans [78]. Several flavonoids act both as bacteriostatic and bactericidal agents by damaging the cytoplasmic membrane, and inhibiting energy metabolism and nucleic acid synthesis of microorganisms [252]. Bridelia is a plant of genus of Phyllanthaceae family, which is used as a pain-relieving medicine in Asia and South Africa. Its extract contains flavonoids like quercetin, gallocatechin-(4′-O-7)-epigallocatechin, myricetin-3-glycosides, and isoflavone. These flavonoids are responsible for its antimalarial, antibacterial, anti-inflammatory activities (Figure 10) [253]. Anthocyanidin plays an important role against tuberculosis and drug-resistant Mycobacterium tuberculosis strains [254]. Studies have shown that hesperidin and hesperetin, two flavonoids, show very good antimicrobial activity [255]. Flavonoids from Cuscuta are reported for many biological activities, including antiproliferative, hepatoprotective, anxiolytic and antimicrobial activities [256]. Turbina corymbose is a natural source of antimicrobial and tyrosinase inhibitor [257,258]. There has been extensive literature available on flavonoid rich plants showing antibacterial activities, such as flowers from Acacia saligna (Labill.), roots and aerial extract of Lamium album [259,260], Tridax procumbens, [261], Tunisian Date Palm seeds [262], Aurone derivatives [263], Chrysoeriol [264], Alanchoe mortagei, K. fedtschenkoi [265], Onion (Allium cepa L.), Asplenium nidus (fern) [266], Trianthema decandra [267], pineapple (Ananas comosus) [268], Pseudarthria hookeri (Fabaceae) [269], Keigairengyoto [270], Quercus brantii L. [271], Acacia saligna (Labill.) [260], or the use of flavonoid rich marine algae such as marine algae Sargassum swartzii [272], all of which show significant antimicrobial activities.
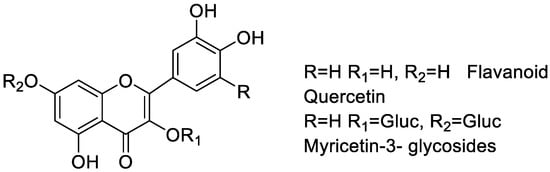
Figure 10.
Flavonoids of Bridelia extract.
2.12. Antidiabetic Effects
It is found that cranberry flavonoids decrease the blood glucose levels and increase insulin sensitivity in animals [273]. Anthocyanins control obesity and consequently can also help in the prevention of type 2 diabetes [274]. Chrysin is natural flavone having many health benefits such as antidiabetic, antiallergic, anticancer, and antitumor effects [275]. Many flavonoids are anti-diabetic by increasing secretion of insulin, improvement of hyperglycemia, reduce resistance to insulin and increase uptake of glucose by skeletal muscles in murine model [276]. Flavonol rich chocolate enhances insulin sensitivity and reduce insulin resistance in healthy subjects [277]. Allium cepa L. (onion) flesh and skin [278] are rich in quercetin derivatives [279] and display protein tyrosine phosphatase 1B (PTP1B) inhibitory activity, decreases its expression, and increases glucose uptake making it a potential antioxidant and antidiabetic agent [280].
Flavonoids have many important functions in gastrointestinal tract i.e glucose homeostasis and lipid metabolism [281]. Anthocyanins obtained from black beans have antidiabetic potential [282]. Melicope lunuankenda leaves extract have O-prenylated flavonoids which shows antidiabetic activity against type 2 diabetes [283]. Flavonoids in black carrots (Daucus carota) are therapeutic agents against obesity and diabetes mellitus [284]. Naringenin and its glycosides found in citrus fruits has antioxidant and anti-diabetic activity [285]. The genus Sterculia have many natural products compounds including flavonoids and polyphenols which have antidiabetic and antimicrobial activities [286].
Kaempferol, a natural flavonoid, acts as antidiabetic agent by inhibiting cell proliferation, decreases PI3K, P63, SREBP-1 expression and phosphorylates insulin resistance substrates [287].
Warionia saharae is a flavonoid rich plant. Its extract was used on animal model to prove its antidiabetic properties. Treptozotocin (STZ) induced diabetic rats were used as test subject and normal rats were used as control group. Oral administration of the extract was continued for 15 days and its histopathological examination of liver along with glucose tolerance test was performed. The state of the liver and pancreas was found to be improved with the test group rats and had the potential to act as an antioxidant as well as an efficient antidiabetic agent [288]. In order to overcome the absorption and bioavailability issues of flavonoids, novel strategies should be developed for their maximum absorption. In one such study, baicalin, which is hydrophilic and poorly absorbed glycosylated flavonoid, was loaded inside nano-structured lipid carriers [289]. These baicalin containing a lipid carrier were tested in diabetic rats and its was observed that they have significant antidiabetic efficacy by halting the lipid peroxidation [289].
Although there are several studies in murine models that showed that, the hesperidin controls the blood glucose level, clinical trials in human showed no such observation [290]. Thus majority of clinical studies hints that hesperidin did not modulate insulin or enzymes of the glucose metabolic pathway. There are some limitation of these clinical trials related to absorption of hesperidin [290].
Methylglyoxal is the metabolite whose concentration increases in the blood during diabetes and causes atherogenesis (a condition where artheromatous plaque develop in arteries, with blockage problems) and it also attaches with nerve ending resulting in nerve damage [91]. Clinical trials on quercetin indicated a decrease in the plasma level of methylglyoxal while epicatechin has no promising effect. This showed the different potency of various flavonoids against specific disease.
2.13. Antifungal Properties
New antifungal agents are required as the currently available antifungal drugs are not effective completely due the development of resistance and undesirable side effects [291]. Leaves of Aquilaria have many bioactive compounds including flavonoids responsible for its antiviral, antifungal, and antitumor activities [292]. Artemisia sacrorum extract have two flavonoids namely sacriflavone A and sacriflavone B and both of them have antifungal potency [293]. Moreover, 2′,4′-dihydroxy-5′-(1‴,1‴-dimethylallyl)-8-prenylpinocembrin (8PP) is a natural prenyl flavonoid isolated from Dalea elegans, which shows antifungal effects against Candida albicans biofilms (Figure 11) [294].
Figure 11.
Chemical structure of 2′,4′-dihydroxy-5′-(1‴,1‴-dimethylallyl)-8-prenyl pinocembrin (8PP).
3. Conclusions
Flavonoids are groups of various compounds found naturally in many plants, such as fruits and vegetables, along with plant products such as coffee, chocolate, and tea. It had been repeatedly reported that flavonoids possess a wide range of health benefits. For example, flavonoids are rich in antioxidants, providing our body with natural immune protections from daily environmental and endogenous toxins. Different classes of flavonoids are so far isolated with several significant biological activities such as anticancer, antibacterial, antifungal, anti-diabetic, antimalarial, neuroprotective, cardio-protective, anti-inflammatory. Thus, including different types of flavonoids in daily diet is highly recommended to stay healthy and to reduce the risk of serval life threatening diseases such as diabetes mellitus, cancer as well as lowering the risk of having stroke and heart attack. The therapeutic effects of flavonoids have been proved in majority of pre-clinical studies in murine models. Different approaches should be used in clinical trials so that the absorption and bioavailability of flavonoids are not compromised. Their production should be increased through expression of their biosynthetic pathway enzymes in other plants and species with rapid growth. The funding agencies should facilitate research on flavonoids due to their versatile role in health and wellness. Further, the conjugates of flavonoids with other important drugs may enhance the potency of those compounds. Decisively, more research work is needed to resolve more the structures opf more flavonoids and to investigate their therapeutic applications. Flavonoids structures will always inspire research for the design and synthesis of new effective drugs for different types of diseases.
