A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro
Abstract
:1. Introduction
2. Results
2.1. LC-MS Analysis of Scabiosa atropurpurea Leaves
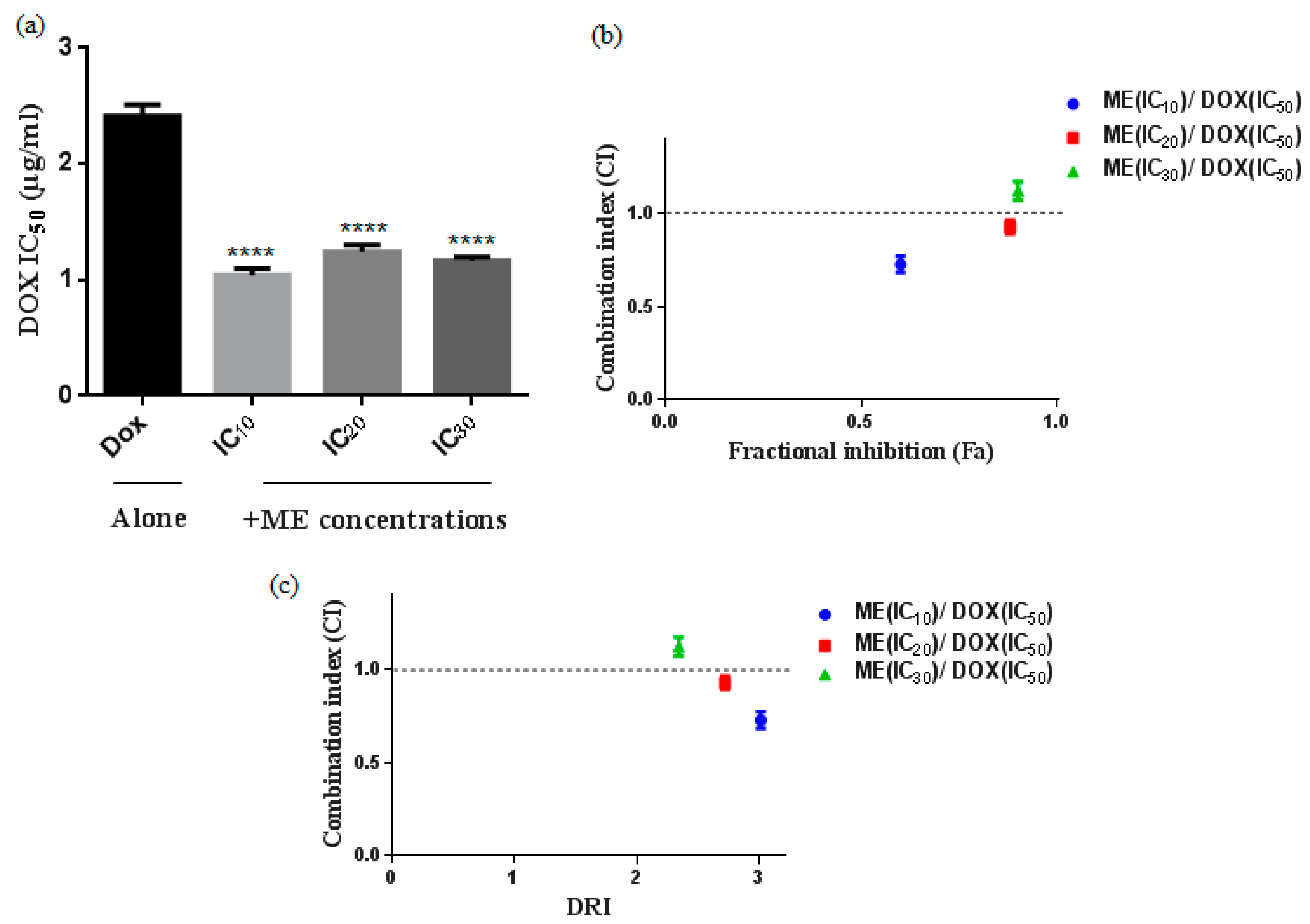
2.2. S. atropurpurea Enhances Dox Cytotoxicity against Caco-2 MDR Colorectal Cancer
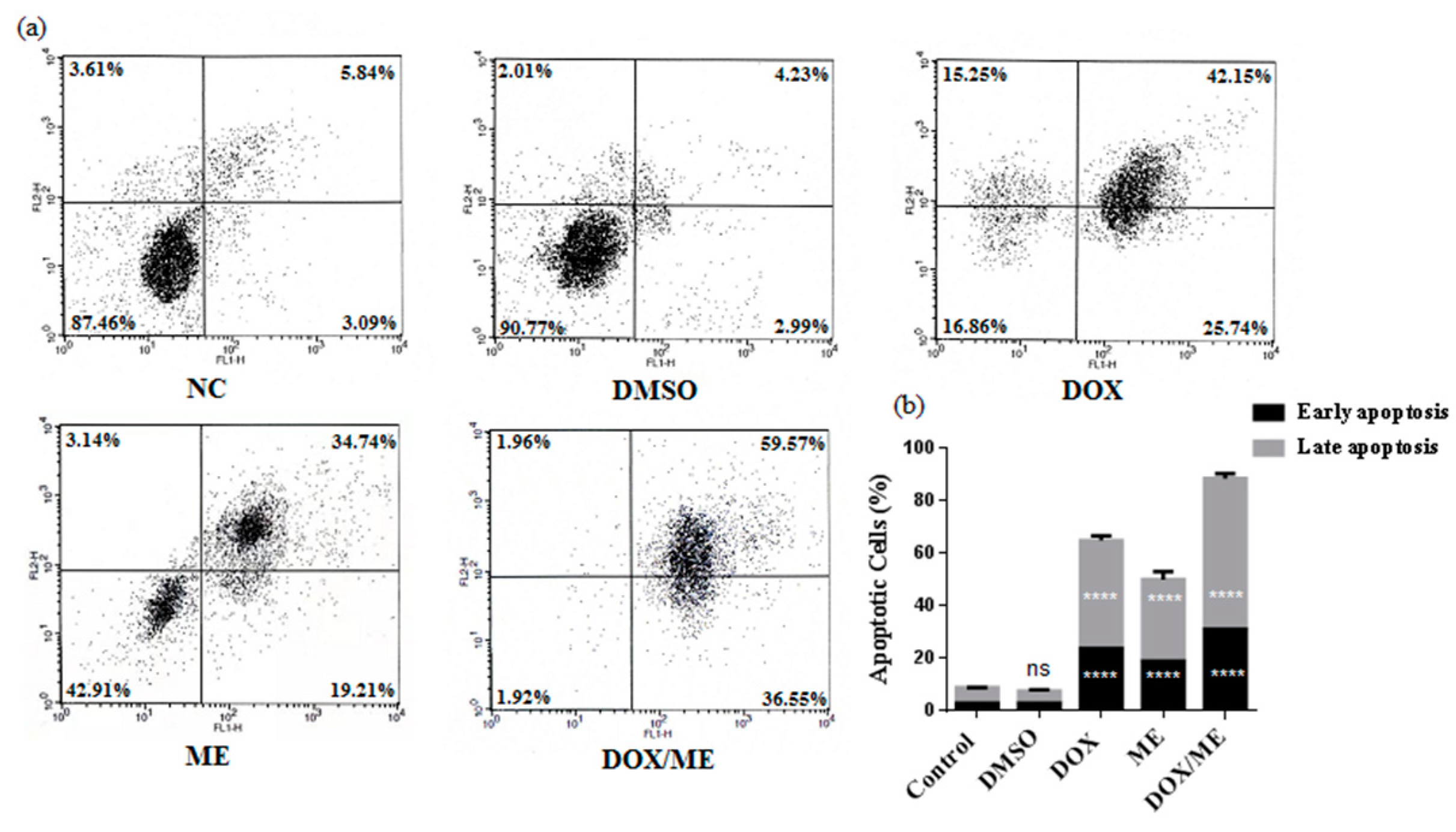
2.3. Apoptotic Effect of ME and Dox against Caco-2 cells
2.4. Multicaspase Assay
2.5. Quantitative Real Time PCR (qPCR) for Bax, Bcl-2, Casp-3 and p21 Expression in Caco-2 Cells
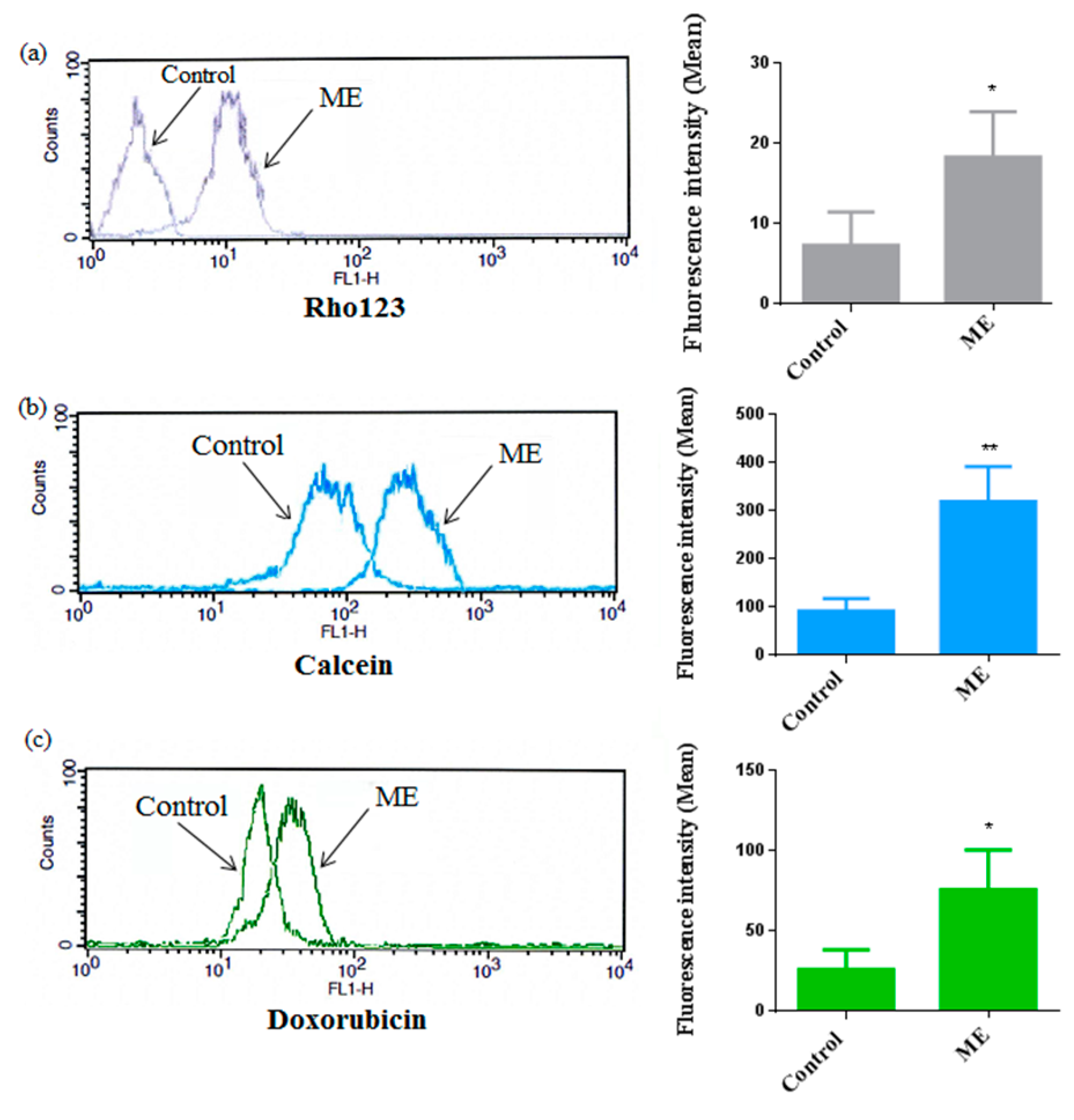
2.6. ME Inhibited ABC Transporter Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Phytochemical Analysis
4.4. MTT Assay
4.5. Drug Combination Assays
4.6. Annexin-V/PI Double-Staining Analysis of Apoptotic Cells
4.7. Multicaspase Assay
4.8. Quantitative Reverse Transcription Real-Time PCR (RT-qPCR)
4.9. ABC Transporter Activity
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ferlay, J.; Steliarova-Foucher, E.; Lortet-Tieulent, J.; Rosso, S.; Coebergh, J.W.W.; Comber, H.; Forman, D.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries in 2012. Eur. J. Cancer 2013, 49, 1374–1403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Lin, S.-R.; Chang, C.-H.; Hsu, C.-F.; Tsai, M.-J.; Cheng, H.; Leong, M.K.; Sung, P.-J.; Chen, J.-C.; Weng, C.-F. Natural compounds as potential adjuvants to cancer therapy: Preclinical evidence. Br. J. Pharmacol. 