Signalling Properties of Inositol Polyphosphates
Abstract
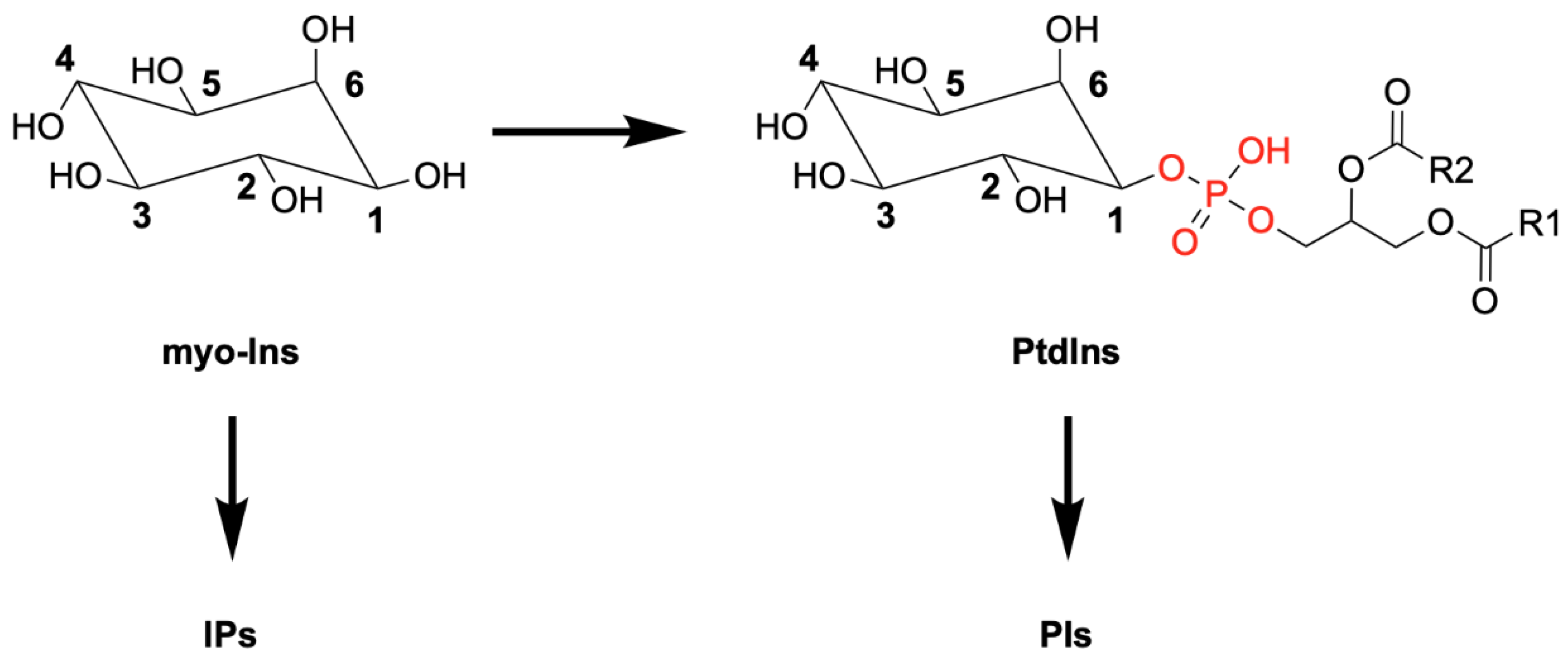
1. Introduction
2. Ins(1,4,5)P3, a Bona Fide Second Messenger
3. Intracellular Processes Regulated by IPs Binding to Proteins
3.1. Endo and Exocytosis
3.2. Nuclear Functions
3.3. Platelet Aggregation
3.4. Reactive Oxygen Species (ROS) Formation and Drug Sensitivity
3.5. Viral Replication
4. Exploiting the Binding Properties of IPs for Therapeutic Purposes
4.1. Exogenous IPs
4.2. Interfering with IPs/Proteins Binding
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Michell, R.H. Inositol derivatives: Evolution and functions. Nat. Rev. Mol. Cell Biol. 2008, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Berrie, C.P.; Falasca, M. Patterns within protein/polyphosphoinositide interactions provide specific targets for therapeutic intervention. FASEB J. 2000, 14, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Resnick, A.C. Inositol polyphosphate multikinase: Metabolic architect of nuclear inositides. Front. Biosci. 2008, 13, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Odom, A.R.; Stahlberg, A.; Wente, S.R.; York, J.D. A Role for Nuclear Inositol 1,4,5-Trisphosphate Kinase in Transcriptional Control. Science 2000, 287, 2026–2029. [Google Scholar] [CrossRef] [PubMed]
- Nalaskowski, M.M.; Deschermeier, C.; Fanick, W.; Mayr, G.W. The human homologue of yeast ArgRIII protein is an inositol phosphate multikinase with predominantly nuclear localization. Biochem. J. 2002, 366, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-C.; Miller, A.L.; Feng, Y.; Wente, S.R.; Majerus, P.W. The Human Homolog of the Rat Inositol Phosphate Multikinase Is an Inositol 1,3,4,6-Tetrakisphosphate 5-Kinase. J. Biol. Chem. 2002, 277, 43836–43843. [Google Scholar] [CrossRef]
- Frederick, J.P.; Mattiske, D.; Wofford, J.A.; Megosh, L.C.; Drake, L.Y.; Chiou, S.-T.; Hogan, B.L.M.; York, J.D. An essential role for an inositol polyphosphate multikinase, Ipk2, in mouse embryogenesis and second messenger production. Proc. Natl. Acad. Sci. USA 2005, 102, 8454–8459. [Google Scholar] [CrossRef]
- Leyman, A.; Pouillon, V.; Bostan, A.; Schurmans, S.; Erneux, C.; Pesesse, X. The absence of expression of the three isoenzymes of the inositol 1,4,5-trisphosphate 3-kinase does not prevent the formation of inositol pentakisphosphate and hexakisphosphate in mouse embryonic fibroblasts. Cell. Signal. 2007, 19, 1497–1504. [Google Scholar] [CrossRef]
- Desfougères, Y.; Wilson, M.S.C.; Laha, D.; Miller, G.J.; Saiardi, A. ITPK1 mediates the lipid-independent synthesis of inositol phosphates controlled by metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 24551–24561. [Google Scholar] [CrossRef]