Funding
Mariusz Jaremko; Benjamin Gabriel Poulson and Abdul-Hamid Emwas would like to thanks King Abdullah University of Science and Technology for the financial support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cavalcante, G.M.; da Silva Cabral, A.E.; Silva, C.C. Leishmanicidal Activity of Flavonoids Natural and Synthetic: A Minireview. Mintage J. Pharm. Med. Sci. 2018, 7, 25–34. [Google Scholar]
- Shan, X.; Cheng, J.; Chen, K.l.; Liu, Y.M.; Juan, L. Comparison of Lipoxygenase, Cyclooxygenase, Xanthine Oxidase Inhibitory Effects and Cytotoxic Activities of Selected Flavonoids. DEStech Trans. Environ. Energy Earth Sci. 2017. [Google Scholar] [CrossRef]
- Feliciano, R.P.; Pritzel, S.; Heiss, C.; Rodriguez-Mateos, A. Flavonoid intake and cardiovascular disease risk. Curr. Opin. Food Sci. 2015, 2, 92–99. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Aleksandra Kozłowska, D.S.-W. Flavonoids-food sources and health benefits. Rocz. Panstw. Zakl. Hig. 2014, 65, 65. [Google Scholar]
- Shkondrov, A.; Krasteva, I.; Pavlova, D.; Zdraveva, P. Determination of flavonoids in related Astragalus species (Sect. Incani) occurring in Bulgaria. Comptes rendus de l’Académie Bulg. des Sci. 2017, 70, 363–366. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Villela, A.; van Vuuren, M.S.; Willemen, H.M.; Derksen, G.C.; van Beek, T.A. Photo-stability of a flavonoid dye in presence of aluminium ions. Dyes Pigment. 2019, 162, 222–231. [Google Scholar] [CrossRef]
- Paramita, V.; Kusumayanti, H.; Amalia, R.; Leviana, W.; Nisa, Q.A. Application of Flavonoid and Anthocyanin Contents from Rambutan (Nephelium lappaceum) Peel as Natural Dyes on Cotton Fabric. Adv. Sci. Lett. 2018, 24, 9853–9855. [Google Scholar] [CrossRef]
- Lanzendörfer, G.; Stäb, F.; Untiedt, S. Cosmetic and Dermatological Preparations with Flavonoids. WO/1996/018379, 20 June 1996. [Google Scholar]
- Danihelová, M.; Viskupičová, J.; Šturdík, E. Lipophilization of flavonoids for their food, therapeutic and cosmetic applications. Acta Chim. Slovaca 2012, 5, 59–69. [Google Scholar] [CrossRef]
- Chuarienthong, P.; Lourith, N.; Leelapornpisid, P. Clinical efficacy comparison of anti-wrinkle cosmetics containing herbal flavonoids. Int. J. Cosmet. Sci. 2010, 32, 99–106. [Google Scholar] [CrossRef]
- Zhao, L.; Yuan, X.; Wang, J.; Feng, Y.; Ji, F.; Li, Z.; Bian, J. A review on flavones targeting serine/threonine protein kinases for potential anticancer drugs. Bioorganic Med. Chem. 2019, 27, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Yuan, Y.; Lin, B.; Miao, Z.; Li, Z.; Guo, Q.; Lu, N. LW-215, a newly synthesized flavonoid, exhibits potent anti-angiogenic activity in vitro and in vivo. Gene 2018, 642, 533–541. [Google Scholar] [CrossRef]
- Camero, C.M.; Germanò, M.P.; Rapisarda, A.; D’Angelo, V.; Amira, S.; Benchikh, F.; Braca, A.; De Leo, M. Anti-angiogenic activity of iridoids from Galium tunetanum. Rev. Bras. de Farmacogn. 2018, 28, 374–377. [Google Scholar] [CrossRef]
- Patel, K.; Kumar, V.; Rahman, M.; Verma, A.; Patel, D.K. New insights into the medicinal importance, physiological functions and bioanalytical aspects of an important bioactive compound of foods ‘Hyperin’: Health benefits of the past, the present, the future. Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 31–42. [Google Scholar] [CrossRef]
- Balasuriya, N.; Rupasinghe, H.V. Antihypertensive properties of flavonoid-rich apple peel extract. Food Chem. 2012, 135, 2320–2325. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, J.; Chen, Z.; Ma, Q.; Guo, Q.; Gao, X.; Chen, H. Antioxidant, antihypertensive, and anticancer activities of the flavonoid fractions from green, oolong, and black tea infusion waste. J. Food Biochem. 2018, 42, e12690. [Google Scholar] [CrossRef]
- Khan, S.; Khan, T.; Shah, A.J. Total phenolic and flavonoid contents and antihypertensive effect of the crude extract and fractions of Calamintha vulgaris. Phytomedicine 2018, 47, 174–183. [Google Scholar] [CrossRef]
- Lagunas-Herrera, H.; Tortoriello, J.; Herrera-Ruiz, M.; Martínez-Henández, G.B.; Zamilpa, A.; Santamaría, L.A.; Lorenzana, M.G.; Lombardo-Earl, G.; Jiménez-Ferrer, E. Acute and Chronic Antihypertensive Effect of Fractions, Tiliroside and Scopoletin from Malva parviflora. Biol. Pharm. Bull. 2019, 42, 18–25. [Google Scholar] [CrossRef]
- Mazidi, M.; Katsiki, N.; Banach, M. A higher flavonoid intake is associated with less likelihood of nonalcoholic fatty liver disease: Results from a multiethnic study. J. Nutr. Biochem. 2019, 65, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, L.M.; Geraldi, M.V.; Cazarin, C.B.B.; Junior, M.R.M. Functional Food Consumption and Its Physiological Effects. In Bioactive Compounds; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 205–225. [Google Scholar]
- Panche, A.; Diwan, A.; Chandra, S. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Lewis, J.R.; Blekkenhorst, L.C.; Bondonno, C.P.; Shin, J.H.; Croft, K.D.; Woodman, R.J.; Wong, G.; Lim, W.H.; Gopinath, B. Association of flavonoids and flavonoid-rich foods with all-cause mortality: The Blue Mountains Eye Study. Clin. Nutr. 2019, 39, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.K.; Zill, E.H.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Khalifa, I.; Zhu, W.; Li, K.-k.; Li, C.-m. Polyphenols of mulberry fruits as multifaceted compounds: Compositions, metabolism, health benefits, and stability—A structural review. J. Funct. Foods 2018, 40, 28–43. [Google Scholar] [CrossRef]
- Wagner, C.E.; Jurutka, P.W.; Marshall, P.A.; Groy, T.L.; Van Der Vaart, A.; Ziller, J.W.; Furmick, J.K.; Graeber, M.E.; Matro, E.; Miguel, B.V. Modeling, synthesis and biological evaluation of potential retinoid X receptor (RXR) selective agonists: Novel analogues of 4-[1-(3,5,5,8,8-pentamethyl-5,6,7,8-tetrahydro-2-naphthyl) ethynyl] benzoic acid (bexarotene). J. Med. Chem. 2009, 52, 5950–5966. [Google Scholar] [CrossRef]
- Wang, T.Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2017. [Google Scholar] [CrossRef]
- Patil, V.M.; Masand, N. Anticancer Potential of Flavonoids: Chemistry, Biological Activities, and Future Perspectives. In Studies in Natural Products Chemistry, 1st ed.; Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 59, pp. 401–430. ISBN 1572-5995. [Google Scholar]
- Silalahi, J. Anticancer and health protective properties of citrus fruit components. Asia Pacific J. Clin. Nutr. 2002, 11, 79–84. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Nabavi, S.F.; Setzer, W.N.; Ahmadi, A.; Mansouri, K.; Nabavi, S.M. Hesperidin: A promising anticancer agent from nature. Ind. Crops Prod. 2015, 76, 582–589. [Google Scholar] [CrossRef]
- Ersoz, M.; Erdemir, A.; Duranoglu, D.; Uzunoglu, D.; Arasoglu, T.; Derman, S.; Mansuroglu, B. Comparative evaluation of hesperetin loaded nanoparticles for anticancer activity against C6 glioma cancer cells. Artificial Cells Nanomed. Biotechnol. 2019, 47, 319–329. [Google Scholar] [CrossRef]
- Alsayari, A.; Muhsinah, A.B.; Hassan, M.Z.; Ahsan, M.J.; Alshehri, J.A.; Begum, N. Aurone: A biologically attractive scaffold as anticancer agent. European J. Med. Chem. 2019, 166, 417–431. [Google Scholar] [CrossRef]
- Darband, S.G.; Kaviani, M.; Yousefi, B.; Sadighparvar, S.; Pakdel, F.G.; Attari, J.A.; Mohebbi, I.; Naderi, S.; Majidinia, M. Quercetin: A functional dietary flavonoid with potential chemo-preventive properties in colorectal cancer. J. Cell. Physiol. 2018, 233, 6544–6560. [Google Scholar] [CrossRef]
- Yang, P.-W.; Lu, Z.-Y.; Pan, Q.; Chen, T.-T.; Feng, X.-J.; Wang, S.-M.; Pan, Y.-C.; Zhu, M.-H.; Zhang, S.-H. MicroRNA-6809–5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine 2019, 57, 18–29. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Devi, K.P.; Rajavel, T.; Habtemariam, S.; Nabavi, S.F.; Nabavi, S.M. Molecular mechanisms underlying anticancer effects of myricetin. Life Sci. 2015, 142, 19–25. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Elhaty, I.A.; Elhaw, M.; Murali, C.; Al Mansoori, A.; Awad, B.; Amin, A. Antioxidant and anticancer activities of chamomile (Matricaria recutita L.). BMC Res. Notes 2019, 12, 3. [Google Scholar] [CrossRef]
- Shahat, A.A.; Hidayathulla, S.; Khan, A.A.; Alanazi, A.M.; Al Meanazel, O.T.; Alqahtani, A.S.; Alsaid, M.S.; Hussein, A.A. Phytochemical profiling, Antioxidant and Anticancer activities of Gastrocotyle hispida growing in Saudi Arabia. Acta Trop. 2019, 191, 243–247. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Guo, S.; Yang, X.-Y.; Zhang, Y.-F.; Shang, M.-Y.; Shang, Y.-H.; Xiao, J.-J.; Cai, S.-Q. Flavonoids isolated from Sinopodophylli Fructus and their bioactivities against human breast cancer cells. Chin. J. Nat. Med. 2017, 15, 225–233. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. 2011, 2, 408–420. [Google Scholar] [CrossRef]
- Tu, S.-H.; Chen, L.-C.; Ho, Y.-S. An apple a day to prevent cancer formation: Reducing cancer risk with flavonoids. J. Food Drug Anal. 2017, 25, 119–124. [Google Scholar] [CrossRef]
- Karabin, M.; Hudcova, T.; Jelinek, L.; Dostalek, P. Biotransformations and biological activities of hop flavonoids. Biotechnol. Adv. 2015, 33, 1063–1090. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.S.; Singh, M.K.; Singh, P.K.; Kumar, V. Traditional knowledge to clinical trials: A review on therapeutic actions of Emblica officinalis. Biomed. Pharmacother. 2017, 93, 1292–1302. [Google Scholar] [CrossRef]
- Huntley, A.L. The health benefits of berry flavonoids for menopausal women: Cardiovascular disease, cancer and cognition. Maturitas 2009, 63, 297–301. [Google Scholar] [CrossRef]
- Walle, T. Methoxylated flavones, a superior cancer chemopreventive flavonoid subclass? Semin. Cancer Biology 2007, 17, 354–362. [Google Scholar] [CrossRef]