2020, 177, 1409–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled drug delivery vehicles for cancer treatment and their performance. Signal Transduct. Target. Ther. 2018, 3, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Glasgow, M.D.K.; Chougule, M.B. Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. J. Biomed. Nanotechnol. 2015, 11, 1859–1898. [Google Scholar] [CrossRef]
- Liboiron, B.D.; Mayer, L.D. Nanoscale particulate systems for multidrug delivery: Towards improved combination chemotherapy. Ther. Deliv. 2014, 5, 149–171. [Google Scholar] [CrossRef]
- Zhou, J.-X.; Wink, M. Reversal of multidrug resistance in human colon cancer and human leukemia cells by three plant extracts and their major secondary metabolites. Medicines 2018, 5, 123. [Google Scholar] [CrossRef] [Green Version]
- Pinto, D.C.G.A.; Rahmouni, N.; Beghidja, N.; Silva, A.M.S. Scabiosa Genus: A Rich Source of Bioactive Metabolites. Medicines 2018, 5, 110. [Google Scholar] [CrossRef] [Green Version]
- Elhawary, S.; Eltantawy, M.; Sleem, A.A.; Abdallah, H.; Mohamed, N.M. Investigation of phenolic content and biological activities of Scabiosa atropurpurea L. World Appl. Sci. J. 2011, 15, 311–317. [Google Scholar]
- El Gueder, D.; Maatouk, M.; Kalboussi, Z.; Daouefi, Z.; Chaaban, H.; Ioannou, I.; Ghedira, K.; Ghedira, L.C.; Luis, J. Heat processing effect of luteolin on anti-metastasis activity of human glioblastoma cells U87. Environ. Sci. Pollut. Res. Int. 2018, 25, 36545–36554. [Google Scholar] [CrossRef]
