Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering
Abstract
1. Introduction
2. Overview: Polysaccharide-Based Porous Materials
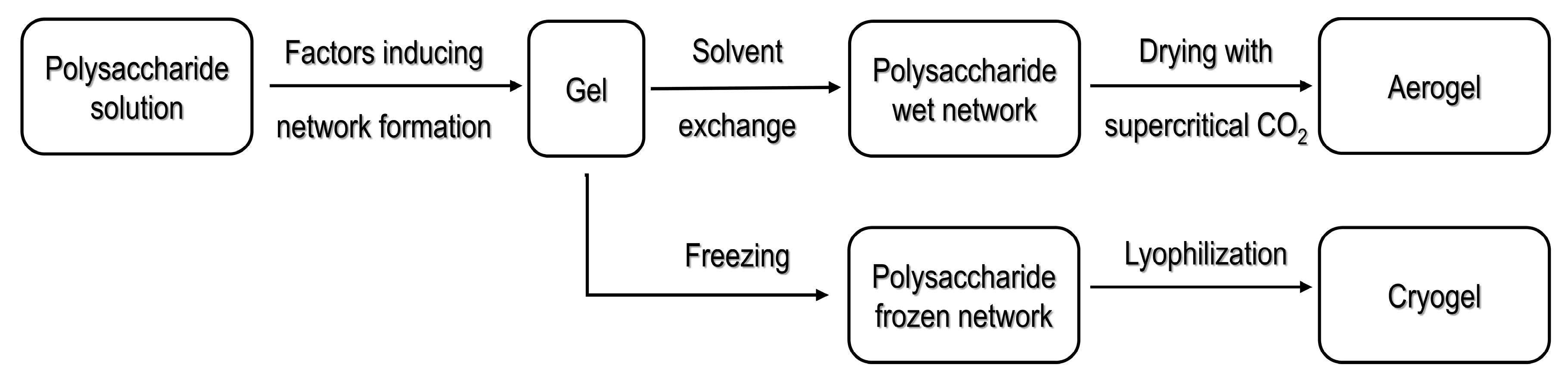
2.1. Processing Strategies for Polysaccharide-Based Aerogels
2.1.1. Processing Using Supercritical Fluid Technology
2.1.2. Cryogels Obtained by Freeze Drying
3. Polysaccharide-Based Porous Materials for Tissue Engineering
3.1. Polysaccharide-Based Porous Materials as Extracellular Matrices
3.2. Influence of the Mechanical Properties of the Scaffold in Cells and Tissues Behavior
3.3. Polysaccharide-Based Porous Materials as Scaffolds for Electrical Stimulation of Cells
3.4. Polysaccharide-Based Porous Materials as Drug-Delivery Systems
3.4.1. Diffusive Phenomena on the Controlled Release of Drugs on Polysaccharide-Based Aerogels
3.4.2. Controlled Drug Release by Electrical Stimulation Employing Conductive Porous Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric scaffolds in tissue engineering application: A review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Stratton, S.; Shelke, N.B.; Hoshino, K.; Rudraiah, S.; Kumbar, S.G. Bioactive polymeric scaffolds for tissue engineering. Bioact. Mater. 2016, 1, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.; Sim, M.; Kim, S.; Yang, S.; Yoo, Y.; Park, J.-H.; Yoon, T.H.; Kim, M.-G.; Lee, J.Y. Electrically conductive graphene/polyacrylamide hydrogels produced by mild chemical reduction for enhanced myoblast growth and differentiation. Acta Biomater. 2017, 48, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Aznar-Cervantes, S.; Pagán, A.; Martínez, J.G.; Bernabeu-Esclapez, A.; Otero, T.F.; Meseguer-Olmo, L.; Paredes, J.I.; Cenis, J.L. Electrospun silk fibroin scaffolds coated with reduced graphene promote neurite outgrowth of PC-12 cells under electrical stimulation. Mater. Sci. Eng. C 2017, 79, 315–325. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Budtova, T.; Duraes, L.; Erkey, C.; Del Gaudio, P.; Gurikov, P.; Koebel, M.; Liebner, F.; Neagu, M.; Smirnova, I. An opinion paper on aerogels for biomedical and environmental applications. Molecules 2019, 24, 1815. [Google Scholar] [CrossRef] [PubMed]
- Shalumon, K.T.; Liao, H.; Kuo, C.; Wong, C.; Li, C.; Mini, P.A.; Chen, J. Rational design of gelatin/nanohydroxyapatite cryogel scaffolds for bone regeneration by introducing chemical and physical cues to enhance osteogenesis of bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2019, 104, 109855. [Google Scholar] [CrossRef]
- Afanasenkau, D.; Kalinina, D.; Lyakhovetskii, V.; Tondera, C.; Gorsky, O.; Moosavi, S.; Pavlova, N.; Merkulyeva, N.; Kalueff, A.V.; Minev, I.R.; et al. Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces. Nat. Biomed. Eng. 2020, 4, 1010–1022. [Google Scholar] [CrossRef] [PubMed]
- Gaharwar, A.K.; Singh, I.; Khademhosseini, A. Engineered biomaterials for in situ tissue regeneration. Nat. Rev. Mater. 2020, 5, 686–705. [Google Scholar] [CrossRef]
- Jahromi, M.; Razavi, S.; Bakhtiari, A. The advances in nerve tissue engineering: From fabrication of nerve conduit to in vivo nerve regeneration assays. J. Tissue Eng. Regen. Med. 2019, 13, 2077–2100. [Google Scholar] [CrossRef]
- Lin, H.; Sohn, J.; Shen, H.; Langhans, M.T.; Tuan, R.S. Bone marrow mesenchymal stem cells: Aging and tissue engineering applications to enhance bone healing. Biomaterials 2019, 203, 96–110. [Google Scholar] [CrossRef]
- Paun, I.A.; Zamfirescu, M.; Luculescu, C.R.; Acasandrei, A.M.; Mustaciosu, C.C.; Mihailescu, M.; Dinescu, M. Electrically responsive microreservoires for controllable delivery of dexamethasone in bone tissue engineering. Appl. Surf. Sci. 2017, 392, 321–331. [Google Scholar] [CrossRef]
- Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C.A. Sterile and dual-porous aerogels scaffolds obtained through a multistep supercritical CO2-based approach. Molecules 2019, 24, 871. [Google Scholar] [CrossRef] [PubMed]
- Francis Suh, J.K.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Hamidouche, Z.; Haÿ, E.; Vaudin, P.; Charbord, P.; Schüle, R.; Marie, P.J.; Fromigué, O. FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/β-catenin signaling-dependent Runx2 expression. FASEB J. 2008, 22, 3813–3822. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.; Weissman, I. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef]
- Sammons, J.; Ahmed, N.; Hassan, H.T. The role of BMP-6, IL-6, and BMP-4 in mesenchymal stem cell-dependent bone development: Effects on osteoblastic differentiation induced by parathyroid hormone and vitamin D3. Stem Cells Dev. 2004, 280, 273–280. [Google Scholar] [CrossRef]