- Andujar, I.; Recio, M.C.; Giner, R.M.; Rios, J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell Longev. 2012, 2012, 906252. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yang, B.; Wang, J.; Liu, Y.; Yu, L.; Jiang, Y. Immunomodulatory and anticancer activities of flavonoids extracted from litchi (Litchi chinensis Sonn) pericarp. Int. Immunopharmacol. 2007, 7, 162–166. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Yang, W.; Bosland, M.C. Soy isoflavones and prostate cancer: A review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014, 140, 116–132. [Google Scholar] [CrossRef]
- McKay, D.L.; Blumberg, J.B. The Role of Tea in Human Health: An Update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Amjadi, M.; Khoshraj, J.M.; Majidi, M.R.; Baradaran, B.; de la Guardia, M. Evaluation of Flavonoid Derivative and Doxorubicin Effects in Lung Cancer Cells (A549) Using Differential Pulse Voltammetry Method. Adv. Pharm. Bull. 2018, 8, 637. [Google Scholar] [CrossRef]
- Aleksandar, P.; Dragana, M.-Ć.; Nebojša, J.; Biljana, N.; Nataša, S.; Branka, V.; Jelena, K.-V. Wild edible onions—Allium flavum and Allium carinatum—successfully prevent adverse effects of chemotherapeutic drug doxorubicin. Biomed. Pharmacother. 2019, 109, 2482–2491. [Google Scholar] [CrossRef]
- de Novais, L.M.; de Arueira, C.C.; Ferreira, L.F.; Ribeiro, T.A.; Sousa Jr, P.T.; Jacinto, M.J.; de Carvalho, M.G.; Judice, W.A.; Jesus, L.O.; de Souza, A.A. 4′-Hydroxy-6, 7-methylenedioxy-3-methoxyflavone: A novel flavonoid from Dulacia egleri with potential inhibitory activity against cathepsins B and L. Fitoterapia 2019, 132, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wang, L.-q.; Liu, Y. Antitumor Activities of Widely-used Chinese Herb—Licorice. Chin. Herbal Med. 2014, 6, 274–281. [Google Scholar] [CrossRef]
- Gong, W.-Y.; Zhao, Z.-X.; Liu, B.-J.; Lu, L.-W.; Dong, J.-C. Exploring the chemopreventive properties and perspectives of baicalin and its aglycone baicalein in solid tumors. Eur. J. Med. Chem. 2017, 126, 844–852. [Google Scholar] [CrossRef]
- Li, S.; Cheng, X.; Wang, C. A review on traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the genus Peganum. J. Ethnopharmacol. 2017, 203, 127–162. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Ribeiro, T.A.N.; Araújo, M.F.; de Carvalho, M.G. Ouratea genus: Chemical and pharmacological aspects. Rev. Bras. Farmacogn. 2014, 24, 1–19. [Google Scholar] [CrossRef]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- McGown, A.; Ragazzon-Smith, A.; Hadfield, J.A.; Potgetier, H.; Ragazzon, P.A. Microwave-Assisted Synthesis of Novel Bis-Flavone Dimers as New Anticancer Agents. Lett. Org. Chem. 2019, 16, 66–75. [Google Scholar] [CrossRef]
- Bailly, C. Molecular and cellular basis of the anticancer activity of the prenylated flavonoid icaritin in hepatocellular carcinoma. Chem. Interact. 2020, 325, 109124. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, P.; Li, X.; Zhu, W.; Sun, X.; Sun, X.; Chen, X.; Xing, L.; Yu, J. A prospective phase II trial of EGCG in treatment of acute radiation-induced esophagitis for stage III lung cancer. Radiother. Oncol. 2015, 114, 351–356. [Google Scholar] [CrossRef]
- Zwicker, J. Targeting protein disulfide isomerase with the flavonoid isoquercetin to improve hypercoagulability in advanced cancer. JCI Insight 2019, 4, 4. [Google Scholar] [CrossRef]
- Garcia-Maceira, P.; Mateo, J. Silibinin inhibits hypoxia-inducible factor-1α and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: Implications for anticancer therapy. Oncogene 2009, 28, 313. [Google Scholar] [CrossRef]
- Agarwal, C.; Wadhwa, R.; Deep, G.; Biedermann, D.; Gažák, R.; Křen, V.; Agarwal, R. Anti-cancer efficacy of silybin derivatives-a structure-activity relationship. PLoS ONE 2013, 8, e60074. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, Y.; Zhang, D.; Liu, Z.; Duan, C.; Jia, L.; Wang, F.; Liu, Y.; Liu, G.; Hao, L. In vitro antitumor activity of silybin nanosuspension in PC-3 cells. Cancer Lett. 2011, 307, 158–164. [Google Scholar] [CrossRef]
- Lin, C.-J.; Sukarieh, R.; Pelletier, J. Silibinin inhibits translation initiation: Implications for anticancer therapy. Mol. Cancer Ther. 2009, 8, 1535–7163. [Google Scholar] [CrossRef]
- Júnior, R.G.O.; Ferraz, C.A.A.; Pereira, E.C.V.; Sampaio, P.A.; Silva, M.F.S.; Pessoa, C.O.; Rolim, L.A.; da Silva Almeida, J.R.G. Phytochemical analysis and cytotoxic activity of Cnidoscolus quercifolius Pohl (Euphorbiaceae) against prostate (PC3 and PC3-M) and breast (MCF-7) cancer cells. Pharmacogn. Mag. 2019, 15, 24. [Google Scholar]
- Durgawale, P.P.; Patil, M.N.; Joshi, S.A.; Korabu, K.S.; Datkhile, K.D. Studies on phytoconstituents, in vitro antioxidant, antibacterial, antiparasitic, antimicrobial, and anticancer potential of medicinal plant Lasiosiphon eriocephalus decne (Family: Thymelaeaceae). J. Nat. Sci. Biol. Med. 2019, 10, 38. [Google Scholar]
- Teekaraman, D.; Elayapillai, S.P.; Viswanathan, M.P.; Jagadeesan, A. Quercetin inhibits human metastatic ovarian cancer cell growth by modulating intrinsic apoptotic pathway in PA-1 cell line. Chem. Interact. 2019, 300, 91–100. [Google Scholar] [CrossRef]
- Elfalleh, W.; Kirkan, B.; Sarikurkcu, C. Antioxidant potential and phenolic composition of extracts from Stachys tmolea: An endemic plant from Turkey. Ind. Crops Prod. 2019, 127, 212–216. [Google Scholar] [CrossRef]
- Kamble, S.S.; Gacche, R.N. Evaluation of anti-breast cancer, anti-angiogenic and antioxidant properties of selected medicinal plants. Eur. J. Integr. Med. 2019, 25, 13–19. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, X. Inhibitory effects of Broccolini leaf flavonoids on human cancer cells. Scanning 2012, 34, 1–5. [Google Scholar] [CrossRef]
- Jaidee, W.; Andersen, R.J.; Chavez, M.A.; Wang, Y.A.; Patrick, B.O.; Pyne, S.G.; Muanprasat, C.; Borwornpinyo, S.; Laphookhieo, S. Amides and Flavonoids from the Fruit and Leaf Extracts of Melodorum siamensis. J. Nat. Prod. 2019, 82, 283–292. [Google Scholar] [CrossRef]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants and developmental regulators: Relative significance in plants and humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Nijveldt, R.J.; Van Nood, E.; Van Hoorn, D.E.; Boelens, P.G.; Van Norren, K.; Van Leeuwen, P.A. Flavonoids: A review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 2001, 74, 418–425. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Chen, D.-F.; Liang, Q.; Guo, R.; Fu, Z.-M. Theoretical studies on the antioxidant activity of pinobanksin and its ester derivatives: Effects of the chain length and solvent. Food Chem. 2018, 240, 323–329. [Google Scholar] [CrossRef]
- Zheng, Y.-Z.; Deng, G.; Guo, R.; Fu, Z.-M.; Chen, D.-F. The influence of the H5⋯OC4 intramolecular hydrogen-bond (IHB) on the antioxidative activity of flavonoid. Phytochemistry 2019, 160, 19–24. [Google Scholar] [CrossRef]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Preethi Soundarya, S.; Sanjay, V.; Haritha Menon, A.; Dhivya, S.; Selvamurugan, N. Effects of flavonoids incorporated biological macromolecules based scaffolds in bone tissue engineering. Int. J. Biol. Macromol. 2017. [Google Scholar] [CrossRef]
- Terao, J. Factors modulating bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Rufatto, L.C.; dos Santos, D.A.; Marinho, F.; Henriques, J.A.P.; Roesch Ely, M.; Moura, S. Red propolis: Chemical composition and pharmacological activity. Asian Pacific J. Trop. Biomed. 2017, 7, 591–598. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Svensson, L.; Sekwati-Monang, B.; Lutz, D.L.; Schieber, A.; Ganzle, M.G. Phenolic acids and flavonoids in nonfermented and fermented red sorghum (Sorghum bicolor (L.) Moench). J. Agric. Food Chem. 2010, 58, 9214–9220. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Cabrera-Ramírez, Á.H.; Rodríguez-Castillo, N.; Campos-Vega, R.; Loarca-Piña, G.; Gaytán-Martínez, M. Impact of cooking and nixtamalization on the bioaccessibility and antioxidant capacity of phenolic compounds from two sorghum varieties. Food Chem. 2020, 309, 125684. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, M.D.; Geleijnse, J.M.; Scheijen, J.L.; Hanssen, N.M.; Dower, J.I.; Afman, L.A.; Stehouwer, C.D.; Hollman, P.C.; Schalkwijk, C.G. Quercetin, but not epicatechin, decreases plasma concentrations of methylglyoxal in adults in a randomized, double-blind, placebo-controlled, crossover trial with pure flavonoids. J. Nutr. 2018, 148, 1911–1916. [Google Scholar] [CrossRef]
- Woodside, J.V.; McGrath, A.J.; Lyner, N.; McKinley, M.C. Carotenoids and health in older people. Maturitas 2015, 80, 63–68. [Google Scholar] [CrossRef]
- Naeimi, A.F.; Alizadeh, M. Antioxidant properties of the flavonoid fisetin: An updated review of in vivo and in vitro studies. Trends Food Sci. Technol. 2017, 70, 34–44. [Google Scholar] [CrossRef]
- Shi, P.; Du, W.; Wang, Y.; Teng, X.; Chen, X.; Ye, L. Total phenolic, flavonoid content, and antioxidant activity of bulbs, leaves, and flowers made from Eleutherine bulbosa (Mill.) Urb. Food Sci. Nutr. 2019, 7, 148–154. [Google Scholar] [CrossRef]
- Amiri, M.; Jelodar, G.; Erjaee, H.; Nazifi, S. The effects of different doses of onion (Allium cepa. L) extract on leptin, ghrelin, total antioxidant capacity, and performance of suckling lambs. Comp. Clin. Pathol. 2019, 28, 1–6. [Google Scholar] [CrossRef]
- Saleh, H.A.-R.; El-Nashar, Y.I.; Serag-El-Din, M.F.; Dewir, Y.H. Plant growth, yield and bioactive compounds of two culinary herbs as affected by substrate type. Sci. Hortic. 2019, 243, 464–471. [Google Scholar] [CrossRef]
- Ielciu, I.; Mouithys-Mickalad, A.; Franck, T.; Angenot, L.; Ledoux, A.; Păltinean, R.; Cieckiewicz, E.; Etienne, D.; Tits, M.; Crişan, G. Flavonoid composition, cellular antioxidant activity and (myelo) peroxidase inhibition of a Bryonia alba L.(Cucurbitaceae) leaves extract. J. Pharm. Pharmacol. 2019, 71, 230–239. [Google Scholar] [CrossRef]
