Monitoring Methylmalonic Aciduria by NMR Urinomics
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. MMA Cases
3.2. Sample Collection
3.3. NMR Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nicholson, J.K.; Buckingham, M.J.; Sadler, P.J. High resolution 1H-NMR studies of vertebrate blood and plasma. Biochem. J. 1983, 211, 605–615. [Google Scholar] [CrossRef]
- Bales, J.R.; Higham, D.P.; Howe, I.; Nicholson, J.K.; Sadler, P.J. Use of high-resolution proton nuclear magnetic resonance spectroscopy for rapid multi-component analysis of urine. Clin. Chem. 1984, 30, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Bales, J.R.; Sadler, P.J.; Nicholson, J.K.; Timbrell, J.A. Urinary excretion of acetaminophen and its metabolites as studied by proton NMR spectroscopy. Clin. Chem. 1984, 30, 1631–1636. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.K.; Sadler, P.J.; Bales, J.R.; Juul, S.M.; MacLeod, A.F.; Sonksen, P.H. Monitoring metabolic disease by proton NMR of urine. Lancet 1984, 324, 751–752. [Google Scholar] [CrossRef]
- Nicholson, J.K.; O’Flynn, M.P.; Sadler, P.J.; Macleod, A.F.; Juul, S.M.; Sonksen, P.H. Proton-nuclear-magnetic-resonance studies of serum, plasma and urine from fasting normal and diabetic subjects. Biochem. J. 1984, 217, 365–375. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Wilson, I.D. High resolution proton magnetic resonance spectroscopy of biological fluids. Prog. NMR Spectrosc. 1989, 21, 449–501. [Google Scholar] [CrossRef]
- Wraith, J.E. Physician’s Guide to the Laboratory Diagnosis of Metabolic Diseases; Springer: Berlin, Germany, 2003. [Google Scholar]
- Blau, N. Physician’s Guide to the Diagnosis, Treatment, and Follow-Up of Inherited Metabolic Disease; Springer: Heidelberg, Germany, 2014. [Google Scholar]
- Saudubray, J.-M.; Baumgartner, M.R.; Walter, J. Inborn Metabolic Diseases. Diagnosis and Treatment, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zuppi, C.; Messana, I.; Forni, F.; Rossi, C.; Pennacchietti, L.; Ferrari, F.; Giardina, B. 1H-NMR spectra of normal urines: Reference ranges of the major metabolites. Clin. Chim. Acta 1997, 265, 85–97. [Google Scholar] [CrossRef]
- Zuppi, C.; Messana, I.; Tapanainen, P.; Knip, M.; Vincenzoni, F.; Giardina, B.; Nuutinen, M. Proton nuclear magnetic resonance spectral profiles of urine from children and adolescents with type 1 diabetes. Clin. Chem. 2002, 48, 660–662. [Google Scholar] [CrossRef] [Green Version]
- Messana, I.; Forni, F.; Ferrari, F.; Rossi, C.; Giardina, B.; Zuppi, C. Proton nuclear magnetic resonance spectral profiles of urine in type II diabetic patients. Clin. Chem. 1998, 44, 1529–1534. [Google Scholar] [CrossRef] [Green Version]
- Zuppi, C.; Messana, I.; Forni, F.; Ferrari, F.; Rossi, C.; Giardina, B. Influence of feeding on metabolite excretion evidenced by urine 1H-NMR spectral profiles: A comparison between subjects living in Rome and subjects living at arctic latitudes (Svaldbard). Clin. Chim. Acta 1998, 278, 75–79. [Google Scholar] [CrossRef]
- Ciurtin, C.; Nicolescu, A.; Stefan, L.I.; Kovacs, E.; Smith, I.C.P.; Deleanu, C. Metabolic profiling of urine by 1H-NMR spectroscopy. A critical assessment of interpreting metabolite concentrations for normal and diabetes groups. Rev. Chim. 2007, 52, 51–55. [Google Scholar]
- Stefan, L.I.; Nicolescu, A.; Popa, S.; Mota, M.; Kovacs, E.; Deleanu, C. 1H-NMR urine metabolic profiling in type 1 diabetes Mellitus. Rev. Roum. Chim. 2010, 55, 1033–1037. [Google Scholar]
- Grama, A.; Blaga, L.; Nicolescu, A.; Deleanu, C.; Militaru, M.; Cainap, S.S.; Pop, I.; Tita, G.; Sîrbe, C.; Fufezan, O.; et al. Novel mutation in GALT gene in galactosemia patient with Ggroup B Streptococcus Meningitis and acute liver failure. Medicina 2019, 55, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulturar, R.; Chis, A.; Baizat, M.; Cozma, A.; Suharoschi, R.; Nicolescu, A.; Deleanu, C. A severe neonatal argininosuccinic aciduria case investigated by 1H-NMR spectroscopy. Rev. Chim. 2020, 71, 210–218. [Google Scholar] [CrossRef]