- Hernandes, L.C.; Machado, A.R.T.; Tuttis, K.; Ribeiro, D.L.; Aissa, A.F.; Dévoz, P.P.; Antunes, L.M.G. Caffeic acid and chlorogenic acid cytotoxicity, genotoxicity and impact on global DNA methylation in human leukemic cell lines. Genet. Mol. Biol. 2020, 43, e20190347. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, M.A.; Sobeh, M.; Rezq, S.; El-Shazly, A.M.; Mahmoud, M.F.; Wink, M. HPLC-ESI-MS/MS profiling of polyphenolics of a leaf extract from Alpinia zerumbet (Zingiberaceae) and its anti-inflammatory, anti-nociceptive, and antipyretic activities in vivo. Molecules 2018, 23, 3238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.-C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sioud, F.; Amor, S.; Toumia, I.B.; Lahmar, A.; Aires, V.; Chekir-Ghedira, L.; Delmas, D. A New Highlight of Ephedra alata Decne Properties as Potential Adjuvant in Combination with Cisplatin to Induce Cell Death of 4T1 Breast Cancer Cells In Vitro and In Vivo. Cells 2020, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sioud, F.; Ben Toumia, I.; Lahmer, A.; Khlifi, R.; Dhaouefi, Z.; Maatouk, M.; Ghedira, K.; Chekir-Ghedira, L. Methanolic extract of Ephedra alata ameliorates cisplatin-induced nephrotoxicity and hepatotoxicity through reducing oxidative stress and genotoxicity. Environ. Sci. Pollut. Res. Int. 2020, 27, 12792–12801. [Google Scholar] [CrossRef]