- Wei, Y.-q.; Sun, M.-m.; Fang, H.-y. Dienzyme-assisted salting-out extraction of flavonoids from the seeds of Cuscuta chinensis Lam. Ind. Crops Prod. 2019, 127, 232–236. [Google Scholar] [CrossRef]
- Das, S.; Ray, A.; Nasim, N.; Nayak, S.; Mohanty, S. Effect of different extraction techniques on total phenolic and flavonoid contents, and antioxidant activity of betelvine and quantification of its phenolic constituents by validated HPTLC method. 3 Biotech 2019, 9, 37. [Google Scholar] [CrossRef]
- Jamzad, M.; Emadi, E. Total Phenolic and Flavonoid Contents, and Antioxidant Activity of Salvia Aristata Aucher ex Benth Extracts. Green Nat. Chem. Res. 2019, 1, 20–23. [Google Scholar]
- Krishna, S.; Chandrasekaran, S.; Dhanasekar, D.; Perumal, A. GCMS analysis, antioxidant and antibacterial activities of ethanol extract of Anisomeles malabarica (L.) R. Br. ex. Sims leaves. Asian J. Pharm. Pharmacol. 2019, 5, 180–187. [Google Scholar] [CrossRef]
- Nile, S.H.; Keum, Y.S.; Nile, A.S.; Jalde, S.S.; Patel, R.V. Antioxidant, anti-inflammatory, and enzyme inhibitory activity of natural plant flavonoids and their synthesized derivatives. J. Biochem. Mol. Toxicol. 2018, 32, e22002. [Google Scholar] [CrossRef]
- Worawalai, W.; Phuwapraisirisan, P. Samin-derived flavonolignans, a new series of antidiabetic agents having dual inhibition against α-glucosidase and free radicals. Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef]
- Li, A.-L.; Li, G.-H.; Li, Y.-R.; Wu, X.-Y.; Ren, D.-M.; Lou, H.-X.; Wang, X.-N.; Shen, T. Lignan and flavonoid support the prevention of cinnamon against oxidative stress related diseases. Phytomedicine 2019, 53, 143–153. [Google Scholar] [CrossRef]
- Ali, M.; Alhazmi, H.A.; Ansari, S.; Hussain, A.; Ahmad, S.; Alam, M.S.; Ali, M.S.; El-Sharkawy, K.A.; Hakeem, K.R. Tamarix aphylla (L.) Karst. Phytochemical and Bioactive Profile Compilations of Less Discussed but Effective Naturally Growing Saudi Plant. In Plant and Human Health; Ozturk, M., Hakeem, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 343–352. [Google Scholar]
- Kartikawati, M.; Purnomo, H. Improving meatball quality using different varieties of rice bran as natural antioxidant. Food Res. 2019, 3, 79–85. [Google Scholar] [CrossRef]
- Hwang, I.-W.; Chung, S.-K. Isolation and Identification of Myricitrin, an Antioxidant Flavonoid, from Daebong Persimmon Peel. Prev. Nutr. Food Sci. 2018, 23, 341. [Google Scholar] [CrossRef]
- Sari, N.; Kuspradini, H.; Amirta, R.; Kusuma, I. Antioxidant activity of an invasive plant, Melastoma malabathricum and its potential as herbal tea product. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Samarinda, East Kalimantan, Indonesia, 9 November 2017. [Google Scholar]
- Abidi, S.; Shaheen, N.; Azher, I.; Mahmood, Z.A. Photoprotective and antioxidant activities along with phytochemical investigation of rose water. Int. J. Pharm. Sci. Res. 2018, 9, 5320–5326. [Google Scholar]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. Bio. Med. Res. Int. 2013, 2013, 1–10. [Google Scholar]
- Kumar, S.; Gupta, A.; Pandey, A.K. Calotropis procera root extract has the capability to combat free radical mediated damage. ISRN Pharmacol. 2013, 2013, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Singh, S.; Singh, A.; Dixit, A.K.; Srivastava, B.; Sidhu, G.K.; Singh, R.; Meena, A.K.; Singh, R.P.; Subhose, V. Phytochemical, Antioxidant, Antimicrobial, and Protein Binding Qualities of Hydro-ethanolic Extract of Tinospora cordifolia. J. Biol. Active Prod. Nat. 2018, 8, 192–200. [Google Scholar] [CrossRef]
- Mahmood, W.; Saleem, H.; Shahid, W.; Ahmad, I.; Zengin, G.; Mahomoodally, M.F.; Ashraf, M.; Ahemad, N. Clinical enzymes inhibitory activities, antioxidant potential and phytochemical profile of Vernonia oligocephala (DC.) Sch. Bip. ex Walp roots. Biocatal. Agric. Biotechnol. 2019, 18, 101039. [Google Scholar] [CrossRef]
- Slavin, J.L.; Lloyd, B. Health benefits of fruits and vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and platelet aggregation: A brief review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef]
- Xie, J.; Xiong, J.; Ding, L.-S.; Chen, L.; Zhou, H.; Liu, L.; Zhang, Z.-F.; Hu, X.-M.; Luo, P.; Qing, L.-S. A efficient method to identify cardioprotective components of Astragali Radix using a combination of molecularly imprinted polymers-based knockout extract and activity evaluation. J. Chromatogr. A 2018, 1576, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signalling molecules? Free Radic. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Hodgson, J.M.; Croft, K.D. Tea flavonoids and cardiovascular health. Mol. Asp. Med. 2010, 31, 495–502. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Cassidy, A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017. [Google Scholar] [CrossRef]
- Corti, R.; Flammer, A.J.; Hollenberg, N.K.; Luscher, T.F. Cocoa and cardiovascular health. Circulation 2009, 119, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Van Dam, R.M.; Naidoo, N.; Landberg, R. Dietary flavonoids and the development of type 2 diabetes and cardiovascular diseases. Curr. Opin. Lipidol. 2013, 24, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Lilamand, M.; Kelaiditi, E.; Guyonnet, S.; Antonelli Incalzi, R.; Raynaud-Simon, A.; Vellas, B.; Cesari, M. Flavonoids and arterial stiffness: Promising perspectives. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 698–704. [Google Scholar] [CrossRef]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Venu Gopal, J. Morin Hydrate: Botanical origin, pharmacological activity and its applications: A mini-review. Pharmacogn. J. 2013, 5, 123–126. [Google Scholar] [CrossRef]
- Yang, J. Brazil nuts and associated health benefits: A review. LWT Food Sci. Technol. 2009, 42, 1573–1580. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Habtemariam, S.; Orhan, I.E.; Daglia, M.; Manayi, A.; Gortzi, O.; Nabavi, S.M. Neuroprotective effects of chrysin: From chemistry to medicine. Neurochem. Int. 2015, 90, 224–231. [Google Scholar] [CrossRef]
- Latypova, G.; Bychenkova, M.; Katayev, V.; Perfilova, V.; Tyurenkov, I.; Mokrousov, I.; Prokofiev, I.; Salikhov, S.M.; Iksanova, G. Composition and cardioprotective effects of Primula veris L. solid herbal extract in experimental chronic heart failure. Phytomedicine 2019, 54, 17–26. [Google Scholar] [CrossRef]
- Nissler, L.; Gebhardt, R.; Berger, S. Flavonoid binding to a multi-drug-resistance transporter protein: An STD-NMR study. Anal. Bioanal. Chem. 2004, 379, 1045–1049. [Google Scholar] [CrossRef]
- Scotti, L.; Fernandes, M.B.; Muramatsu, E.; Emereciano, V.d.P.; Tavares, J.F.; Silva, M.S.d.; Scotti, M.T. 13C NMR spectral data and molecular descriptors to predict the antioxidant activity of flavonoids. Braz. J. Pharm. Sci. 2011, 47, 241–249. [Google Scholar] [CrossRef]
- Blunder, M.; Orthaber, A.; Bauer, R.; Bucar, F.; Kunert, O. Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chem. 2017, 218, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.K.; Malik, S.; Narayanan, S.P.; Mutneja, E.; Sahu, A.K.; Bhatia, J.; Arya, D.S. Role of MAPK/NF-κB pathway in cardioprotective effect of Morin in isoproterenol induced myocardial injury in rats. Mol. Biol. Rep. 2019, 46, 1139–1148. [Google Scholar] [CrossRef]
- Gvozdjakova, A.; Singh, R.; Singh, R.B.; Takahashi, T.; Fedacko, J.; Hristova, K.; Wilczynska, A.; Mojtová, M.; Mojto, V. Cocoa Consumption and Prevention of Cardiometabolic Diseases and Other Chronic Diseases. In The Role of Functional Food Security in Global Health; Watson, R., Singh, R., Takahashi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 317–345. [Google Scholar]
- Zięba, K.; Makarewicz-Wujec, M.; Kozłowska-Wojciechowska, M. Cardioprotective Mechanisms of Cocoa. J. Am. Coll. Nutr. 2019, 38, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct. 2019, 10, 203–215. [Google Scholar] [CrossRef]
- Petruzzellis, V.; Troccoli, T.; Candiani, C.; Guarisco, R.; Lospalluti, M.; Belcaro, G.; Dugall, M. Oxerutins (Venoruton®): Efficacy in Chronic Venous Insufficiency: A Double-Blind, Randomized, Controlled Study. Angiology 2002, 53, 257–263. [Google Scholar] [CrossRef]
- Massimo, C.; Alunni, F.D.; Giuseppe, P.; Spedale, V.M.D.; Italia, C.A. Comparison of Centella with Flavonoids for Treatment of Symptoms in Hemorrhoidal Disease and After Surgical Intervention: A Randomized Clinical Trial. Sci. Rep. 2020, 10, 1–14. [Google Scholar]
- Corsale, I.; Carrieri, P.; Martellucci, J.; Piccolomini, A.; Verre, L.; Rigutini, M.; Panicucci, S. Flavonoid mixture (diosmin, troxerutin, rutin, hesperidin, quercetin) in the treatment of I–III degree hemorroidal disease: A double-blind multicenter prospective comparative study. Int. J. Colorectal Dis. 2018, 33, 1595–1600. [Google Scholar] [CrossRef]
- Di Visconte, M.S.; Nicolì, F.; Del Giudice, R.; Cipolat Mis, T. Effect of a mixture of diosmin, coumarin glycosides, and triterpenes on bleeding, thrombosis, and pain after stapled anopexy: A prospective, randomized, placebo-controlled clinical trial. Int. J. Colorectal Dis. 2016, 32, 425–431. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, J.; Li, H.; Kong, X.; Liu, Y.; Guo, R.; Li, G.; Yang, B.; Pei, M. Cardioprotective effects of total flavonoids from Jinhe Yangxin prescription by activating the PI3K/Akt signaling pathway in myocardial ischemia injury. Biomed. Pharmacother. 2018, 98, 308–317. [Google Scholar] [CrossRef]
- Zeng, C.; Jiang, W.; Yang, X.; He, C.; Wang, W.; Xing, J. Pretreatment with Total Flavonoid Extract from Dracocephalum Moldavica L. Attenuates Ischemia Reperfusion-induced Apoptosis. Sci. Rep. 2018, 8, 17491. [Google Scholar] [CrossRef]