- Wojak-Ćwik, I.M.; Rumian, Ł.; Krok-Borkowicz, M.; Hess, R.; Bernhardt, R.; Dobrzyński, P.; Möller, S.; Schnabelrauch, M.; Hintze, V.; Scharnweber, D.; et al. Synergistic effect of bimodal pore distribution and artificial extracellular matrices in polymeric scaffolds on osteogenic differentiation of human mesenchymal stem cells. Mater. Sci. Eng. C 2019, 97, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Cheema, U.; Yang, S.Y.; Mudera, V.; Goldspink, G.G.; Brown, R.A. 3-D in vitro model of early skeletal muscle development. Cell Motil. Cytoskelet. 2003, 54, 226–236. [Google Scholar] [CrossRef]
- He, H.; Sofman, M.; Wang, A.J.S.; Ahrens, C.C.; Wang, W.; Griffith, L.G.; Hammond, P.T. Engineering helical modular polypeptide-based hydrogels as synthetic extracellular matrices for cell culture. Biomacromolecules 2020, 21, 566–580. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef]
- Kistler, S.S. Coherent expanded aerogels. J. Phys. Chem. 1932, 36, 52–64. [Google Scholar] [CrossRef]
- Korkmaz, S.; Kariper, A. Graphene and graphene oxide based aerogels: Synthesis, characteristics and supercapacitor applications. J. Energy Storage 2020, 27, 101038. [Google Scholar] [CrossRef]
- Shewale, P.M.; Rao, A.V.; Gurav, J.L.; Rao, A.P. Synthesis and characterization of low density and hydrophobic silica aerogels dried at ambient pressure using sodium silicate precursor. J. Porous Mater. 2009, 16, 101–108. [Google Scholar] [CrossRef]
- Zhu, F. Starch based aerogels: Production, properties and applications. Trends Food Sci. Technol. 2019, 89, 1–10. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical applications of biodegradable polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Wang, Y.; Su, Y.; Wang, W.; Fang, Y.; Riffat, S.B.; Jiang, F. The advances of polysaccharide-based aerogels: Preparation and potential application. Carbohydr. Polym. 2019, 226, 115242. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels-promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef]
- García-González, C.A.; Jin, M.; Gerth, J.; Alvarez-Lorenzo, C.; Smirnova, I. Polysaccharide-based aerogel microspheres for oral drug delivery. Carbohydr. Polym. 2015, 117, 797–806. [Google Scholar] [CrossRef]
- Rosa, M.F.; Medeiros, E.S.; Malmonge, J.A.; Gregorski, K.S.; Wood, D.F.; Mattoso, L.H.C.; Glenn, G.; Orts, W.J.; Imam, S.H. Cellulose nanowhiskers from coconut husk fibers: Effect of preparation conditions on their thermal and morphological behavior. Carbohydr. Polym. 2010, 81, 83–92. [Google Scholar] [CrossRef]
- Sussman, E.M.; Halpin, M.C.; Muster, J.; Moon, R.T.; Ratner, B.D. Porous implants modulate healing and induce shifts in local macrophage polarization in the foreign body reaction. Ann. Biomed. Eng. 2014, 42, 1508–1516. [Google Scholar] [CrossRef]
- Loh, Q.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef]
- Takahashi, Y.; Tabata, Y. Effect of the fiber diameter and porosity of non- woven PET fabrics on the osteogenic differentiation of mesenchymal stem cells. J. Biomater. Sci. Polym. Ed. 2012, 15, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Pingguan-Murphy, B.; Abu Osman, N.A. Progress of key strategies in development of electrospun scaffolds: Bone tissue. Sci. Technol. Adv. Mater. 2012, 13, 43002. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Ondeck, M.G.; Chirasatitsin, S.; Ngamkham, K.; Reilly, G.C.; Engler, A.J.; Battaglia, G. 3D surface topology guides stem cell adhesion and differentiation. Biomaterials 2015, 52, 140–147. [Google Scholar] [CrossRef]
- Shuaib, A.; Motan, D.; Bhattacharya, P.; McNabb, A.; Skerry, T.M.; Lacroix, D. Heterogeneity in the mechanical properties of integrins determines mechanotransduction dynamics in bone osteoblasts. Sci. Rep. 2019, 9, 13113. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Gurikov, P.; Poejo, J.; Matias, A.A.; Heinrich, S.; Duarte, C.M.M.; Smirnova, I. Alginate-based hybrid aerogel microparticles for mucosal drug delivery. Eur. J. Pharm. Biopharm. 2016, 107, 160–170. [Google Scholar] [CrossRef]
- Ramírez-Sánchez, K.; Ledezma-Espinoza, A.; Sánchez-Kopper, A.; Avendaño-Soto, E.; Prado, M.; Starbird Perez, R. Polysaccharide κ-carrageenan as doping agent in conductive coatings for electrochemical controlled release of dexamethasone at therapeutic doses. Molecules 2020, 25, 2139. [Google Scholar] [CrossRef]
- Chen, C.; Yuen, D.; Ng, W.; Weil, T. Progress in polymer science polymer bioconjugates: Modern design concepts toward precision hybrid materials. Prog. Polym. Sci. 2020, 105, 101241. [Google Scholar] [CrossRef]
- Pires, F.; Ferreira, Q.; Rodrigues, C.A.V.; Morgado, J.; Ferreira, F.C. Neural stem cell differentiation by electrical stimulation using a cross-linked PEDOT substrate: Expanding the use of biocompatible conjugated conductive polymers for neural tissue engineering. BBA-Gen. Subj. 2015, 1850, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, I.; Mamic, J.; Arlt, W. Adsorption of drugs on silica aerogels. Langmuir 2003, 19, 8521–8525. [Google Scholar] [CrossRef]