- Lehbili, M.; Alabdul Magid, A.; Hubert, J.; Kabouche, A.; Voutquenne-Nazabadioko, L.; Renault, J.-H.; Nuzillard, J.-M.; Morjani, H.; Abedini, A.; Gangloff, S.C.; et al. Two new bis-iridoids isolated from Scabiosa stellata and their antibacterial, antioxidant, anti-tyrosinase and cytotoxic activities. Fitoterapia 2018, 125, 41–48. [Google Scholar] [CrossRef]
- Eid, S.Y.; El-Readi, M.Z.; Wink, M. Synergism of three-drug combinations of sanguinarine and other plant secondary metabolites with digitonin and doxorubicin in multi-drug resistant cancer cells. Phytomedicine 2012, 19, 1288–1297. [Google Scholar] [CrossRef]
- Nakagawa, S.; Shiraishi, T.; Kihara, S.; Tabuchi, K. Detection of DNA strand breaks associated with apoptosis in human brain tumors. Virchows Arch. 1995, 427, 175–179. [Google Scholar] [CrossRef]
- Joselin, A.P.; Schulze-Osthoff, K.; Schwerk, C. Loss of Acinus inhibits oligonucleosomal DNA fragmentation but not chromatin condensation during apoptosis. J. Biol. Chem. 2006, 281, 12475–12484. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Kwok, R.T.K.; Liu, J.; Xing, B.; Tang, B.Z.; Liu, B. Real-time monitoring of cell apoptosis and drug screening using fluorescent light-up probe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Z.-Q.; Li, M.-H.; Qin, Y.-M.; Jiang, H.-Y.; Zhang, X.; Wu, M.-H. Luteolin inhibits tumorigenesis and induces apoptosis of non-small cell lung cancer cells via regulation of MicroRNA-34a-5p. Int. J. Mol. Sci. 2018, 19, 447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.-Y.; Chen, M.-H.; May, B.H.; Liao, X.-Z.; Liu, J.-H.; Tao, L.