- Lin, Q.; Chen, X.-Y.; Zhang, J.; Yuan, Y.-L.; Zhao, W.; Wei, B. Upregulation of SIRT1 contributes to the cardioprotective effect of Rutin against myocardial ischemia-reperfusion injury in rats. J. Funct. Foods 2018, 46, 227–236. [Google Scholar] [CrossRef]
- Mohamed, M.K.; Anaytulla, P.A.; Rahman, M.M.; Malik, T.K.; Hasan, M.M.; Azad, A.K. Evaluation of Ex-Vivo Cardioprotective and Anti-inflammatory Investigation of Bangladeshi Plants Extract. J. Sci. Res. Rep. 2015, 7, 58–66. [Google Scholar] [CrossRef]
- Kammoun, I.; Ben Salah, H.; Ben Saad, H.; Cherif, B.; Droguet, M.; Magné, C.; Kallel, C.; Boudawara, O.; Hakim, A.; Gharsallah, N. Hypolipidemic and cardioprotective effects of Ulva lactuca ethanolic extract in hypercholesterolemic mice. Arch. Physiol. Biochem. 2018, 124, 313–325. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Chen, R.-C.; Sun, G.-B.; Yang, L.-P.; Xu, X.-D.; Sun, X.-B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Jiang, R.; Guo, Y.; Chen, N.; Gao, C.; Ding, Z.; Jin, B. Total Flavonoids from Carya cathayensis Sarg. Leaves Alleviate H9c2 Cells Hypoxia/Reoxygenation Injury via Effects on miR-21 Expression, PTEN/Akt, and the Bcl-2/Bax Pathway. Evid. Based Complementary Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Du, Z.; Li, Y.; Wang, L.; Gao, P.; Gao, X.; Li, C.; Zhao, M.; Jiang, Y.; Tu, P. Integration of metabolomics with pharmacodynamics to elucidate the anti-myocardial ischemia effects of combination of notoginseng total saponins and safflower total flavonoids. Front. Pharmacol. 2018, 9, 667. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.-Y.; Xu, Q.-H.; Peng, G.; Chen, Z.-W. The protective effect of total flavones from Rhododendron simsii Planch. on myocardial ischemia/reperfusion injury and its underlying mechanism. Evid. Based Complementary Altern. Med. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Enayati, A.; Yassa, N.; Mazaheri, Z.; Rajaei, M.; Pourabouk, M.; Ghorghanlu, S.; Basiri, S.; Khori, V. Cardioprotective and anti-apoptotic effects of Potentilla reptans L. root via Nrf2 pathway in an isolated rat heart ischemia/reperfusion model. Life Sci. 2018, 215, 216–226. [Google Scholar] [CrossRef]
- Pradeepkumar, B.; Sudheer, A.; Reddy, T.S.; Reddy, K.S.; Narayana, G.; Veerabhadrappa, K. Cardioprotective Activity of Flavonoid Fraction of Gymnema Sylvestre Leaves on Doxorubicin Induced Cardiac Damage. J. Young Pharm. 2018, 10, 422–426. [Google Scholar] [CrossRef]
- Orhan, I.; Daglia, M.; Nabavi, S.; Loizzo, M.; Sobarzo-Sánchez, E.; Nabavi, S. Flavonoids and dementia: An update. Curr. Med. Chem. 2015, 22, 1004–1015. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y.; Yamada, K. Anti-dementia activity of nobiletin, a citrus flavonoid: A review of animal studies. Clin. Psychopharmacol. Neurosci. 2014, 12, 75. [Google Scholar] [CrossRef]
- Datla, K.P.; Christidou, M.; Widmer, W.W.; Rooprai, H.K.; Dexter, D.T. Tissue distribution and neuroprotective effects of citrus flavonoid tangeretin in a rat model of Parkinson’s disease. Neuroreport 2001, 12, 3871–3875. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cassidy, A.; Schwarzschild, M.; Rimm, E.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.K.; Haleagrahara, N. Protective mechanisms of flavonoids in Parkinson’s disease. Oxidative Med. Cell. Longev. 2015, 2015, 1–14. [Google Scholar] [CrossRef]
- Bakhtiari, M.; Panahi, Y.; Ameli, J.; Darvishi, B. Protective effects of flavonoids against Alzheimer’s disease-related neural dysfunctions. Biomed. Pharmacother. 2017, 93, 218–229. [Google Scholar] [CrossRef]
- Ozcan, T.; Akpinar-Bayizit, A.; Yilmaz-Ersan, L.; Delikanli, B. Phenolics in Human Health. Int. J. Chem. Eng. Appl. 2014, 5, 393–396. [Google Scholar] [CrossRef]
- Bursal, E.; Aras, A.; Kılıç, Ö.; Taslimi, P.; Gören, A.C.; Gülçin, İ. Phytochemical content, antioxidant activity, and enzyme inhibition effect of Salvia eriophora Boiss. & Kotschy against acetylcholinesterase, α-amylase, butyrylcholinesterase, and α-glycosidase enzymes. J. Food Biochem. 2019, 43, e12776. [Google Scholar]
- Gao, Z.; Gao, W.; Zeng, S.-L.; Li, P.; Liu, E.H. Chemical structures, bioactivities and molecular mechanisms of citrus polymethoxyflavones. J. Funct. Foods 2018, 40, 498–509. [Google Scholar] [CrossRef]
- Spencer, J.P.E.; Vafeiadou, K.; Williams, R.J.; Vauzour, D. Neuroinflammation: Modulation by flavonoids and mechanisms of action. Mol. Asp. Med. 2012, 33, 83–97. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Neuropharmacological properties and pharmacokinetics of the citrus flavonoids hesperidin and hesperetin—A mini-review. Life Sci. 2014, 113, 1–6. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef]
- Lamuela-Raventós, R.M.; Romero-Pérez, A.I.; Andrés-Lacueva, C.; Tornero, A. Review: Health Effects of Cocoa Flavonoids. Food Sci. Technol. Int. 2016, 11, 159–176. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef]
- Wei, Q.; Zhang, R.; Wang, Q.; Yan, X.J.; Yu, Q.W.; Yan, F.X.; Li, C.; Pei, Y.H. Iridoid, phenylethanoid and flavonoid glycosides from Forsythia suspensa. Nat. Prod. Res. 2019, 34, 1320–1325. [Google Scholar] [CrossRef]
- Botalova, A.; Bombela, T.; Zubov, P.; Segal, M.; Korkotian, E. The flavonoid acetylpectolinarin counteracts the effects of low ethanol on spontaneous network activity in hippocampal cultures. J. Ethnopharmacol. 2019, 229, 22–28. [Google Scholar] [CrossRef]
- Narenjkar, J.; Roghani, M.; Alambeygi, H.; Sedaghati, F. The effect of the flavonoid quercetin on pain sensation in diabetic rats. Basic Clin. Neurosci. 2011, 2, 51–57. [Google Scholar]
- Shahid, M.; Subhan, F.; Ahmad, N.; Sewell, R.D. The flavonoid 6-methoxyflavone allays cisplatin-induced neuropathic allodynia and hypoalgesia. Biomed. Pharmacother. 2017, 95, 1725–1733. [Google Scholar] [CrossRef]
- Cuello, A.C. Intracellular and extracellular Aβ, a tale of two neuropathologies. Brain Pathol. 2005, 15, 66–71. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. South Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Pohanka, M. Inhibitors of acetylcholinesterase and butyrylcholinesterase meet immunity. Int. J. Mol. Sci. 2014, 15, 9809–9825. [Google Scholar] [CrossRef]
- Eruygur, N.; Ucar, E.; Akpulat, H.A.; Shahsavari, K.; Safavi, S.M.; Kahrizi, D. In vitro antioxidant assessment, screening of enzyme inhibitory activities of methanol and water extracts and gene expression in Hypericum lydium. Mol. Biol. Rep. 2019, 46, 2121–2129. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, L.; Yang, C.; Li, Z.; Rong, S. Procyanidins and Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 5556–5567. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Kirkan, B.; Sarikurkcu, C. Phenolic ingredients and therapeutic potential of Stachys cretica subsp. smyrnaea for the management of oxidative stress, Alzheimer’s disease, hyperglycemia, and melasma. Ind. Crops Prod. 2019, 127, 82–87. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Anticholinergic effects of Actinidia arguta fruits and their polyphenol content determined by liquid chromatography-photodiode array detector-quadrupole/time of flight-mass spectrometry (LC-MS-PDA-Q/TOF). Food Chem. 2019, 271, 216–223. [Google Scholar] [CrossRef]
- Katalinić, M.; Rusak, G.; Barović, J.D.; Šinko, G.; Jelić, D.; Antolović, R.; Kovarik, Z. Structural aspects of flavonoids as inhibitors of human butyrylcholinesterase. Eur. J. Med. Chem. 2010, 45, 186–192. [Google Scholar] [CrossRef]
- Luo, W.; Chen, Y.; Wang, T.; Hong, C.; Chang, L.-P.; Chang, C.-C.; Yang, Y.-C.; Xie, S.-Q.; Wang, C.-J. Design, synthesis and evaluation of novel 7-aminoalkyl-substituted flavonoid derivatives with improved cholinesterase inhibitory activities. Bioorganic Med. Chem. 2016, 24, 672–680. [Google Scholar] [CrossRef]
- Kubínová, R.; Gazdová, M.; Hanáková, Z.; Jurkaninová, S.; Dall’Acqua, S.; Cvačka, J.; Humpa, O. New diterpenoid glucoside and flavonoids from Plectranthus scutellarioides (L.) R. Br. South Afr. J. Bot. 2019, 120, 286–290. [Google Scholar] [CrossRef]
- Kobus-Cisowska, J.; Szymanowska, D.; Maciejewska, P.; Kmiecik, D.; Gramza-Michałowska, A.; Kulczyński, B.; Cielecka-Piontek, J. In vitro screening for acetylcholinesterase and butyrylcholinesterase inhibition and antimicrobial activity of chia seeds (Salvia hispanica). Electron. J. Biotechnol. 2019, 37, 1–10. [Google Scholar] [CrossRef]
- Bose, B.; Tripathy, D.; Chatterjee, A.; Tandon, P.; Kumaria, S. Secondary metabolite profiling, cytotoxicity, anti-inflammatory potential and in vitro inhibitory activities of Nardostachys jatamansi on key enzymes linked to hyperglycemia, hypertension and cognitive disorders. Phytomedicine 2019, 55, 58–69. [Google Scholar] [CrossRef]
- Karakaya, S.; Koca, M.; Sytar, O.; Duman, H. The natural phenolic compounds and their antioxidant and anticholinesterase potential of herb Leiotulus dasyanthus (K. Koch) Pimenov & Ostr. Nat. Prod. Res. 2019, 34, 1303–1305. [Google Scholar]
- Orhan, I.E.; Akkol, E.K.; Suntar, I.; Yesilada, E. Assessment of anticholinesterase and antioxidant properties of the extracts and (+)-catechin obtained from Arceuthobium oxycedri (DC) M. Bieb (dwarf mistletoe). S. Afr. J. Bot. 2019, 120, 309–312. [Google Scholar] [CrossRef]
- Orhan, I.E.; Senol, F.S.; Ercetin, T.; Kahraman, A.; Celep, F.; Akaydin, G.; Sener, B.; Dogan, M. Assessment of anticholinesterase and antioxidant properties of selected sage (Salvia) species with their total phenol and flavonoid contents. Ind. Crops Prod. 2013, 41, 21–30. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, Y.T. Food-derived natural compounds for pain relief in neuropathic pain. Bio. Med. Res. Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef]
- Blackburn, K.; Warren, K. A Case of Peripheral Neuropathy Due to Pyridoxine Toxicity in Association with NOS Energy Drink Consumption (P4. 043). Available online: https://n.neurology.org/content/88/16_Supplement/P4.043 (accessed on 18 April 2017).