- Dovey, C.M.; Diep, J.; Clarke, B.P.; Hale, A.T.; McNamara, D.E.; Guo, H.; Brown, N.W.; Cao, J.Y.; Grace, C.R.; Gough, P.J.; et al. MLKL Requires the Inositol Phosphate Code to Execute Necroptosis. Mol. Cell 2018, 70, 936–948. [Google Scholar] [CrossRef]
- Lee, S.; Kim, M.G.; Ahn, H.; Kim, S. Inositol Pyrophosphates: Signaling Molecules with Pleiotropic Actions in Mammals. Molecules 2020, 25, 2208. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Rapid accumulation of inositol trisphosphate reveals that agonists hydrolyse polyphosphoinositides instead of phosphatidylinositol. Biochem. J. 1983, 212, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J. Inositol trisphosphate and diacylglycerol as second messengers. Biochem. J. 1984, 220, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Pedi, L.; Diver, M.M.; Long, S.B. Crystal structure of the calcium release-activated calcium channel Orai. Science 2012, 338, 1308–1313. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.; Lewis, R.S. Physiological CRAC channel activation and pore properties require STIM1 binding to all six Orai1 subunits. J. Gen. Physiol. 2018, 150, 1373–1385. [Google Scholar] [CrossRef] [PubMed]
- Prole, D.L.; Taylor, C.W. Structure and Function of IP3 Receptors. Cold Spring Harb. Perspect. Biol. 2019, 11, a035063. [Google Scholar] [CrossRef]
- Clapham, D.E. A STIMulus Package puts orai calcium channels to work. Cell 2009, 136, 814–816. [Google Scholar] [CrossRef][Green Version]
- Macbeth, M.R.; Schubert, H.L.; VanDeMark, A.P.; Lingam, A.T.; Hill, C.P.; Bass, B.L. Inositol Hexakisphosphate Is Bound in the ADAR2 Core and Required for RNA Editing. Science 2005, 309, 1534–1539. [Google Scholar] [CrossRef]
- Wang, Q.; Vogan, E.M.; Nocka, L.M.; Rosen, C.E.; Zorn, J.A.; Harrison, S.C.; Kuriyan, J. Autoinhibition of Bruton’s tyrosine kinase (Btk) and activation by soluble inositol hexakisphosphate. eLife 2015, 4, e06074. [Google Scholar] [CrossRef]
- Lee, W.-K.; Son, S.H.; Jin, B.-S.; Na, J.-H.; Kim, S.-Y.; Kim, K.-H.; Kim, E.E.; Yu, Y.G.; Lee, H.H. Structural and functional insights into the regulation mechanism of CK2 by IP6 and the intrinsically disordered protein Nopp140. Proc. Natl. Acad. Sci. USA 2013, 110, 19360–19365. [Google Scholar] [CrossRef]
- Zhang, Z.-M.; Ma, K.-W.; Yuan, S.; Luo, Y.; Jiang, S.; Hawara, E.; Pan, S.; Ma, W.; Song, J. Structure of a pathogen effector reveals the enzymatic mechanism of a novel acetyltransferase family. Nat. Struct. Mol. Biol. 2016, 23, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Scherer, P.C.; Ding, Y.; Liu, Z.; Xu, J.; Mao, H.; Barrow, J.C.; Wei, N.; Zheng, N.; Snyder, S.H.; Rao, F. Inositol hexakisphosphate (IP6) generated by IP5K mediates cullin-COP9 signalosome interactions and CRL function. Proc. Natl. Acad. Sci. USA 2016, 113, 3503–3508. [Google Scholar] [CrossRef] [PubMed]