-T.; Man-Yuen Sze, D.; Zhang, A.L.; Mo, S.-L. Matrine induces apoptosis in multiple colorectal cancer cell lines in vitro and inhibits tumour growth with minimum side effects in vivo via Bcl-2 and caspase-3. Phytomedicine 2018, 51, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef] [PubMed]
- Adel, N.; El-Mesery, M.; El-Karef, A.; Eissa, L.; Gayar, A. Chlorogenic acid potentiates antitumor effect of doxorubicin through upregulation of death receptors in solid Ehrlich carcinoma model in mice. Egypt. J. Basic Appl. Sci. 2019, 6, 158–172. [Google Scholar]
- Pezzani, R.; Salehi, B.; Vitalini, S.; Iriti, M.; Zuñiga, F.A.; Sharifi-Rad, J.; Martorell, M.; Martins, N. Synergistic effects of plant derivatives and conventional chemotherapeutic agents: An update on the cancer perspective. Medicina 2019, 55, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of arctigenin and matairesinol against the T-cell lymphoma cell line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Eid, S.; Ashour, M.L.; Tahrani, A.; Wink, M. Modulation of multidrug resistance in cancer cells by chelidonine and Chelidonium majus alkaloids. Phytomedicine 2013, 20, 282–294. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds and the plant materials are available from the authors. |
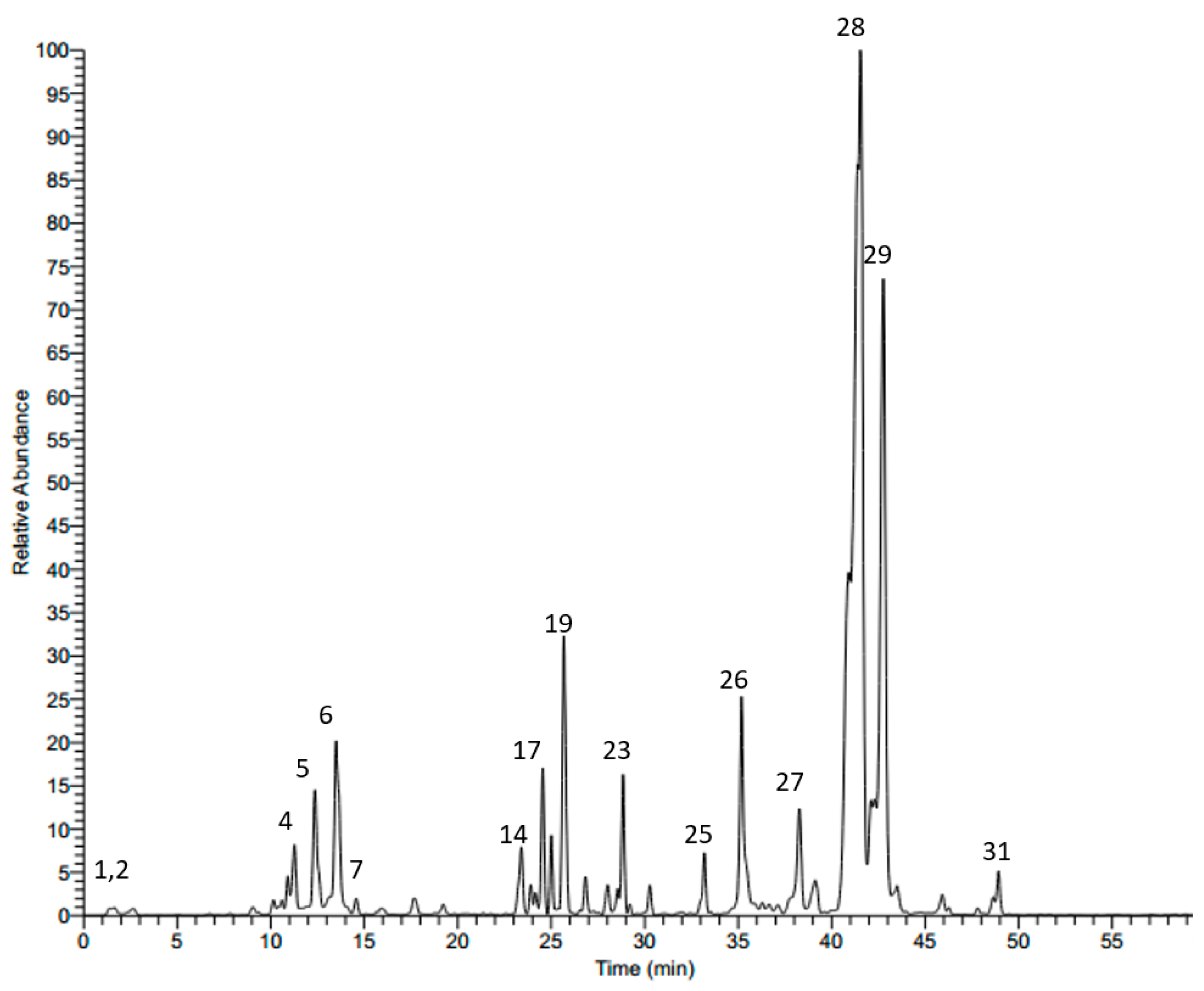



| No. | Rt | M − H | MS/MS | Tentatively Identified Compound |
|---|---|---|---|---|
| 1 | 1.43 | 191 | 127, 173 | Quinic acid a |
| 2 | 7.71 | 315 | 153 | Protocatechuic acid 3-glucoside a |