- Molema, F.; Gleich, F.; Burgard, P.; van der Ploeg, A.T.; Summar, M.L.; Chapman, K.A.; Baric, I.; Lund, A.M.; Kolker, S.; Williams, M.; et al. Evaluation of dietary treatment and amino acid supplementation in organic acidurias and urea-cycle disorders: On the basis of information from a European multicenter registry. J. Inherit. Metab. Dis. 2019, 42, 1162–1175. [Google Scholar] [CrossRef] [Green Version]
- Molema, F.; Gleich, F.; Burgard, P.; van der Ploeg, A.T.; Summar, M.L.; Chapman, K.A.; Lund, A.M.; Rizopoulos, D.; Kolker, S.; Williams, M.; et al. Decreased plasma L-arginine levels in organic acidurias (MMA and PA) and decreased plasma branched-chain amino acid levels in urea cycle disorders as a potential cause of growth retardation: Options for treatment. Mol. Genet. Metab. 2019, 126, 397–405. [Google Scholar] [CrossRef]
- Nicolescu, A.; Dolenko, B.; Bezabeh, T.; Stefan, L.-I.; Ciurtin, C.; Kovacs, E.; Smith, I.C.P.; Simionescu, B.C.; Deleanu, C. Diagnosis of type II diabetes based on non-glucose regions of 1H-NMR spectra of urine: A metabonomic approach. Rev. Chim. 2011, 62, 1150–1153. [Google Scholar]
- EUROCORDIS, Rare Diseases Europe, Patient-Driven NGO. Available online: www.eurordis.org (accessed on 25 August 2020).
- Baldovino, S.; Moliner, A.M.; Taruscio, D.; Daina, E.; Roccatello, D. Rare diseases in Europe: From a wide to a local perspective. Isr. Med. Assoc. J. 2016, 18, 359–363. [Google Scholar]
- Nguengang Wakap, S.; Lambert, D.M.; Olry, A.; Rodwell, C.; Gueydan, C.; Lanneau, V.; Murphy, D.; Le Cam, Y.; Rath, A. Estimating cumulative point prevalence of rare diseases: Analysis of the Orphanet database. Eur. J. Hum. Genet. 2020, 28, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Aygen, S.; Durr, U.; Hegele, P.; Kunig, J.; Spraul, M.; Schäfer, H.; Krings, D.; Cannet, C.; Fang, F.; Schutz, B.; et al. NMR-Based screening for Inborn Errors of Metabolism: Initial results from a study on Turkish neonates. JIMD Rep. 2014, 16, 101–111. [Google Scholar]
- Therrell, B.L.; Padilla, C.D.; Loeber, J.G.; Kneisser, I.; Saadallah, A.; Borrajo, G.J.C.; Adams, J. Current status of newborn screening worldwide: 2015. Seminars Perinatol. 2015, 39, 171–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groselj, U.; Tansek, M.Z.; Smon, A.; Angelkova, N.; Anton, D.; Baric, I.; Djordjevic, M.; Grimci, L.; Ivanova, M.; Kadamh, A.; et al. Newborn screening in southeastern Europe. Mol. Genet. Metab. 2014, 113, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Tansek, M.Z.; Groselj, U.; Angelkova, N.; Anton, D.; Baric, I.; Djordjevic, M.; Grimci, L.; Ivanova, M.; Kadam, A.; Kotori, V.; et al. Phenylketonuria screening and management in southeastern Europe—Survey results from 11 countries. Orphanet J. Rare Dis. 2015, 10, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherry, E.B.; Lee, P.; Choi, I.-Y. In vivo NMR studies of the brain with hereditary or acquired metabolic disorders. Neurochem. Res. 2015, 40, 2647–2685. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Neidich, J.A.; Salazar, D.; Thomas-Johnson, E.; Ferreira, B.F.; Kwong, A.M.; Lin, A.M.; Jonas, A.J.; Levine, S.; Lorey, F.; et al. Asymptomatic maternal combined homocystinuria and methylmalonic aciduria (cblC) detected through low carnitine levels on newborn screening. J. Pediatr. 2009, 155, 924–927. [Google Scholar] [CrossRef]
- Hannah-Shmouni, F.; Cruz, V.; Schulze, A.; Mercimek-Andrews, S. Transcobalamin receptor defect: Identification of two new cases through positive newborn screening for propionic/methylmalonic aciduria and long-term outcome. Am. J. Med. Gen. 2018, 176, 1411–1415. [Google Scholar] [CrossRef]