- Durães, L.; Hajar, M.; Vareda, J.P.; Lamy-Mendes, A.; Portugal, A. Exploring the versatile surface chemistry of silica aerogels for multipurpose application. MRS Adv. 2017, 2, 3511–3519. [Google Scholar] [CrossRef]
- Rezaei, S.; Jalali, A.; Zolali, A.M.; Alshrah, M.; Karamikamkar, S.; Park, C.B. Robust, ultra-insulative and transparent polyethylene-based hybrid silica aerogel with a novel non-particulate structure. J. Colloid Interface Sci. 2019, 548, 206–216. [Google Scholar] [CrossRef]
- Ziegler, C.; Wolf, A.; Liu, W.; Herrmann, A.K.; Gaponik, N.; Eychmüller, A. Modern inorganic aerogels. Angew. Chem. Int. Ed. 2017, 56, 13200–13221. [Google Scholar] [CrossRef]
- Nagai, Y.; Yokoi, H.; Kaihara, K.; Naruse, K. The mechanical stimulation of cells in 3D culture within a self-assembling peptide hydrogel. Biomaterials 2012, 33, 1044–1051. [Google Scholar] [CrossRef]
- García-González, C.A.; Carenza, E.; Zeng, M.; Smirnova, I.; Roig, A. Design of biocompatible magnetic pectin aerogel monoliths and microspheres. RSC Adv. 2012, 2, 9816–9823. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Scognamiglio, M.; Reverchon, E. A new tool to produce alginate-based aerogels for medical applications, by supercritical gel drying. J. Supercrit. Fluids 2019, 146, 152–158. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- Baudron, V.; Gurikov, P.; Smirnova, I.; Whitehouse, S. Porous starch materials via supercritical-and freeze-drying. Gels 2019, 5, 12. [Google Scholar] [CrossRef]
- Starbird, R.; García-González, C.A.; Smirnova, I.; Krautschneider, W.H.; Bauhofer, W. Synthesis of an organic conductive porous material using starch aerogels as template for chronic invasive electrodes. Mater. Sci. Eng. C 2014, 37, 177–183. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Ardao, I.; Starbird, R.; García-González, C.A. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Avraamidou, S.; Baratsas, S.G.; Tian, Y.; Pistikopoulos, E.N. Circular economy—A challenge and an opportunity for Process Systems Engineering. Comput. Chem. Eng. 2020, 133, 106629. [Google Scholar] [CrossRef]
- Ganesan, K.; Budtova, T.; Ratke, L.; Gurikov, P.; Baudron, V.; Preibisch, I.; Niemeyer, P.; Smirnova, I.; Milow, B. Review on the production of polysaccharide aerogel particles. Materials 2018, 11, 2144. [Google Scholar] [CrossRef]
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of materials for regenerative medicine using supercritical fluid technology. Bioconjug. Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef]
- Knez, Ž.; Pantić, M.; Cör, D.; Novak, Z.; Knez Hrnčič, M. Are supercritical fluids solvents for the future? Chem. Eng. Process. Process Intensif. 2019, 141, 107532. [Google Scholar] [CrossRef]
- Wilhelm Oetjen, G. Freeze Drying; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1999; ISBN 3-527-29571-2. [Google Scholar]
- Liao, W.; Zhao, H.B.; Liu, Z.; Xu, S.; Wang, Y.Z. On controlling aerogel microstructure by freeze casting. Compos. Part B Eng. 2019, 173, 107036. [Google Scholar] [CrossRef]
- Ni, X.; Ke, F.; Xiao, M.; Wu, K.; Kuang, Y.; Corke, H.; Jiang, F. The control of ice crystal growth and effect on porous structure of konjac glucomannan-based aerogels. Int. J. Biol. Macromol. 2016, 92, 1130–1135. [Google Scholar] [CrossRef]
- García-gonzález, C.A.; Camino-rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Kenar, J.A.; Eller, F.J.; Felker, F.C.; Jackson, A.; Fanta, G.F. Starch aerogel beads obtained from inclusion complexes prepared from high amylose starch and sodium palmitate. Green Chem. 2014, 16, 1921–1930. [Google Scholar] [CrossRef]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch aerogels: A member of the family of thermal super-insulating materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Ren, H.; Bi, Y.; Shi, X.; Wang, B.; Zhang, L. A novel starch-enhanced melamine-formaldehyde aerogel with low volume shrinkage and high toughness. J. Porous Mater. 2017, 24, 1303–1307. [Google Scholar] [CrossRef]
- Ubeyitogullari, A.; Brahma, S.; Rose, D.J.; Ciftci, O. In vitro digestibility of nanoporous wheat starch aerogels. J. Agric. Food Chem. 2018, 66, 9490–9497. [Google Scholar] [CrossRef]
- Mohammadi, A.; Moghaddas, J. Mesoporous tablet-shaped potato starch aerogels for loading and release of the poorly water-soluble drug celecoxib. Chin. J. Chem. Eng. 2020, 28, 1778–1787. [Google Scholar] [CrossRef]
- Ganesan, K.; Ratke, L. Facile preparation of monolithic κ-carrageenan aerogels. Soft Matter 2014, 10, 3218–3224. [Google Scholar] [CrossRef]
- Nedelec, J.-M.; Pircher, N.; Strauß, C.; Carbajal, L.; Kasper, C.; Rosenau, T.; Fischhuber, D.; Liebner, F. Preparation and reinforcement of dual-porous biocompatible cellulose scaffolds for tissue engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar]