- Kelly, R.D.W.; Chandru, A.; Watson, P.J.; Song, Y.; Blades, M.; Robertson, N.S.; Jamieson, A.G.; Schwabe, J.W.R.; Cowley, S.M. Histone deacetylase (HDAC) 1 and 2 complexes regulate both histone acetylation and crotonylation in vivo. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Blind, R.D. Structural analyses of inositol phosphate second messengers bound to signaling effector proteins. Adv. Biol. Regul. 2020, 75, 100667. [Google Scholar] [CrossRef] [PubMed]
- Voglmaier, S.M.; Keen, J.H.; Murphy, J.-E.; Ferris, C.D.; Prestwich, G.D.; Snyder, S.H.; Theibert, A.B. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem. Biophys. Res. Commun. 1992, 187, 158–163. [Google Scholar] [CrossRef]
- Norris, F.A.; Ungewickell, E.; Majerus, P.W. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP-3/AP180) and inhibits clathrin cage assembly in vitro. J Biol Chem. 1995, 270, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K.; Pulvermüller, A.; Buczylko, J.; Gutmann, C.; Hofmann, K.P. Binding of inositol phosphates to arrestin. FEBS Lett. 1991, 295, 195–199. [Google Scholar] [CrossRef]
- Larsson, O. Inhibition of Phosphatases and Increased Ca2+ Channel Activity by Inositol Hexakisphosphate. Science 1997, 278, 471–474. [Google Scholar] [CrossRef]
- Efanov, A.M.; Zaitsev, S.V.; Berggren, P.-O. Inositol hexakisphosphate stimulates non-Ca2+-mediated and primes Ca2+-mediated exocytosis of insulin by activation of protein kinase C. Proc. Natl. Acad. Sci. USA 1997, 94, 4435–4439. [Google Scholar] [CrossRef]
- Høy, M.; Efanov, A.M.; Bertorello, A.M.; Zaitsev, S.V.; Olsen, H.L.; Bokvist, K.; Leibiger, B.; Leibiger, I.B.; Zwiller, J.; Berggren, P.-O.; et al. Inositol hexakisphosphate promotes dynamin I- mediated endocytosis. Proc. Natl. Acad. Sci. USA 2002, 99, 6773–6777. [Google Scholar] [CrossRef]
- Barker, C.J.; Berggren, P.-O. New Horizons in Cellular Regulation by Inositol Polyphosphates: Insights from the Pancreaticβ-Cell. Pharmacol. Rev. 2013, 65, 641–669. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, S.S.; Kim, J.; Gaboardi, G.-C.; Gromada, J.; Shears, S.B.; Dos Santos, K.T.; Nolasco, E.L.; Ferreira, S.D.S.; Illies, C.; Köhler, M.; et al. Inositol hexakisphosphate kinase 1 is a metabolic sensor in pancreatic β-cells. Cell. Signal. 2018, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hilton, J.M.; Plomann, M.; Ritter, B.; Modregger, J.; Freeman, H.N.; Falck, J.R.; Krishna, U.M.; Tobin, A.B. Phosphorylation of a Synaptic Vesicle-associated Protein by an Inositol Hexakisphosphate-regulated Protein Kinase. J. Biol. Chem. 2001, 276, 16341–16347. [Google Scholar] [CrossRef] [PubMed]