- Smithard, D.; Roberts, R.; Iles, R.A. Analysis of organic acidurias by proton-spin-echo nuclear magnetic resonance (1H-NMR). Clin. Sci. 1983, 65, 22P. [Google Scholar] [CrossRef]
- Iles, R.A.; Buckinghamt, M.J.; Hawkes, G.E. Spin-echo proton-nuclear-magnetic-resonance detection of normal and abnormal metabolites in plasma and urine. Biochem. Soc. Trans. 1983, 11, 374–375. [Google Scholar] [CrossRef]
- Hind, A.J.; Chalmers, R.A.; Iles, R.A. Methylmalonic and propionic aciduria studied by proton-nuclear-magnetic resonance spectroscopy. Biochem. Soc. Trans. 1985, 13, 201–202. [Google Scholar] [CrossRef]
- Iles, R.A.; Chalmers, R.A.; Hind, A.J. Methylmalonic aciduria and propionic acidaemia studied by proton nuclear magnetic resonance spectroscopy. Clin. Chim. Acta 1986, 173, 173–189. [Google Scholar] [CrossRef]
- Iles, R.A.; Hind, A.J.; Chalmers, R.A. The use of proton nuclear magnetic resonance spectroscopy for detection and study of organic acidurias. Clin. Chem. 1985, 31, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Kodama, S.; Sugiura, M.; Nakao, H.; Kobayashi, K.; Miyoshi, M.; Yoshii, K.; Komatsu, M.; Sakurai, T. 1H-NMR studies of urine propionic academia and methylmalonic academia. Acta Pediatr. Jpn. 1991, 33, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Wikoff, W.R.; Gangoiti, J.A.; Barshop, B.A.; Siuzdak, G. Metabolomics identifies perturbations in human disorders of propionate metabolism. Clin. Chem. 2007, 53, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Commodari, F.; Arnold, D.L.; Sactury, B.C.; Shoubridge, E.A. 1H-NMR characterization of normal human cerebrospinal fluid and the detection of methylmalonic acid in a vitamin B12 deficient patient. NMR Biomed. 1991, 4, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Trinh, B.C.; Melhem, E.R.; Barker, P.B. Multi-slice proton MR spectroscopy and diffusion-weighted imaging in methylmalonic academia: Report of two cases and review of the literature. Am. J. Neuroradiol. 2001, 22, 831–833. [Google Scholar]
- Takeuchi, M.; Harada, M.; Matsuzaki, K.; Hisaoka, S.; Nishitani, H.; Mori, K. Magnetic resonance imaging and spectroscopy in a patient with treated methylmalonic academia. J. Comput. Assist. Tomogr. 2003, 27, 547–551. [Google Scholar] [CrossRef]
- Oberholzer, V.G.; Levin, B.; Burgess, E.A.; Young, W.F. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch. Dis. Child. 1967, 42, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Morrow, G.; Barness, L.A.; Cardinale, G.J.; Abeles, R.H.; Flaks, J.G. Congenital methylmalonic academia: Enzymatic evidence for two forms of the disease. Proc. Nat. Acad. Sci. USA 1969, 63, 191–197. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, L.E.; Lilljeqvist, A.-C.; Hsia, Y.E.; Rosenbloom, F.M. Vitamin B12 dependent methylmalonic aciduria: Defective B12 metabolism in cultured fibroblasts. Biochem. Biophys. Res. Commun. 1969, 36, 607–614. [Google Scholar] [CrossRef]
- Haijes, H.A.; van Hasselt, P.M.; Jans, J.J.M.; Verhoeven-Duif, N.M. Pathophysiology of propionic and methylmalonic acidemias. Part 2: Treatment strategies. J. Inherit. Metab. Dis. 2019, 42, 745–761. [Google Scholar] [CrossRef]
- Hannibal, L.; Lysne, V.; Bjørke-Monsen, A.-L.; Behringer, S.; Grünert, S.C.; Spiekerkoetter, U.; Jacobsen, D.W.; Blom, H.J. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front. Mol. Biosci. 2016, 3, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowler, B.; Leonard, J.V.; Baumgartner, M.R. Causes of and diagnostic approach to methylmalonic acidurias. J. Inherit. Metab. Dis. 2008, 31, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Lentner, C.; Wink, A. Units of measurement, Body fluids, Composition of the body, Nutrition. Geigy Sci. Tables 1981, 1, 228–231. [Google Scholar]
- METAGENE, Metabolic and Genetic Information Center. Available online: www.metagene.de (accessed on 30 August 2020).