- Raman, S.P.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluids 2015, 106, 23–33. [Google Scholar] [CrossRef]
- Betz, M.; García-González, C.A.; Subrahmanyam, R.P.; Smirnova, I.; Kulozik, U. Preparation of novel whey protein-based aerogels as drug carriers for life science applications. J. Supercrit. Fluids 2012, 72, 111–119. [Google Scholar] [CrossRef]
- Goimil, L.; Braga, M.E.M.; Dias, A.M.A.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; De Sousa, H.C.; García-González, C.A. Supercritical processing of starch aerogels and aerogel-loaded poly(ε-caprolactone) scaffolds for sustained release of ketoprofen for bone regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
- Theocharis, A.D.; Skandalis, S.S.; Gialeli, C.; Karamanos, N.K. Extracellular matrix structure. Adv. Drug Deliv. Rev. 2016, 97, 4–27. [Google Scholar] [CrossRef]
- Brien, F.J.O. Biomaterials & scaffolds every day thousands of surgical procedures are performed to replace. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Gleeson, J.P.; Brien, F.J.O. Composite scaffolds for orthopaedic regenerative medicine. In Advances in Composite Materials for Medicine and Nanotechnology; InTech Open Access: Rijeka, Croatia, 2011; Volume 10, pp. 39–59. [Google Scholar]
- Chen, H.; Zhong, J.; Wang, J.; Huang, R.; Qiao, X.; Wang, H.; Tan, Z. Enhanced growth and differentiation of myoblast cells grown on E-jet 3D printed platforms. Int. J. Nanomed. 2019, 14, 937–950. [Google Scholar] [CrossRef]
- Miroslaw, L.; Capila, I.; Kaundinya, G. Mass spectrometric methods for the analysis of heparin and heparan sulfate. Glycosaminoglycans Methods Mol. Biol. 2014, 1229, 119–128. [Google Scholar]
- Park, J.S.; Lim, H.J.; Yi, S.W.; Park, K.H. Stem cell differentiation-related protein-loaded PLGA microspheres as a novel platform micro-typed scaffold for chondrogenesis. Biomed. Mater. 2016, 11, 55003. [Google Scholar] [CrossRef]
- Simann, M.; Schneider, V.; Le Blanc, S.; Dotterweich, J.; Zehe, V.; Krug, M.; Jakob, F.; Schilling, T.; Schütze, N. Heparin affects human bone marrow stromal cell fate: Promoting osteogenic and reducing adipogenic differentiation and conversion. Bone 2015, 78, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Solakyildirim, K. Recent advances in glycosaminoglycan analysis by various mass spectrometry techniques. Anal. Bioanal. Chem. 2019, 411, 3731–3741. [Google Scholar] [CrossRef]
- Uygun, B.E.; Stojsih, S.E.; Matthew, H.W.T. Effects of immobilized glycosaminoglycans on the proliferation and differentiation of mesenchymal stem cells. Tissue Eng. Part A 2009, 15, 3499–3512. [Google Scholar] [CrossRef]
- Benoit, D.S.W.; Durney, A.R.; Anseth, K.S. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials 2007, 28, 66–77. [Google Scholar] [CrossRef]
- Seto, S.P.; Casas, M.E.; Temenoff, J.S. Differentiation of mesenchymal stem cells in heparin-containing hydrogels via coculture with osteoblasts. Cell Tissue Res. 2012, 347, 589–601. [Google Scholar] [CrossRef]
- Farina, M.; Chua, C.Y.X.; Ballerini, A.; Thekkedath, U.; Alexander, J.F.; Rhudy, J.R.; Torchio, G.; Fraga, D.; Pathak, R.R.; Villanueva, M.; et al. Transcutaneously refillable, 3D-printed biopolymeric encapsulation system for the transplantation of endocrine cells. Biomaterials 2018, 177, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Paez-Mayorga, J.; Capuani, S.; Farina, M.; Lotito, M.L.; Niles, J.A.; Salazar, H.F.; Rhudy, J.; Esnaola, L.; Chua, C.Y.X.; Taraballi, F.; et al. Enhanced in vivo vascularization of 3D-printed cell encapsulation device using platelet-rich plasma and mesenchymal stem cells. Adv. Healthc. Mater. 2020, 2000670, 1–11. [Google Scholar] [CrossRef]
- Erdem, A.; Darabi, M.A.; Nasiri, R.; Sangabathuni, S.; Ertas, Y.N.; Alem, H.; Hosseini, V.; Shamloo, A.; Nasr, A.S.; Ahadian, S.; et al. 3D bioprinting of oxygenated cell-laden gelatin methacryloyl constructs. Adv. Healthc. Mater. 2020, 9, e1901794. [Google Scholar] [CrossRef]
- Blakney, A.K.; Swartzlander, M.D.; Bryant, S.J. The effects of substrate stiffness on the in vitro activation of macrophages and in vivo host response to poly (ethylene glycol)-based hydrogels. Soc. Biomater. 2012, 100, 1375–1386. [Google Scholar]
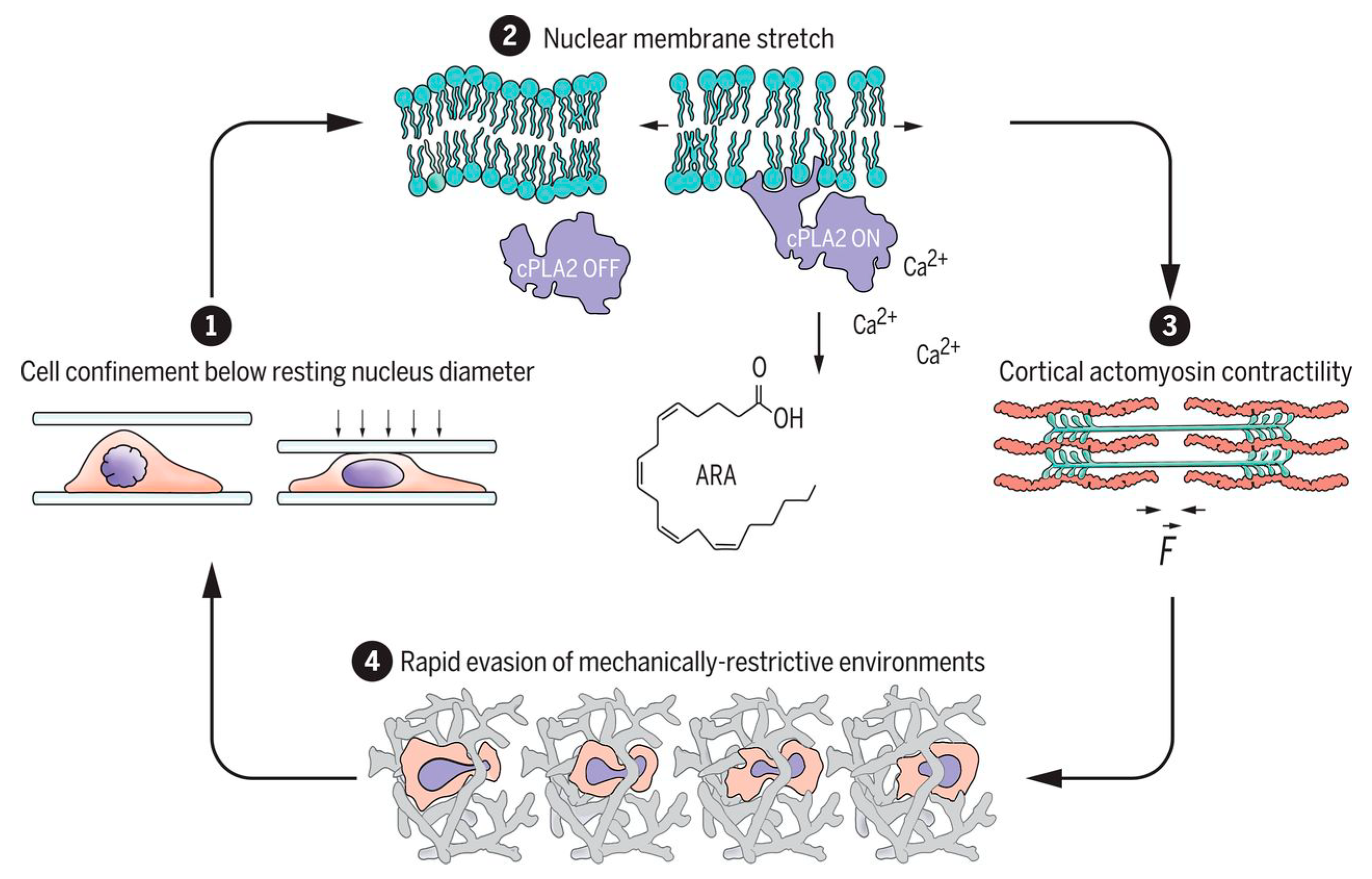
- Venturini, V.; Pezano, F.; Frederic, C.; Hakkinen, H.-M.; Jiménez-delgado, S.; Colomer-rosell, M.; Marro, M.; Tolosa-ramon, Q.; Paz-lópez, S.; Valverde, M.A.; et al. The nucleus measures shape changes for cellular proprioception to control dynamic cell behavior. Science 2020, 370, 2644. [Google Scholar] [CrossRef]
- Lomakin, A.; Cattin, C.; Cuvelier, D.; Alraies, Z.; Molina, M.; Nader, G.P.; Srivastava, N.; Saez, P.; Garcia-Arcos, J.M.; Zhitnyak, I.Y.; et al. The nucleus acts as a ruler tailoring cell responses to spatial constraints. Science 2020, 370, 2894. [Google Scholar] [CrossRef]