- De Ruijter, A.J.; Van Gennip, A.H.; Caron, H.N.; Kemp, S.; Van Kuilenburg, A.B. Histone deacetylases (HDACs): Characterization of the classical HDAC family. Biochem. J. 2003, 370, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.J.; Fairall, L.; Santos, G.M.; Schwabe, J.W.R. Structure of HDAC3 bound to co-repressor and inositol tetraphosphate. Nat. Cell Biol. 2012, 481, 335–340. [Google Scholar] [CrossRef]
- Millard, C.J.; Watson, P.J.; Celardo, I.; Gordiyenko, Y.; Cowley, S.M.; Robinson, C.V.; Fairall, L.; Schwabe, J.W.R. Class I HDACs Share a Common Mechanism of Regulation by Inositol Phosphates. Mol. Cell 2013, 51, 57–67. [Google Scholar] [CrossRef]
- Watson, P.J.; Millard, C.J.; Riley, A.M.; Robertson, N.S.; Wright, L.C.; Godage, H.Y.; Cowley, S.M.; Jamieson, A.G.; Potter, B.V.L.; Schwabe, J.W.R. Insights into the activation mechanism of class I HDAC complexes by inositol phosphates. Nat. Commun. 2016, 7, 11262. [Google Scholar] [CrossRef]
- Jamaladdin, S.; Kelly, R.D.W.; O’Regan, L.; Dovey, O.M.; Hodson, G.E.; Millard, C.J.; Portolano, N.; Fry, A.M.; Schwabe, J.W.R.; Cowley, S.M. Histone deacetylase (HDAC) 1 and 2 are essential for accurate cell division and the pluripotency of embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 9840–9845. [Google Scholar] [CrossRef]
- Ouyang, Z.; Zheng, G.; Tomchick, D.R.; Luo, X.; Yu, H. Structural Basis and IP6 Requirement for Pds5-Dependent Cohesin Dynamics. Mol. Cell 2016, 62, 248–259. [Google Scholar] [CrossRef]
- Hanakahi, L.A.; Bartlet-Jones, M.; Chappell, C.; Pappin, D.; West, S.C. Binding of Inositol Phosphate to DNA-PK and Stimulation of Double-Strand Break Repair. Cell 2000, 102, 721–729. [Google Scholar] [CrossRef]
- York, J.D. A Phospholipase C-Dependent Inositol Polyphosphate Kinase Pathway Required for Efficient Messenger RNA Export. Science 1999, 285, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L.; Suntharalingam, M.; Johnson, S.L.; Audhya, A.; Emr, S.D.; Wente, S.R. Cytoplasmic Inositol Hexakisphosphate Production Is Sufficient for Mediating the Gle1-mRNA Export Pathway. J. Biol. Chem. 2004, 279, 51022–51032. [Google Scholar] [CrossRef] [PubMed]