| 3 | 10.35 | 325 | 119, 163 | p-Coumaric acid 3-glucoside a |
| 4 | 11.28 | 353 | 179, 191 | Neochlorogenic acid |
| 5 | 12.46 | 353 | 179, 191 | Cryptochlorogenic acid |
| 6 | 13.53 | 353 | 179, 191 | Chlorogenic acid a,b |
| 7 | 14.28 | 373 | 149, 193 | Ferrulic acid derivative |
| 8 | 15.82 | 625 | 301, 463 | Quercetin diglucoside |
| 9 | 16.07 | 337 | 119, 163, 191 | p-Coumeroylquinic acid a |
| 10 | 17.12 | 609 | 285, 327, 447 | Luteolin-7,3-diglucoside a |
| 11 | 17.52 | 625 | 179, 255, 301, 463 | Quercetin 3,4’-diglucoside a |
| 12 | 17.62 | 367 | 127, 173, 191 | 3-O-Caffeoylquinic acid methyl ester |
| 13 | 19.16 | 609 | 285, 327, 447 | Luteolin-7,3’-diglucoside a |
| 14 | 22.95 | 515 | 353 | 3,4-Dicaffeoylquinic acid |
| 15 | 23.35 | 579 | 285, 447 | Luteolin-pentosyl-glucoside |
| 16 | 23.89 | 579 | 285, 447 | Luteolin-pentosyl-glucoside |
| 17 | 24.43 | 515 | 179, 353 | 3,5-Dicaffeoylquinic acid |
| 18 | 25.05 | 593 | 285, 447 | Luteolin 7-rutinoside a |
| 19 | 25.71 | 447 | 285 | Luteolin 7-O-β-d-glucoside b |
| 20 | 26.88 | 447 | 179, 285 | Luteolin 3’-glucoside b |
| 21 | 27.79 | 593 | 299 | Diosmetin pentosyl-glucoside |
| 22 | 28.12 | 745 | 583 | Cantleyoside |
| 23 | 28.60 | 431 | 269 | Apigenin 7-glucoside a |
| 24 | 30.28 | 461 | 299 | Diosmetin-7-O-glucoside |
| 25 | 33.18 | 489 | 285, 447 | Luteolin acetylglucoside |
| 26 | 35.22 | 285 | 151 | Luteolin b |
| 27 | 38.48 | 911 | 471, 603, 749 | Maslinic acid-pentosyl-rhamnosyl-glucoside |