- Fahy, N.; Alini, M.; Stoddart, M.J. Mechanical stimulation of mesenchymal stem cells: Implications for cartilage tissue engineering. J. Orthop. Res. 2018, 36, 52–63. [Google Scholar] [CrossRef]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef]
- Pedersen, J.; Swartz, M. Mechanobiology in the third dimension. Ann. Biomed. Eng. 2005, 33, 1469–1490. [Google Scholar] [CrossRef] [PubMed]
- Terraciano, V.; Hwang, N.; Moroni, L.; Park, H.B.; Zhang, Z.; Mizrahi, J.; Seliktar, D.; Elisseeff, J. Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 2007, 25, 2730–2738. [Google Scholar] [CrossRef]
- Zhang, W.; Kong, C.W.; Tong, M.H.; Chooi, W.H.; Huang, N.; Li, R.A.; Chan, B.P. Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: Effects of niche cell supplementation and mechanical stimulation. Acta Biomater. 2017, 49, 204–217. [Google Scholar] [CrossRef]
- Barnes, J.M.; Przybyla, L.; Weaver, V.M. Tissue mechanics regulate brain development, homeostasis and disease. J. Cell Sci. 2017, 130, 71–82. [Google Scholar] [CrossRef]
- Liu, C.; Cui, X.; Ackermann, T.M.; Flamini, V.; Chen, W.; Castillo, A.B. Osteoblast-derived paracrine factors regulate angiogenesis in response to mechanical stimulation. Integr. Biol. 2016, 8, 785–794. [Google Scholar] [CrossRef]
- Bono, N.; Pezzoli, D.; Levesque, L.; Loy, C.; Candiani, G.; Fiore, G.B.; Mantovani, D. Unraveling the role of mechanical stimulation on smooth muscle cells: A comparative study between 2D and 3D models. Biotechnol. Bioeng. 2016, 113, 2254–2263. [Google Scholar] [CrossRef]
- Hasanzadeh, E.; Amoabediny, G.; Haghighipour, N.; Gholami, N.; Mohammadnejad, J.; Shojaei, S.; Salehi-Nik, N. The stability evaluation of mesenchymal stem cells differentiation toward endothelial cells by chemical and mechanical stimulation. Vitr. Cell. Dev. Biol.—Anim. 2017, 53, 818–826. [Google Scholar] [CrossRef]
- Gaub, B.M.; Mu, D.J. Mechanical stimulation of piezo1 receptors depends on extracellular matrix proteins and directionality of force. Nano Lett. 2017, 17, 2064–2072. [Google Scholar] [CrossRef]
- Godau, B. Determining the Effect of Structure and Function on 3D Bioprinted Hydrogel Scaffolds for Applications in Tissue Engineering. Bachelor´s Thesis, University of Victoria, Victoria, BC, Canada, 2019. [Google Scholar]
- Peeters, E.A.G. Monitoring the biomechanical response of individual cells under compression: A new compression device. Med. Biol. Eng. Comput. 2003, 41, 498–503. [Google Scholar] [CrossRef]
- Wang, N.; Butler, J.P.; Ingber, D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H.; Calderon, C.; Burgos-Bravo, F.; Kobler, O.; Zuschratter, W.; Ramirez, O.; Härtel, S.; Schneider, P.; Quest, A.F.G.; Herrera-Molina, R.; et al. Astrocyte-to-neuron communication through integrin-engaged Thy-1/CBP/Csk/Src complex triggers neurite retraction via the RhoA/ROCK pathway. Biochim. Biophys. Acta—Mol. Cell Res. 2017, 1864, 243–254. [Google Scholar] [CrossRef]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, S.; Wang, Z.; Wang, F.; Cao, X.; Wu, Q.; Zhao, C.; Ma, H.; Ye, F.; Wang, H.; et al. Supervillin promotes epithelial- mesenchymal transition and metastasis of hepatocellular carcinoma in hypoxia via activation of the RhoA/ROCK-ERK/p38 pathway. J. Exp. Clin. Cancer Res. 2018, 37, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Glücksmann, A. Studies on bone mechanics in vitro. I. Influence of pressure on orientation of structure. Anat. Rec. 1938, 72, 97–113. [Google Scholar] [CrossRef]
- Gelmi, A.; Cieslar-Pobuda, A.; de Muinck, E.; Los, M.; Rafat, M.; Jager, E.W.H. Direct Mechanical stimulation of stem cells: A beating electromechanically active scaffold for cardiac tissue engineering. Adv. Healthc. Mater. 2016, 5, 1471–1480. [Google Scholar] [CrossRef]
- Ghezzi, C.E.; Marelli, B.; Donelli, I.; Alessandrino, A.; Freddi, G.; Nazhat, S.N. The role of physiological mechanical cues on mesenchymal stem cell differentiation in an airway tract-like dense collagen-silk fibroin construct. Biomaterials 2014, 35, 6236–6247. [Google Scholar] [CrossRef]
- Núñez-Toldrà, R.; Vasquez-Sancho, F.; Barroca, N.; Catalan, G. Investigation of the cellular response to bone fractures: Evidence for flexoelectricity. Sci. Rep. 2020, 10, 254. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, X.; Yue, Y.; Mei, C.; Huang, C.; Jiang, S.; Wu, Q.; Han, J. Nanocellulose-mediated electroconductive viscoelasticity, stretchability, and biocompatibility toward multifunctional applications. ACS Appl. Mater. Interfaces 2018, 10, 27987–28002. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-bialy, T.; Saldaña, M.D.A. High-intensity ultrasound-assisted formation of cellulose nanofiber scaffold with low and high lignin content and their cytocompatibility with gingival fibroblast cells. Ultrason. Sonochem. 2019, 64, 104759. [Google Scholar] [CrossRef]