- Hasannejad, F.; Ansar, M.M.; Rostampour, M.; Fikijivar, E.M.; Taleghani, B.K. Improvement of pyridoxine-induced peripheral neuropathy by Cichorium intybus hydroalcoholic extract through GABAergic system. J. Physiol. Sci. 2019, 69, 465–476. [Google Scholar] [CrossRef]
- Testa, R.; Bonfigli, A.; Genovese, S.; De Nigris, V.; Ceriello, A. The possible role of flavonoids in the prevention of diabetic complications. Nutrients 2016, 8, 310. [Google Scholar] [CrossRef]
- Li, R.; Zhang, Y.; Rasool, S.; Geetha, T.; Babu, J.R. Effects and Underlying Mechanisms of Bioactive Compounds on Type 2 Diabetes Mellitus and Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 1–25. [Google Scholar] [CrossRef]
- Bayram, E.H.; Sezer, A.D.; Elçioğlu, H.K.b. Diabetic Neuropathy and Treatment Strategy–New Challenges and Applications. In Smart Drug Delivery System; Sezer, A.D., Ed.; InTechOpen: Rijeka, Croatia, 2016. [Google Scholar] [CrossRef]
- Visnagri, A.; Kandhare, A.D.; Chakravarty, S.; Ghosh, P.; Bodhankar, S.L. Hesperidin, a flavanoglycone attenuates experimental diabetic neuropathy via modulation of cellular and biochemical marker to improve nerve functions. Pharm. Biol. 2014, 52, 814–828. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Matsuzaki, K.; Nakata, N.; Onozuka, H.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; Ohizumi, Y. Nobiletin, a citrus flavonoid, reverses learning impairment associated with N-methyl-D-aspartate receptor antagonism by activation of extracellular signal-regulated kinase signaling. J. Pharmacol. Exp. Ther. 2007, 321, 784–790. [Google Scholar] [CrossRef]
- Nakajima, A.; Yamakuni, T.; Haraguchi, M.; Omae, N.; Song, S.-Y.; Kato, C.; Nakagawasai, O.; Tadano, T.; Yokosuka, A.; Mimaki, Y. Nobiletin, a citrus flavonoid that improves memory impairment, rescues bulbectomy-induced cholinergic neurodegeneration in mice. J. Pharmacol. Sci. 2007, 105, 122–126. [Google Scholar] [CrossRef]
- Parkar, N.; Addepalli, V. Effect of nobiletin on diabetic neuropathy in experimental rats. J. Pharmacol. Ther. 2014, 2, 1028. [Google Scholar]
- Rajkumari Sahane*, P.N. Flavonoid Rich Fraction of Helicteres Isora Fruits Ameliorate Streptozotocin and High Fat Diet Induced Diabetic Neuropathy in Sprague Dawley Rats. J. Nat. Prod. Plant Resour. 2018, 8, 8–16. [Google Scholar]
- Azevedo, M.I.; Pereira, A.F.; Nogueira, R.B.; Rolim, F.E.; Brito, G.A.; Wong, D.V.T.; Lima-Júnior, R.C.; de Albuquerque Ribeiro, R.; Vale, M.L. The antioxidant effects of the flavonoids rutin and quercetin inhibit oxaliplatin-induced chronic painful peripheral neuropathy. Mol. Pain 2013, 9, 53. [Google Scholar] [CrossRef]
- Ishii, N.; Matsuoka, Y.; Omiya, H.; Taniguchi, A.; Kaku, R.; Morita, K. The flavonoid quercetin suppreses the development of neuropathic pain behavior in rats: 14AP4-3. Eur. J. Anaesthesiol. (EJA) 2013, 30, 214. [Google Scholar] [CrossRef]
- Wang, J.; Huang, L.; Cheng, C.; Li, G.; Xie, J.; Shen, M.; Chen, Q.; Li, W.; He, W.; Qiu, P. Design, synthesis and biological evaluation of chalcone analogues with novel dual antioxidant mechanisms as potential anti-ischemic stroke agents. Acta Pharm. Sin. B 2019, 9, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Acosta, S.A.; Lee, J.Y.; Nguyen, H.; Kaneko, Y.; Borlongan, C.V. Endothelial Progenitor Cells Modulate Inflammation-Associated Stroke Vasculome. Stem Cell Rev. Rep. 2019, 15, 256–275. [Google Scholar] [CrossRef]
- Gelderblom, M.; Leypoldt, F.; Lewerenz, J.; Birkenmayer, G.; Orozco, D.; Ludewig, P.; Thundyil, J.; Arumugam, T.V.; Gerloff, C.; Tolosa, E. The flavonoid fisetin attenuates postischemic immune cell infiltration, activation and infarct size after transient cerebral middle artery occlusion in mice. J. Cereb. Blood Flow Metab. 2012, 32, 835–843. [Google Scholar] [CrossRef]
- Rodrigues, A.M.G.; dos Santos Marcilio, F.; Muzitano, M.F.; Giraldi-Guimarães, A. Therapeutic potential of treatment with the flavonoid rutin after cortical focal ischemia in rats. Brain Res. 2013, 1503, 53–61. [Google Scholar] [CrossRef]
- Kim, H.; Yi, J.-W.; Sung, Y.-H.; Kim, C.-J.; Kim, C.-S.; Kang, J.-M. Delayed preconditioning effect of isoflurane on spinal cord ischemia in rats. Neurosci. Lett. 2008, 440, 211–216. [Google Scholar] [CrossRef]
- Uslusoy, F.; Nazıroğlu, M.; Övey, İ.S.; Sönmez, T.T. Hypericum perforatum L. supplementation protects sciatic nerve injury-induced apoptotic, inflammatory and oxidative damage to muscle, blood and brain in rats. J. Pharm. Pharmacol. 2019, 71, 83–92. [Google Scholar] [CrossRef]
- Song-Tao, M.; Dong-lian, L.; Jing-jing, D.; Yan-juan, P. Protective effect of mulberry flavonoids on sciatic nerve in alloxan-induced diabetic rats. Brazilian J. Pharm. Sci. 2014, 50, 765–771. [Google Scholar] [CrossRef]
- Valsecchi, A.E.; Franchi, S.; Panerai, A.E.; Sacerdote, P.; Trovato, A.E.; Colleoni, M. Genistein, a natural phytoestrogen from soy, relieves neuropathic pain following chronic constriction sciatic nerve injury in mice: Anti-inflammatory and antioxidant activity. J. Neurochem. 2008, 107, 230–240. [Google Scholar] [CrossRef]
- Raafat, K.M. Anti-inflammatory and anti-neuropathic effects of a novel quinic acid derivative from Acanthus syriacus. Avicenna J. Phytomedicine 2019, 9, 221–236. [Google Scholar]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Muhammad, A.; Khan, B.; Iqbal, Z.; Khan, A.Z.; Khan, I.; Khan, K.; Alamzeb, M.; Ahmad, N.; Khan, K.; Lal Badshah, S. Viscosine as a Potent and Safe Antipyretic Agent Evaluated by Yeast-Induced Pyrexia Model and Molecular Docking Studies. ACS Omega 2019, 4, 14188–14192. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, S.; DasSharma, S.; Banik, R.; Chakraborty, A.; Dinda, M. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur. J. Med. Chem. 2017, 131, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, X.-Y.; Martin, C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016, 61, 1391–1398. [Google Scholar] [CrossRef] [PubMed]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Perez-Guerrero, C.; Lopez-Lazaro, M. A Review on the Dietary Flavonoid Kaempferol. Mini-Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Zeinali, M.; Rezaee, S.A.; Hosseinzadeh, H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed. Pharmacother. 2017, 92, 998–1009. [Google Scholar] [CrossRef] [PubMed]
- Fang, J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Van, Q.T.T.; Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Cuong, N.T.; Thao, N.P.; Thuan, N.H.; Dang, N.H.; Thanh, N.V.; Cuong, N.X. Acylated flavonoid glycosides from Barringtonia racemosa. Nat. Prod. Res. 2019, 34, 1276–1281. [Google Scholar] [CrossRef]
- Abu-Qatouseh, L.; Mallah, E.; Mansour, K. Evaluation of Anti-Propionibacterium Acnes and Anti-Inflammatory Effects of Polyphenolic Extracts of Medicinal Herbs in Jordan. Biomed. Pharmacol. J. 2019, 12, 211–217. [Google Scholar] [CrossRef]
- Chen, G.-L.; Fan, M.-X.; Wu, J.-L.; Li, N.; Guo, M.-Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food Chem. 2019, 277, 706–712. [Google Scholar] [CrossRef]
- Ma, Q.; Jiang, J.-G.; Yuan, X.; Qiu, K.; Zhu, W. Comparative antitumor and anti-inflammatory effects of flavonoids, saponins, polysaccharides, essential oil, coumarin and alkaloids from Cirsium japonicum DC. Food Chem. Toxicol. 2019, 125, 422–429. [Google Scholar] [CrossRef]
- Dong, X.; Huang, Y.; Wang, Y.; He, X. Anti-inflammatory and antioxidant jasmonates and flavonoids from lychee seeds. J. Funct. Foods 2019, 54, 74–80. [Google Scholar] [CrossRef]
- Abubakar, S.; Al-Mansoub, M.A.; Murugaiyah, V.; Chan, K.L. The phytochemical and anti-inflammatory studies of Dillenia suffruticosa leaves. Phytother. Res. 2019, 33, 660–675. [Google Scholar] [CrossRef]
- Escribano-Ferrer, E.; Queralt Regué, J.; Garcia-Sala, X.; Boix Montañés, A.; Lamuela-Raventos, R.M. In Vivo Anti-inflammatory and Antiallergic Activity of Pure Naringenin, Naringenin Chalcone, and Quercetin in Mice. J. Nat. Prod. 2019, 82, 177–182. [Google Scholar] [CrossRef]