| 28 | 41.24 | 1351 | 455, 587, 733, 865, 1027 | Oleanolic acid-pentosyl-rhamnosyl-pentosyl-glucosyl-di-glucoside |
| 29 | 41.70 | 1381 | 455, 587, 733, 895, 1057 | Oleanolic acid-pentosyl-rhamnosyl-glucosyl-glucosyl-di-glucoside |
| 30 | 46.15 | 537 | 375 | Loganic acid glucoside |
| 31 | 49.11 | 537 | 375 | Loganic acid galactoside |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Toumia, I.; Sobeh, M.; Ponassi, M.; Banelli, B.; Dameriha, A.; Wink, M.; Chekir Ghedira, L.; Rosano, C. A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules 2020, 25, 5265. https://doi.org/10.3390/molecules25225265
Ben Toumia I, Sobeh M, Ponassi M, Banelli B, Dameriha A, Wink M, Chekir Ghedira L, Rosano C. A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules. 2020; 25(22):5265. https://doi.org/10.3390/molecules25225265
Chicago/Turabian StyleBen Toumia, Imene, Mansour Sobeh, Marco Ponassi, Barbara Banelli, Anas Dameriha, Michael Wink, Leila Chekir Ghedira, and Camillo Rosano. 2020. "A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro" Molecules 25, no. 22: 5265. https://doi.org/10.3390/molecules25225265
APA StyleBen Toumia, I., Sobeh, M., Ponassi, M., Banelli, B., Dameriha, A., Wink, M., Chekir Ghedira, L., & Rosano, C. (2020). A Methanol Extract of Scabiosa atropurpurea Enhances Doxorubicin Cytotoxicity against Resistant Colorectal Cancer Cells In Vitro. Molecules, 25(22), 5265. https://doi.org/10.3390/molecules25225265