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2017, 150, 60–86. [Google Scholar] [CrossRef]
- Jaatinen, L. The Effect of an Applied Electric Current on Cell Proliferation, Viability, Morphology, Adhesion, and Stem Cell Differentiation. Ph.D. Thesis, Tampere University of Technology, Tampere, Finland, 2017. [Google Scholar]
- Taghian, T.; Narmoneva, D.A.; Kogan, A.B. Modulation of cell function by electric field: A high-resolution analysis. J. R. Soc. Interface 2015, 12, 21–25. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Y.; Jin, H.; Steinacker, J. Electrical stimulation induced Hsp70 response in C2C12 cells. Exerc. Immunol. Rev. 2010, 16, 86–97. [Google Scholar]
- Ghasemi-mobarakeh, L.; Prabhakaran, M.P.; Morshed, M. Application of conductive polymers, scaffolds and electrical stimulation for nerve tissue engineering. J. Tissue Eng. Regen. Med. 2011, 5, 17–35. [Google Scholar] [CrossRef]
- Sherrell, P.C.; Thompson, B.C.; Wassei, J.K.; Gelmi, A.A.; Higgins, M.J.; Kaner, R.B.; Wallace, G.G. Maintaining cytocompatibility of biopolymers through a graphene layer for electrical stimulation of nerve cells. Adv. Funct. Mater. 2014, 24, 769–776. [Google Scholar] [CrossRef]
- Aleem, I.S.; Aleem, I.; Evaniew, N.; Busse, J.W.; Yaszemski, M.; Agarwal, A.; Einhorn, T.; Bhandari, M. Efficacy of electrical stimulators for bone healing: A meta-analysis of randomized sham-controlled trials. Sci. Rep. 2016, 6, 31724. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.W.H.; Bergquist, A.J.; Aldayel, A.; Czitron, J.; Collins, D.F. Interleaved neuromuscular electrical stimulation reduces muscle fatigue. Muscle Nerve 2017, 55, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ye, T.; Lee, S.J.; Cui, H.; Miao, S.; Zhou, X.; Shuai, D.; Zhang, L.G. Enhanced neural stem cell functions in conductive annealed carbon nanofibrous scaffolds with electrical stimulation. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 2485–2494. [Google Scholar] [CrossRef]
- Yuan, X.; Arkonac, D.E.; Chao, P.G.; Vunjak-novakovic, G. Electrical stimulation enhances cell migration and integrative repair in the meniscus. Sci. Rep. 2014, 4, 3674. [Google Scholar] [CrossRef]
- Tai, G.; Reid, B.; Cao, L.; Zhao, M. Electrotaxis and wound healing: Experimental methods to study electric fields as a directional signal for cell migration. Methods Mol. Biol. 2009, 571, 77–97. [Google Scholar]
- Bajaj, P.; Bobby, R.; Millet, L.; Wei, C.; Zorlutuna, P.; Baoe, G.; Bashir, R. Patterning the differentiation of C2C12 skeletal myoblasts. Integr. Biol. 2011, 3, 897–909. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, X.; Yu, X.; Wang, S.; Qiu, J.; Tang, W.; Li, L.; Liu, H.; Wang, Z.L. Self-powered electrical stimulation for enhancing neural differentiation of mesenchymal stem cells on graphene− Poly(3,4-ethylenedioxythiophene) hybrid microfibers. ASC Nano 2016, 10, 5086–5095. [Google Scholar] [CrossRef]
- Wang, J.; Tian, L.; Chen, N.; Ramakrishna, S.; Mo, X. The cellular response of nerve cells on poly-L-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation. Mater. Sci. Eng. C 2018, 91, 715–726. [Google Scholar] [CrossRef]
- Boehler, C.; Kleber, C.; Martini, N.; Xie, Y.; Dryg, I.; Stieglitz, T.; Hofmann, U.G.; Asplund, M. Actively controlled release of Dexamethasone from neural microelectrodes in a chronic in vivo study. Biomaterials 2017, 129, 176–187. [Google Scholar] [CrossRef]
- Hao, H.B.; Yuan, L.; Fu, Z.B.; Wang, C.Y.; Yang, X.; Zhu, J.; Qu, J.; Chen, H.; Schiraldi, D. Biomass-based mechanically-strong and electrically-conductive polymer aerogels and their application for supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 9917–9924. [Google Scholar]
- You, I.; Jeong, U. Electromechanical decoupling by porous aerogel conducting polymer. Matter 2019, 1, 24–25. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, D.; Liu, J.; Luo, Y. Conducting polymer aerogels from supercritical CO2 drying PEDOT-PSS hydrogels. J. Mater. Chem. 2010, 20, 5080–5085. [Google Scholar] [CrossRef]
- Bertucci, C.; Koppes, R.; Dumont, C.; Koppes, A. Neural responses to electrical stimulation in 2D and 3D in vitro environments. Brain Res. Bull. 2019, 152, 265–284. [Google Scholar] [CrossRef]
- Hernández-Suarez, P.; Ramírez, K.; Alvarado, F.; Avendaño, E.; Starbird, R. Electrochemical characterization of poly(3,4-ethylenedioxythiophene)/κ-carrageenan as a biocompatible conductive coat for biologic applications. MRS Commun. 2018, 9, 218–223. [Google Scholar] [CrossRef]
- Ryan, E.M.; Breslin, C.B. Formation of polypyrrole with dexamethasone as a dopant: Its cation and anion exchange properties. J. Electroanal. Chem. 2018, 824, 188–194. [Google Scholar] [CrossRef]