- Alcázar-Román, A.R.; Tran, E.J.; Guo, S.; Wente, S.R. Inositol hexakisphosphate and Gle1 activate the DEAD-box protein Dbp5 for nuclear mRNA export. Nat. Cell Biol. 2006, 8, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xiao, H.; Ranallo, R.; Wu, W.-H. Modulation of ATP-Dependent Chromatin-Remodeling Complexes by Inositol Polyphosphates. Science 2002, 299, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Steger, D.J.; Haswell, E.S.; Miller, A.L.; Wente, S.R.; O’Shea, E.K.; Oòshea, E.K. Regulation of Chromatin Remodeling by Inositol Polyphosphates. Science 2002, 299, 114–116. [Google Scholar] [CrossRef]
- Brehm, M.A.; Klemm, U.; Rehbach, C.; Erdmann, N.; Kolšek, K.; Lin, H.; Aponte-Santamaría, C.; Gräter, F.; Rauch, B.H.; Riley, A.M.; et al. Inositol hexakisphosphate increases the size of platelet aggregates. Biochem. Pharmacol. 2019, 161, 14–25. [Google Scholar] [CrossRef]
- Grint, T.; Riley, A.M.; Mills, S.J.; Potter, B.V.L.; Safrany, S.T. Fibrinogen—A Possible Extracellular Target for Inositol Phosphates. Messenger 2012, 1, 160–166. [Google Scholar] [CrossRef][Green Version]
- Pan, C.; Jin, L.; Wang, X.; Li, Y.; Chun, J.; Boese, A.C.; Li, D.; Kang, H.; Zhang, G.; Zhou, L.; et al. Inositol-triphosphate 3-kinase B confers cisplatin resistance by regulating NOX4-dependent redox balance. J. Clin. Investig. 2019, 129, 2431–2445. [Google Scholar] [CrossRef]
- Erneux, C.; Ghosh, S.; Koenig, S. Inositol(1,4,5)P 3 3-kinase isoenzymes: Catalytic properties and importance of targeting to F-actin to understand function. Adv. Biol. Regul. 2016, 60, 135–143. [Google Scholar] [CrossRef]
- Mallery, D.L.; Faysal, K.R.; Kleinpeter, A.; Wilson, M.S.; Vaysburd, M.; Fletcher, A.J.; Novikova, M.; Böcking, T.; Freed, E.O.; Saiardi, A.; et al. Cellular IP6 Levels Limit HIV Production while Viruses that Cannot Efficiently Package IP6 Are Attenuated for Infection and Replication. Cell Rep. 2019, 29, 3983–3996.e4. [Google Scholar] [CrossRef]
- Dick, R.A.; Zadrozny, K.K.; Xu, C.; Schur, F.K.M.; Lyddon, T.D.; Ricana, C.L.; Wagner, J.M.; Perilla, J.R.; Ganser-Pornillos, B.K.; Johnson, M.C.; et al. Inositol phosphates are assembly co-factors for HIV-1. Nat. Cell Biol. 2018, 560, 509–512. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.A.; Mallery, D.L.; Vogt, V.M.; James, L.C. IP6 Regulation of HIV Capsid Assembly, Stability, and Uncoating. Viruses 2018, 10, 640. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.; Lee, E.M.Y.; Jin, J.; Voth, G.A. Atomic-scale characterization of mature HIV-1 capsid stabilization by inositol hexakisphosphate (IP6). Sci. Adv. 2020, 6, eabc6465. [Google Scholar] [CrossRef] [PubMed]
- Mallery, D.L.; Márquez, C.L.; McEwan, W.A.; Dickson, C.F.; Jacques, D.A.; Anandapadamanaban, M.; Bichel, K.; Towers, G.J.; Saiardi, A.; Böcking, T.; et al. IP6 is an HIV pocket factor that prevents capsid collapse and promotes DNA synthesis. eLife 2018, 7, e35335. [Google Scholar] [CrossRef]