- Truong, D.-H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Han, Q.-T.; Ren, Y.; Li, G.-S.; Xiang, K.-L.; Dai, S.-J. Flavonoid alkaloids from Scutellaria moniliorrhiza with anti-inflammatory activities and inhibitory activities against aldose reductase. Phytochemistry 2018, 152, 91–96. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tait, A.R.; Kitts, D.D. Flavonoid composition of orange peel and its association with antioxidant and anti-inflammatory activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Chen, H.; Pu, J.; Liu, D.; Yu, W.; Shao, Y.; Yang, G.; Xiang, Z.; He, N. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L.). PLoS ONE 2016, 11, e0153080. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Campolo, M.; Gugliandolo, E.; Esposito, E.; Benedetto, F.; Cuzzocrea, S.; Navarra, M. Anti-inflammatory and antioxidant effects of flavonoid-rich fraction of bergamot juice (BJe) in a mouse model of intestinal ischemia/reperfusion injury. Front. Pharmacol. 2016, 7, 203. [Google Scholar] [CrossRef]
- Muthaura, C.N.; Keriko, J.M.; Derese, S.; Yenesew, A.; Rukunga, G.M. Investigation of some medicinal plants traditionally used for treatment of malaria in Kenya as potential sources of antimalarial drugs. Exp. Parasitol. 2011, 127, 609–626. [Google Scholar] [CrossRef]
- Badshah, S.L.; Ullah, A.; Ahmad, N.; Almarhoon, Z.M.; Mabkhot, Y. Increasing the strength and production of artemisinin and its derivatives. Molecules 2018, 23, 100. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Amin, H.; Ullah, A.; Saba, S.; Rafique, J.; Khan, K.; Ahmad, N.; Badshah, S.L. Antioxidant and antiplasmodial activities of bergenin and 11-O-galloylbergenin isolated from Mallotus philippensis. Oxidative Med. Cell. Longev. 2016, 2016, 1–6. [Google Scholar] [CrossRef]
- Memvanga, P.B.; Tona, G.L.; Mesia, G.K.; Lusakibanza, M.M.; Cimanga, R.K. Antimalarial activity of medicinal plants from the Democratic Republic of Congo: A review. J. Ethnopharmacol. 2015, 169, 76–98. [Google Scholar] [CrossRef] [PubMed]
- Henciya, S.; Seturaman, P.; James, A.R.; Tsai, Y.-H.; Nikam, R.; Wu, Y.-C.; Dahms, H.-U.; Chang, F.R. Biopharmaceutical potentials of Prosopis spp. (Mimosaceae, Leguminosa). J. Food Drug Anal. 2017, 25, 187–196. [Google Scholar] [CrossRef]
- Mahadeo, K.; Grondin, I.; Kodja, H.; Soulange Govinden, J.; Jhaumeer Laulloo, S.; Frederich, M.; Gauvin-Bialecki, A. The genus Psiadia: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 210, 48–68. [Google Scholar] [CrossRef] [PubMed]
- Zongo, F.; Ribuot, C.; Boumendjel, A.; Guissou, I. Botany, traditional uses, phytochemistry and pharmacology of Waltheria indica L. (syn. Waltheria americana): A review. J. Ethnopharmacol. 2013, 148, 14–26. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mina, P.R.; Kumar, Y.; Verma, A.K.; Khan, F.; Tandon, S.; Pal, A.; Darokar, M.P. Silymarin, a polyphenolic flavonoid impede Plasmodium falciparum growth through interaction with heme. Nat. Prod. Res. 2019, 34, 2647–2651. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Al-Shaebi, E.M.; Al-Quraishy, S. Effect of Indigofera oblongifolia on the Hepatic Oxidative Status and Expression of Inflammatory and Apoptotic Genes during Blood-Stage Murine Malaria. Oxidative Med. Cell. Longev. 2019, 2019, 8264861–8264867. [Google Scholar] [CrossRef]
- Badshah, S.L.; Ullah, A.; Badshah, S.H.; Ahmad, I. Spread of Novel Coronavirus by Returning Pilgrims from Iran to Pakistan. J. Travel Med. 2020, 27. [Google Scholar] [CrossRef]
- Villa, T.G.; Feijoo-Siota, L.; Rama, J.L.R.; Ageitos, J.M. Antivirals against animal viruses. Biochem. Pharmacol. 2017, 133, 97–116. [Google Scholar] [CrossRef]
- Chiow, K.H.; Phoon, M.C.; Putti, T.; Tan, B.K.H.; Chow, V.T. Evaluation of antiviral activities of Houttuynia cordata Thunb. extract, quercetin, quercetrin and cinanserin on murine coronavirus and dengue virus infection. Asian Pacific J. Trop. Med. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Brodowska, K.M. Natural flavonoids: Classification, potential role, and application of flavonoid analogues. Eur. J. Biol. Res. 2017, 7, 108–123. [Google Scholar]
- Sanchez, I.; Gómez-Garibay, F.; Taboada, J.; Ruiz, B. Antiviral effect of flavonoids on the dengue virus. Phytother. Res. 2000, 14, 89–92. [Google Scholar] [CrossRef]
- Song, J.-M.; Lee, K.-H.; Seong, B.-L. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 2005, 68, 66–74. [Google Scholar] [CrossRef]
- Gramza-Michałowska, A.; Sidor, A.; Kulczyński, B. Berries as a potential anti-influenza factor–A review. J. Funct. Foods 2017, 37, 116–137. [Google Scholar] [CrossRef]
- Lani, R.; Hassandarvish, P.; Shu, M.-H.; Phoon, W.H.; Chu, J.J.H.; Higgs, S.; Vanlandingham, D.; Abu Bakar, S.; Zandi, K. Antiviral activity of selected flavonoids against Chikungunya virus. Antivir. Res. 2016, 133, 50–61. [Google Scholar] [CrossRef]
- Seo, D.J.; Jeon, S.B.; Oh, H.; Lee, B.-H.; Lee, S.-Y.; Oh, S.H.; Jung, J.Y.; Choi, C. Comparison of the antiviral activity of flavonoids against murine norovirus and feline calicivirus. Food Control 2016, 60, 25–30. [Google Scholar] [CrossRef]
- Wu, Q.; Yu, C.; Yan, Y.; Chen, J.; Zhang, C.; Wen, X. Antiviral flavonoids from Mosla scabra. Fitoterapia 2010, 81, 429–433. [Google Scholar] [CrossRef]
- Kim, N.; Park, S.; Nhiem, N.X.; Song, J.-H.; Ko, H.-J.; Kim, S.H. Cycloartane-type triterpenoid derivatives and a flavonoid glycoside from the burs of Castanea crenata. Phytochemistry 2019, 158, 135–141. [Google Scholar] [CrossRef]
- Sadati, S.M.; Gheibi, N.; Ranjbar, S.; Hashemzadeh, M.S. Docking study of flavonoid derivatives as potent inhibitors of influenza H1N1 virus neuraminidase. Biomed. Rep. 2019, 10, 33–38. [Google Scholar] [CrossRef]
- Khalil, H.; Abd El Maksoud, A.I.; Roshdey, T.; El-Masry, S. Guava flavonoid glycosides prevent influenza A virus infection via rescue of P53 activity. J. Med. Virol. 2019, 91, 45–55. [Google Scholar] [CrossRef]
- Zhi, H.-J.; Zhu, H.-Y.; Zhang, Y.-Y.; Lu, Y.; Li, H.; Chen, D.-F. In vivo effect of quantified flavonoids-enriched extract of Scutellaria baicalensis root on acute lung injury induced by influenza A virus. Phytomedicine 2019, 57, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Badshah, S.L.; Muska, M.; Ahmad, N.; Khan, K. The current case of quinolones: Synthetic approaches and antibacterial activity. Molecules 2016, 21, 268. [Google Scholar] [CrossRef]
- Badshah, S.L.; Ullah, A. New developments in non-quinolone-based antibiotics for the inhibiton of bacterial gyrase and topoisomerase IV. Eur. J. Med. Chem. 2018, 152, 393–400. [Google Scholar] [CrossRef]
- Ahmad, A.; Kaleem, M.; Ahmed, Z.; Shafiq, H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—A review. Food Res. Int. 2015, 77, 221–235. [Google Scholar] [CrossRef]
- Ngueyem, T.A.; Brusotti, G.; Caccialanza, G.; Finzi, P.V. The genus Bridelia: A phytochemical and ethnopharmacological review. J. Ethnopharmacol. 2009, 124, 339–349. [Google Scholar] [CrossRef]
- Chinsembu, K.C. Tuberculosis and nature’s pharmacy of putative anti-tuberculosis agents. Acta Trop. 2016, 153, 46–56. [Google Scholar] [CrossRef]
- Iranshahi, M.; Rezaee, R.; Parhiz, H.; Roohbakhsh, A.; Soltani, F. Protective effects of flavonoids against microbes and toxins: The cases of hesperidin and hesperetin. Life Sci. 2015, 137, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Tandon, S.; Xuan, T.D.; Nooreen, Z. A Review on Phytoconstituents and Biological activities of Cuscuta species. Biomed. Pharmacother. 2017, 92, 772–795. [Google Scholar] [CrossRef]
- Ngankeu Pagning, A.L.; Tamokou, J.-d.-D.; Lateef, M.; Tapondjou, L.A.; Kuiate, J.-R.; Ngnokam, D.; Ali, M.S. New triterpene and new flavone glucoside from Rhynchospora corymbosa (Cyperaceae) with their antimicrobial, tyrosinase and butyrylcholinesterase inhibitory activities. Phytochem. Lett. 2016, 16, 121–128. [Google Scholar] [CrossRef]
- Loredana, L.; Giuseppina, A.; Filomena, N.; Florinda, F.; Donatella, A. Biochemical, antioxidant properties and antimicrobial activity of different onion varieties in the Mediterranean area. J. Food Meas. Charact. 2019, 13, 1232–1241. [Google Scholar] [CrossRef]