- Goimil, L.; Santos-Rosales, V.; Delgado, A.; Évora, C.; Reyes, R.; Lozano-Pérez, A.A.; Aznar-Cervantes, S.D.; Cenis, J.L.; Gómez-Amoza, J.L.; Concheiro, A.; et al. ScCO2-foamed silk fibroin aerogel/poly(ϵ-caprolactone) scaffolds containing dexamethasone for bone regeneration. J. CO2 Util. 2019, 31, 51–64. [Google Scholar] [CrossRef]
- Costa, P.F.; Puga, A.M.; Díaz-Gomez, L.; Concheiro, A.; Busch, D.H.; Alvarez-Lorenzo, C. Additive manufacturing of scaffolds with dexamethasone controlled release for enhanced bone regeneration. Int. J. Pharm. 2015, 496, 541–550. [Google Scholar] [CrossRef]
- Liu, Q.; Cen, L.; Zhou, H.; Yin, S.; Liu, G.; Liu, W.; Cao, Y.; Cui, L. The role of the extracellular signal-related kinase signaling pathway in osteogenic differentiation of human adipose-derived stem cells and in adipogenic transition initiated by dexamethasone. Tissue Eng. Part A 2009, 15, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Danckwerts, M.; Fassihi, A. Implantable controlled release drug delivery system: A review. Drug Dev. Ind. Pharm. 1991, 7, 1465–1502. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A review of drug release mechanisms from nanocarrier systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Franco, P.; Marco, I. De supercritical CO2 adsorption of non-steroidal anti-inflammatory drugs into biopolymer aerogels. J. CO2 Util. 2020, 36, 40–53. [Google Scholar] [CrossRef]
- Lovskaya, D.D.; Lebedev, A.E.; Menshutina, N.V. Aerogels as drug delivery systems: In vitro and in vivo evaluations. J. Supercrit. Fluids 2015, 106, 5–11. [Google Scholar] [CrossRef]
- De Marco, I.; Reverchon, E. Starch aerogel loaded with poorly water-soluble vitamins through supercritical CO2 adsorption. Chem. Eng. Res. Des. 2017, 119, 221–230. [Google Scholar] [CrossRef]
- García-González, C.A.; Uy, J.J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–36. [Google Scholar] [CrossRef]
- Krukiewicz, K. Tailorable drug capacity of dexamethasone-loaded conducting polymer matrix. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 12202. [Google Scholar] [CrossRef]
- Löffler, S.; Seyock, S.; Nybom, R.; Jacobson, G.B.; Richter-Dahlfors, A. Electrochemically triggered release of acetylcholine from scCO2 impregnated conductive polymer films evokes intracellular Ca2+ signaling in neurotypic SH-SY5Y cells. J. Control. Release 2016, 243, 283–290. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Alshammary, B.; Walsh, F.C.; Herrasti, P.; Ponce de Leon, C. Electrodeposited conductive polymers for controlled drug release: Polypyrrole. J. Solid State Electrochem. 2016, 20, 839–859. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Gniazdowska, B.; Jarosz, T.; Herman, A.P.; Boncel, S.; Turczyn, R. Effect of immobilization and release of ciprofloxacin and quercetin on electrochemical properties of poly(3,4-ethylenedioxypyrrole) matrix. Synth. Met. 2019, 249, 52–62. [Google Scholar] [CrossRef]
- Alizadeh, N.; Shamaeli, E. Electrochemically controlled release of anticancer drug methotrexate using nanostructured polypyrrole modified with cetylpyridinium: Release kinetics investigation. Electrochim. Acta 2014, 130, 488–496. [Google Scholar] [CrossRef]
- Boehler, C.; Oberueber, F.; Asplund, M. Tuning drug delivery from conducting polymer films for accurately controlled release of charged molecules. J. Control. Release 2019, 304, 173–180. [Google Scholar] [CrossRef]
- Svirskis, D.; Sharma, M.; Yu, Y.; Garg, S. Electrically switchable polypyrrole film for the tunable release of progesterone. Ther. Deliv. 2013, 4, 307–313. [Google Scholar] [CrossRef]
- Kim, B.D.; Richardson-burns, S.M.; Hendricks, J.L.; Sequera, C.; Martin, D.C. Effect of immobilized nerve growth factor on conductive polymers: Electrical properties and cellular response. Adv. Funct. Mater. 2007, 17, 79–86. [Google Scholar] [CrossRef]
- Leprince, L.; Dogimont, A.; Magnin, D.; Demoustier-Champagne, S. Dexamethasone electrically controlled release from polypyrrole-coated nanostructured electrodes. J. Mater. Sci. Mater. Med. 2010, 21, 925–930. [Google Scholar] [CrossRef]
- Stevenson, G.; Moulton, S.E.; Innis, P.C.; Wallace, G.G. Polyterthiophene as an electrostimulated controlled drug release material of therapeutic levels of dexamethasone. Synth. Met. 2010, 160, 1107–1114. [Google Scholar] [CrossRef]
- Qu, J.; Liang, Y.; Shi, M.; Guo, B.; Gao, Y.; Yin, Z. Biocompatible conductive hydrogels based on dextran and aniline trimer as electro-responsive drug delivery system for localized drug release. Int. J. Biol. Macromol. 2019, 140, 255–264. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef]
- Lin, H.; Zhu, B.; Wu, Y.; Sekine, J.; Nakao, A.; Luo, S. Dynamic poly(3,4 ethylenedioxythiophene)s integrate low impedance with redox-switchable biofunction. Adv. Funct. Mater. 2018, 28, 1703890. [Google Scholar] [CrossRef]
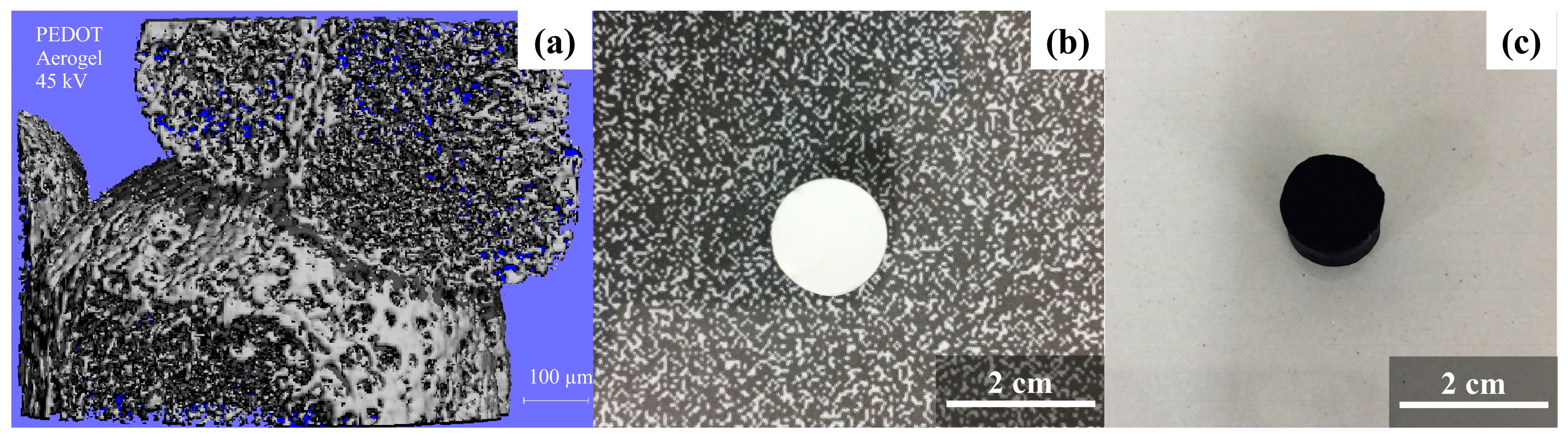