- Ricana, C.L.; Lyddon, T.D.; Dick, R.A.; Johnson, M.C. Primate lentiviruses require Inositol hexakisphosphate (IP6) or inositol pentakisphosphate (IP5) for the production of viral particles. PLoS Pathog. 2020, 16, e1008646. [Google Scholar] [CrossRef] [PubMed]
- Dick, R.A.; Xu, C.; Morado, D.R.; Kravchuk, V.O.; Ricana, C.L.; Lyddon, T.D.; Broad, A.M.; Feathers, J.R.; Johnson, M.C.; Vogt, V.M.; et al. Structures of immature EIAV Gag lattices reveal a conserved role for IP6 in lentivirus assembly. PLoS Pathog. 2020, 16, e1008277. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kanematsu, T.; Misumi, Y.; Sakane, F.; Konishi, H.; Kikkawa, U.; Watanabe, Y.; Katan, M.; Hirata, M. Distinct specificity in the binding of inositol phosphates by pleckstrin homology domains of pleckstrin, RAC-protein kinase, diacylglycerol kinase and a new 130 kDa protein. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 1997, 1359, 275–285. [Google Scholar] [CrossRef]
- Razzini, G.; Ingrosso, A.; Brancaccio, A.; Sciacchitano, S.; Esposito, D.L.; Falasca, M. Different subcellular localization and phosphoinositides binding of insulin receptor substrate protein pleckstrin homology domains. Mol. Endocrinol. 2000, 14, 823–836. [Google Scholar] [CrossRef]
- Lemmon, M.A.; Ferguson, K.M.; O’Brien, R.; Sigler, P.B.; Schlessinger, J. Specific and high-affinity binding of inositol phosphates to an isolated pleckstrin homology domain. Proc. Natl. Acad. Sci. USA 1995, 92, 10472–10476. [Google Scholar] [CrossRef]
- Razzini, G.; Berrie, C.P.; Vignati, S.; Broggini, M.; Mascetta, G.; Brancaccio, A.; Falasca, M. Novel functional PI 3-kinase antagonists inhibit cell growth and tumorigenicity in human cancer cell lines. FASEB J. 2000, 14, 1179–1187. [Google Scholar] [CrossRef]
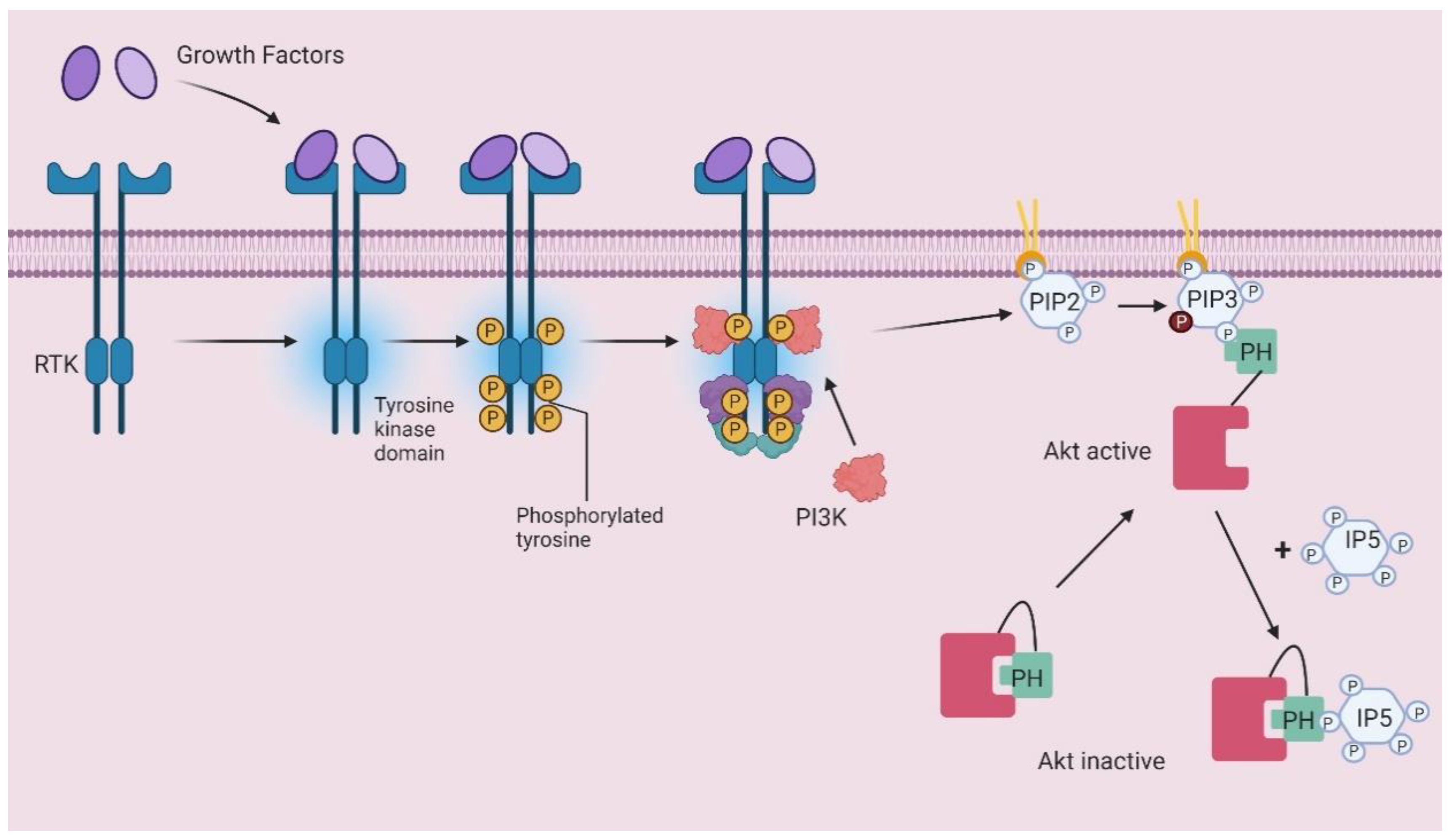
- Jia, Y.; Subramanian, K.K.; Erneux, C.; Pouillon, V.; Hattori, H.; Jo, H.; You, J.; Zhu, D.; Schurmans, S.; Luo, H.R. Inositol 1,3,4,5-Tetrakisphosphate Negatively Regulates Phosphatidylinositol-3,4,5- Trisphosphate Signaling in Neutrophils. Immunity 2007, 27, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, E.; Vignati, S.; Maffucci, T.; Innominato, P.F.; Riley, A.M.; Potter, B.V.L.; Pandolfi, P.P.; Broggini, M.; Iacobelli, S.; Innocenti, P.; et al. Inositol pentakisphosphate promotes apoptosis through the PI 3-K/Akt pathway. Oncogene 2004, 23, 1754–1765. [Google Scholar] [CrossRef]