- Fathi, H.; Gholipour, A.; Ebrahimzadeh, M.A.; Yasari, E.; Ahanjan, M.; Parsi, B. In-vitro evaluation of the antioxidant potential, total phenolic and flavonoid contents and antibacterial activity of lamium album extracts. Int. J. Pharm. Sci. Res. 2018, 9, 4210–4219. [Google Scholar]
- Al-Huqail, A.A.; Behiry, S.I.; Salem, M.Z.; Ali, H.M.; Siddiqui, M.H.; Salem, A.Z. Antifungal, Antibacterial, and Antioxidant Activities of Acacia Saligna (Labill.) HL Wendl. Flower Extract: HPLC Analysis of Phenolic and Flavonoid Compounds. Molecules 2019, 24, 700. [Google Scholar] [CrossRef]
- Andriana, Y.; Xuan, T.D.; Quy, T.N.; Minh, T.N.; Van, T.M.; Viet, T.D. Antihyperuricemia, Antioxidant, and Antibacterial Activities of Tridax procumbens L. Foods 2019, 8, 21. [Google Scholar] [CrossRef]
- Metoui, M.; Essid, A.; Bouzoumita, A.; Ferchichi, A. Chemical Composition, Antioxidant and Antibacterial Activity of Tunisian Date Palm Seed. Polish J. Environ. Stud. 2019, 28, 267–274. [Google Scholar] [CrossRef]
- Olleik, H.; Yahiaoui, S.; Roulier, B.; Courvoisier-Dezord, E.; Perrier, J.; Pérès, B.; Hijazi, A.; Baydoun, E.; Raymond, J.; Boumendjel, A. Aurone derivatives as promising antibacterial agents against resistant Gram-positive pathogens. Eur. J. Med. Chem. 2019, 165, 133–141. [Google Scholar] [CrossRef]
- Bashyal, P.; Parajuli, P.; Pandey, R.P.; Sohng, J.K. Microbial Biosynthesis of Antibacterial Chrysoeriol in Recombinant Escherichia coli and Bioactivity Assessment. Catalysts 2019, 9, 112. [Google Scholar] [CrossRef]
- Richwagen, N.; Lyles, J.T.; Dale, B.; Quave, C.L.; Dale, B.L.F. Antibacterial activity of Kalanchoe mortagei and K. fedtschenkoi against ESKAPE pathogens. Front. Pharmacol. 2019, 10, 67. [Google Scholar] [CrossRef]
- Jarial, R.; Thakur, S.; Sakinah, M.; Zularisam, A.; Sharad, A.; Kanwar, S.; Singh, L. Potent anticancer, antioxidant and antibacterial activities of isolated flavonoids from Asplenium nidus. J. King Saud Univ. Sci. 2018, 30, 185–192. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sundaramurthi, J.C.; Sarada, D.V. Antibacterial activity of flavonoid isolated from Trianthema decandra against Pseudomonas aeruginosa and molecular docking study of FabZ. Microb. Pathog. 2018, 121, 87–92. [Google Scholar] [CrossRef]
- Loon, Y.K.; Satari, M.H.; Dewi, W. Antibacterial effect of pineapple (Ananas comosus) extract towards Staphylococcus aureus. Padjadjaran J. Dent. 2018, 30, 30. [Google Scholar] [CrossRef]
- Dzoyem, J.; Tchamgoue, J.; Tchouankeu, J.; Kouam, S.; Choudhary, M.; Bakowsky, U. Antibacterial activity and cytotoxicity of flavonoids compounds isolated from Pseudarthria hookeri Wight & Arn.(Fabaceae). S. Afr. J. Bot. 2018, 114, 100–103. [Google Scholar]
- Matsumoto, T.; Kaneko, A.; Koseki, J.; Matsubara, Y.; Aiba, S.; Yamasaki, K. Pharmacokinetic Study of Bioactive Flavonoids in the Traditional Japanese Medicine Keigairengyoto Exerting Antibacterial Effects against Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 328. [Google Scholar] [CrossRef] [PubMed]
- Aleebrahim-Dehkordy, E.; Rafieian-Kopaei, M.; Amini-Khoei, H.; Abbasi, S. In Vitro Evaluation of Antioxidant Activity and Antibacterial Effects and Measurement of Total Phenolic and Flavonoid Contents of Quercus brantii L. Fruit Extract. J. Diet. Suppl. 2018, 16, 408–416. [Google Scholar] [CrossRef]
- Sujatha, R.; Siva, D.; Nawas, P. Screening of phytochemical profile and antibacterial activity of various solvent extracts of marine algae Sargassum swartzii. World Sci. News 2019, 115, 27–40. [Google Scholar]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-Etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-Ray, A.; Vita, J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Madana, R.M.; Gogoi, R.; Barua, C.C. Chemopreventive and therapeutic potential of chrysin in cancer: Mechanistic perspectives. Toxicol. Lett. 2015, 233, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Babu, P.V.A.; Liu, D.; Gilbert, E.R. Recent advances in understanding the anti-diabetic actions of dietary flavonoids. J. Nutr. Biochem. 2013, 24, 1777–1789. [Google Scholar] [CrossRef] [PubMed]
- Latif, R. Chocolate/cocoa and human health: A review. Neth. J. Med. 2013, 71, 63–68. [Google Scholar]
- Milea, Ș.-A.; Aprodu, I.; Vasile, A.M.; Barbu, V.; Râpeanu, G.; Bahrim, G.E.; Stănciuc, N. Widen the functionality of flavonoids from yellow onion skins through extraction and microencapsulation in whey proteins hydrolysates and different polymers. J. Food Eng. 2019, 251, 29–35. [Google Scholar] [CrossRef]
- Pucciarini, L.; Ianni, F.; Petesse, V.; Pellati, F.; Brighenti, V.; Volpi, C.; Gargaro, M.; Natalini, B.; Clementi, C.; Sardella, R. Onion (Allium cepa L.) Skin: A Rich Resource of Biomolecules for the Sustainable Production of Colored Biofunctional Textiles. Molecules 2019, 24, 634. [Google Scholar] [CrossRef]
- Yang, S.J.; Paudel, P.; Shrestha, S.; Seong, S.H.; Jung, H.A.; Choi, J.S. In vitro protein tyrosine phosphatase 1B inhibition and antioxidant property of different onion peel cultivars: A comparative study. Food Sci. Nutr. 2019, 7, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018. [Google Scholar] [CrossRef]
- Mojica, L.; Berhow, M.; Gonzalez de Mejia, E. Black bean anthocyanin-rich extracts as food colorants: Physicochemical stability and antidiabetes potential. Food Chem. 2017, 229, 628–639. [Google Scholar] [CrossRef]
- George, S.; Ajikumaran Nair, S.; Johnson, A.J.; Venkataraman, R.; Baby, S. O-prenylated flavonoid, an antidiabetes constituent in Melicope lunu-ankenda. J. Ethnopharmacol. 2015, 168, 158–163. [Google Scholar] [CrossRef]
- Akhtar, S.; Rauf, A.; Imran, M.; Qamar, M.; Riaz, M.; Mubarak, M.S. Black carrot (Daucus carota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends Food Sci. Technol. 2017, 66, 36–47. [Google Scholar] [CrossRef]
- Nyane, N.A.; Tlaila, T.B.; Malefane, T.G.; Ndwandwe, D.E.; Owira, P.M.O. Metformin-like antidiabetic, cardio-protective and non-glycemic effects of naringenin: Molecular and pharmacological insights. Eur. J. Pharmacol. 2017, 803, 103–111. [Google Scholar] [CrossRef]
- El-Sherei, M.M.; Ragheb, A.Y.; Kassem, M.E.S.; Marzouk, M.M.; Mosharrafa, S.A.; Saleh, N.A.M. Phytochemistry, biological activities and economical uses of the genus Sterculia and the related genera: A reveiw. Asian Pacific J. Trop. Dis. 2016, 6, 492–501. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phytother. Res. 2019, 33, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Ajebli, M.; Eddouks, M. Flavonoid-enriched extract from desert plant Warionia saharae improves glucose and cholesterol levels in diabetic rats. Cardiovasc. Hematol. Agents Med. Chem. 2019, 17, 28–39. [Google Scholar] [CrossRef]
- Shi, F.; Wei, Z.; Zhao, Y.; Xu, X. Nanostructured lipid carriers loaded with baicalin: An efficient carrier for enhanced antidiabetic effects. Pharmacogn. Mag. 2016, 12, 198. [Google Scholar]
- Shams-Rad, S.; Mohammadi, M.; Ramezani-Jolfaie, N.; Zarei, S.; Mohsenpour, M.; Salehi-Abargouei, A. Hesperidin supplementation has no effect on blood glucose control: A systematic review and meta-analysis of randomized controlled clinical trials. British J. Clin. Pharmacol. 2020, 86, 13–22. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Adam, A.Z.; Lee, S.Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herbal Med. 2017, 10, 37–44. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Wu, J.-S.; Wu, R.-J.; Han, N.-R.-C.-K.-T.; Dai, N.-Y.-T. Two new flavonoids from Artemisa sacrorum Ledeb and their antifungal activity. J. Mol. Struct. 2015, 1088, 34–37. [Google Scholar] [CrossRef]
- Peralta, M.A.; da Silva, M.A.; Ortega, M.G.; Cabrera, J.L.; Paraje, M.G. Antifungal activity of a prenylated flavonoid from Dalea elegans against Candida albicans biofilms. Phytomedicine 2015, 22, 975–980. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).