| Raw Material | Fabrication Method | Specific Surface Area (m2/g) | Porosity (%) | Reference |
|---|---|---|---|---|
| Corn starch | scCO2 | 130–183 | 80–89 | [13] |
| scCO2 | 102–274 | N.R. | [61] | |
| scCO2 | 221–234 | 85–90 | [62] | |
| scCO2 | 79–87 | N.R. | [52] | |
| scCO2 | 183–197 | 61–73 | [51] | |
| FD | 0.6–7.7 | >80 | [51] | |
| scCO2 | 223–247 | 87 | [53] | |
| scCO2 | 313–362 | N.R. | [63] | |
| scCO2 | 254 | N.R. | [64] | |
| scCO2 | 370 | N.R. | [65] | |
| Wheat starch | scCO2 | 52.6–57.9 | N.R. | [66] |
| scCO2 | 34.7–60.9 | 91–93 | [66] | |
| Pea starch | scCO2 | 204–230 | 84–92 | [62] |
| scCO2 | 221 | N.R. | [64] | |
| Potato starch | scCO2 | 42–70 | N.R. | [67] |
| scCO2 | 85–88 | N.R. | [64] | |
| Starch/κ-carrageenan | scCO2 | 194–231 | 78–85 | [53] |
| κ-carrageenan | scCO2 | ≈ 230 | N.R. | [68] |
| Chitosan | scCO2 | >250 | >96 | [48] |
| Cellulose | scCO2 | 287–303 | 92–96 | [50] |
| FD | 297 | 96.4 | [50] | |
| scCO2 | 20–246 | 91–99 | [69] | |
| Alginate/chitosan | scCO2 | 127.4–192.3 | N.R. | [49] |
| Alginate composites | scCO2 | 200–800 | N.R. | [70] |
| Whey protein isolate | scCO2 | 14–447 | N.R. | [71] |
| FD | <5 | N.R. | [71] | |
| Poly (ϵ-caprolactone) | scCO2 | N.R. | 54–58.8 | [72] |
| Raw Material | Mechanical Test | Result | Reference |
|---|---|---|---|
| Gelatin/nanohydro-xiapatite cryogels | Compressive mechanical stimulation of cryogels for 14 days in a bioreactor containing 150 mL of cultured medium at 30% compression strain. | Mesenchymal stem cells were attached to the scaffold and a higher extent of osteogenic differentiation was obtained after compression. | [7] |
| Self-assembled peptide hydrogel (arginine, leucine, aspartic acid, and alanine) | The hydrogel containing cells was placed into a hand-control stretch device for 120 h. | Smooth muscle cells resulting in a tight adhesion in the porous structure and a lineal cell proliferation rate were reported. | [46] |
| Poly(lactic-co-glycolic acid) fiber coated with polypyrrole | The electrical stimulation of the matrix induced their volume modification, causing changes in the mechanical strain. | The direct dual electrical and mechanical stimulation of the pluripotent stem cells cultured in the scaffold caused a faster expression of cardiomyocytes genes, important for myocardial regeneration. | [107] |
| Collagen matrix reinforced with rings of electrospun silk fi-broin mat | Dynamic stimulation with pulsatile or laminar flow. Pulsatile flow was induced with a gear pump which supply a steady flow (75 mL/min) in series with a pulsatile manifold. Laminar flow was carried out of steady flow of 75 mL/min. | Chondrogenic differentiation of MSCs was observed in the presence of chondrogenic supplements in laminar flow cultures. Pulsatile flow resulted in preferential cellular orientation, as dictated by dynamic circumferential strain, and induced MSC contractile phenotype expression. | [108] |
| Silicon tubes with inner surfaces modified with collagen type I solutions | Cells cultured on collagen-coated silicon tubes were exposed for 24 hours to the shear stress created when culture medium passes through the tube. | Mechanical stimulation caused by shear stress on adipose-derived mesenchymal stem cells depicted significantly higher gene expression of osteoblasts and adipogenic lineages. Moreover, mechanical stimulus induced endothelial differentiation after the addition of VEGF on cultured medium. | [98] |
| Microcracked hydroxyapatite substrates | Bending the top surface of the cracked substrate in a piezoelectric actuator using a force of 50 N at 5 Hz for 150 s. | Flexoelectricity caused by mechanical stimulation on a hydroxyapatite substrate induced apoptotic responses on osteoblasts and osteocytes. Apoptosis was followed by proliferation of the cells adjacent to the crack, better attachment on the substrate, and an increased expression of osteocytes markers. | [109] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Hidalgo, F.; Ramírez-Sánchez, K.; Starbird-Perez, R. Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering. Molecules 2020, 25, 5286. https://doi.org/10.3390/molecules25225286
Alvarado-Hidalgo F, Ramírez-Sánchez K, Starbird-Perez R. Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering. Molecules. 2020; 25(22):5286. https://doi.org/10.3390/molecules25225286
Chicago/Turabian StyleAlvarado-Hidalgo, Fernando, Karla Ramírez-Sánchez, and Ricardo Starbird-Perez. 2020. "Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering" Molecules 25, no. 22: 5286. https://doi.org/10.3390/molecules25225286
APA StyleAlvarado-Hidalgo, F., Ramírez-Sánchez, K., & Starbird-Perez, R. (2020). Smart Porous Multi-Stimulus Polysaccharide-Based Biomaterials for Tissue Engineering. Molecules, 25(22), 5286. https://doi.org/10.3390/molecules25225286