- Maffucci, T. Inhibition of the Phosphatidylinositol 3-Kinase/Akt Pathway by Inositol Pentakisphosphate Results in Antiangiogenic and Antitumor Effects. Cancer Res. 2005, 65, 8339–8349. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Song, Y.; Wen, Z.; Lu, X.; Cui, L. Inositol Hexaphosphate and Inositol Inhibit Colorectal Cancer Metastasis to the Liver in BALB/c Mice. Nutrients 2016, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I. Anticancer Properties of Inositol Hexaphosphate and Inositol: An Overview. J. Nutr. Sci. Vitaminol. 2019, 65, S18–S22. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, C.; Dhanalakshmi, S.; Singh, R.P.; Agarwal, R. Inositol Hexaphosphate Inhibits Growth and Induces G1 Arrest and Apoptotic Death of Androgen-Dependent Human Prostate Carcinoma LNCaP Cells1. Neoplasia 2004, 6, 646–659. [Google Scholar] [CrossRef][Green Version]
- Gu, M.; Roy, S.; Raina, K.; Agarwal, C.; Agarwal, R. Inositol hexaphosphate suppresses growth and induces apoptosis in prostate carcinoma cells in culture and nude mouse xenograft: PI3K-Akt pathway as potential target. Cancer Res. 2009, 69, 9465–9472. [Google Scholar] [CrossRef]
- Raina, K.; Ravichandran, K.; Rajamanickam, S.; Huber, K.M.; Serkova, N.J.; Agarwal, R. Inositol hexaphosphate inhibits tumor growth, vascularity, and metabolism in TRAMP mice: A multiparametric magnetic resonance study. Cancer Prev. Res. 2012, 6, 40–50. [Google Scholar] [CrossRef]
- Tantivejkul, K.; Vucenik, I.; Eiseman, J.; Shamsuddin, A.M. Inositol hexaphosphate (IP6) enhances the anti-proliferative effects of adriamycin and tamoxifen in breast cancer. Breast Cancer Res. Treat. 2003, 79, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Ramakrishna, G.; Tantivejkul, K.; Anderson, L.M.; Ramljak, D. Inositol hexaphosphate (IP6) blocks proliferation of human breast cancer cells through a PKCdelta-dependent increase in p27Kip1 and decrease in retinoblastoma protein (pRb) phosphorylation. Breast Cancer Res Treat. 2005, 91, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kandzari, S.J.; Riggs, D.; Jackson, B.; Luchey, A.; Oliver, C.; Zaslau, S. In vitro regulation of cell growth and angiogenesis by inositol hexaphosphate in bladder cancer. Curr. Urol. 2013, 6, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yang, F.; Liu, C.; Cui, L.; Fu, M.; Song, Y. Inositol hexaphosphate hydrolysate competitively binds to AKT to inhibit the proliferation of colon carcinoma. Oncol. Rep. 2017, 38, 2901–2910. [Google Scholar] [CrossRef] [PubMed]
- Kapral, M.; Wawszczyk, J.; Jesse, K.; Paul-Samojedny, M.; Kuśmierz, D.; Węglarz, L. Inositol Hexaphosphate Inhibits Proliferation and Induces Apoptosis of Colon Cancer Cells by Suppressing the AKT/mTOR Signaling Pathway. Molecules 2017, 22, 1657. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I.; Shamsuddin, A.M. Cancer Inhibition by Inositol Hexaphosphate (IP6) and Inositol: From Laboratory to Clinic. J. Nutr. 2003, 133, 3778S–3784S. [Google Scholar] [CrossRef]
- Singh, R.P.; Agarwal, R. Inositol hexaphosphate inhibits growth, and induces G1 arrest and apoptotic death of prostate carcinoma DU145 cells: Modulation of CDKI-CDK-cyclin and pRb-related protein-E2F complexes. Carcinogenesis 2003, 24, 555–563. [Google Scholar] [CrossRef]
- Liu, G.; Song, Y.; Cui, L.; Wen, Z.; Lu, X. Inositol hexaphosphate suppresses growth and induces apoptosis in HT-29 colorectal cancer cells in culture: PI3K/Akt pathway as a potential target. Int. J. Clin. Exp. Pathol. 2015, 8, 1402–1410. [Google Scholar]
- Kapral, M.; Wawszczyk, J.; Węglarz, L. Regulation of MicroRNA-155 and Its Related Genes Expression by Inositol Hexaphosphate in Colon Cancer Cells. Molecules 2019, 24, 4153. [Google Scholar] [CrossRef]
- Masunaga, T.; Murao, N.; Tateishi, H.; Koga, R.; Ohsugi, T.; Otsuka, M.; Efujita, M. Anti-cancer activity of the cell membrane-permeable phytic acid prodrug. Bioorganic Chem. 2019, 92, 103240. [Google Scholar] [CrossRef]
- Ferry, S.; Matsuda, M.; Yoshida, H.; Hirata, M. Inositol hexakisphosphate blocks tumor cell growth by activating apoptotic machinery as well as by inhibiting the Akt/NFkappaB-mediated cell survival pathway. Carcinogenesis 2002, 23, 2031–2041. [Google Scholar] [CrossRef]
- Ishizuka, S.; Saitoh, K.-I.; Suzuki, T.; Lee, J.-S.; Hara, H. A partially degraded product of phytate suppresses the proliferation of HCT116 colorectal cancer cells. Food Chem. 2011, 125, 1219–1225. [Google Scholar] [CrossRef][Green Version]
- Omoruyi, F.O.; Stennett, D.; Foster, S.; Dilworth, L.L. New Frontiers for the Use of IP6 and Inositol Combination in Treating Diabetes Mellitus: A Review. Molecules 2020, 25, 1720. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzym. Regul. 2010, 50, 324–337. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maffucci, T.; Falasca, M. Signalling Properties of Inositol Polyphosphates. Molecules 2020, 25, 5281. https://doi.org/10.3390/molecules25225281
Maffucci T, Falasca M. Signalling Properties of Inositol Polyphosphates. Molecules. 2020; 25(22):5281. https://doi.org/10.3390/molecules25225281
Chicago/Turabian StyleMaffucci, Tania, and Marco Falasca. 2020. "Signalling Properties of Inositol Polyphosphates" Molecules 25, no. 22: 5281. https://doi.org/10.3390/molecules25225281
APA StyleMaffucci, T., & Falasca, M. (2020). Signalling Properties of Inositol Polyphosphates. Molecules, 25(22), 5281. https://doi.org/10.3390/molecules25225281