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The Human Metabolome Database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vazquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Casado, M.; Sierra, C.; Batllori, M.; Artuch, R.; Ormazabal, A. A targeted metabolomic procedure for amino acid analysis in different biological specimens by ultra-high-performance liquid chromatography-tandem mass spectrometry. Metabolomics 2018, 14, 76. [Google Scholar] [CrossRef]
- Caterino, M.; Ruoppolo, M.; Villani, G.R.D.; Marchese, E.; Costanzo, M.; Sotgiu, G.; Dore, S.; Franconi, F.; Campesi, I. Influence of sex on urinary organic acids: A cross-sectional study in children. Int. J. Mol. Sci. 2020, 21, 582. [Google Scholar] [CrossRef] [Green Version]
- López-Hernández, Y.; Oropeza-Valdez, J.J.; Blanco-Sandate, J.O.; Herrera-Van Oostdam, A.S.; Zheng, J.; Guo, A.C.; Lima-Rogel, V.; Rajabzadeh, R.; Salgado-Bustamante, M.; Adrian-Lopez, J.; et al. The urinary metabolome of healthy newborns. Metabolites 2020, 10, 165. [Google Scholar] [CrossRef] [Green Version]
- Marcell, P.D.; Stabler, S.P.; Podell, S.P.; Allen, R.H. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas-chromatography-mass spectrometry. Anal. Biochem. 1985, 150, 58–66. [Google Scholar] [CrossRef]
- Rasmussen, K. Studies on methylmalonic acid in humans I. Concentrations in serum and urinary excretion in normal subjects after feeding and during fasting, and after loading with protein, fat, sugar, isoleucine and valine. Clin. Chem. 1989, 35, 2271–2276. [Google Scholar] [CrossRef]
- Rasmussen, K.; Moleby, L.; Jensen, M.K. Studies on methylmalonic acid in humans II. Relationships between concentrations in serum and urinary excretion and the correlation between serum cobalamin and accumulation of methylmalonic acid. Clin. Chem. 1989, 35, 2277–2280. [Google Scholar] [CrossRef]
- Frick, M.A.; Barba, I.; Fenoy-Alejandre, M.; López-López, P.; Baquero-Artigao, F.; Rodríguez-Molino, P.; Noguera-Julian, A.; Nicolás-López, M.; de la Fuente-Juárez, A.; Codina-Grau, M.G.; et al. 1H-NMR urinary metabolic profile, a promising tool for the management of infants with human cytomegalovirus-infection. Metabolites 2019, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The human urine metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auray-Blais, C.; Maranda, B.; Lavoie, P. High-throughput tandem mass spectrometry multiplex analysis for newborn urinary screening of creatine synthesis and transport disorders, Triple H syndrome and OTC deficiency. Clin. Chim. Acta 2014, 436, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Porcu, S.; Corda, M.; Lilliu, F.; Contini, L.; Era, B.; Traldi, P.; Fais, A. Increase in urinary purines and pyrimidines in patients with methylmalonic aciduria combined with homocystinuria. Clin. Chim. Acta 2010, 411, 853–858. [Google Scholar] [CrossRef]
- Roe, C.R.; Hoppel, C.L.; Stacey, T.E.; Chalmers, R.A.; Tracey, B.M.; Millington, D.S. Metabolic response to carnitine in methylmalonic aciduria. Arch. Dis. Child. 1983, 58, 916–920. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, R.A.; Roe, C.R.; Stacey, T.E. Urinary excretion of L-carnitine and acylcarnitines in patients with disorders of organic acid metabolism: Evidence for secondary insufficiency of l-carnitine. Pediatr. Res. 1984, 18, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Di Donato, S.; Rimoldi, M.; Garavaglia, B.; Uziel, G. Propionylcarnitine excretion in propionic acidaemia and methylmalonic aciduria: A cause of carnitine deficiency. Clin. Chim. Acta 1984, 139, 13–21. [Google Scholar] [CrossRef]
- Iles, R.A.; Jago, J.R.; Williams, S.R.; Stacey, T.E.; de Sousa, C.; Chalmers, R.A. Human carnitine metabolism studied by 1H-nuclear magnetic resonance spectroscopy. Biochem. Soc. Trans. 1986, 14, 702–703. [Google Scholar] [CrossRef]
- Davies, S.E.C.; Iles, R.A.; Stacey, T.E.; de Sousa, C.; Chalmers, R.A. Carnitine therapy and metabolism in the disorders of propionyl-CoA metabolism studied using 1H-NMR spectroscopy. Clin. Chim. Acta 1991, 204, 263–278. [Google Scholar] [CrossRef]
- Ando, T.; Rasmussen, K.; Wright, J.M.; Nyhan, W.L. Isolation and identification of methylcitrate, a major metabolic product of propionate in patients with propionic academia. J. Biol. Chem. 1972, 247, 2200–2204. [Google Scholar] [PubMed]
- Cheema-Dhadi, S.; Leznoff, C.C.; Halperin, M.L. Effect of 2-methylcitrate on citrate metabolism, implications for the management of patients with propionic academia and methylmalonic aciduria. Pediatr. Res. 1975, 9, 905–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.E.C.; Chalmers, R.A.; Iles, R.A. Influences on the stereochemistry of citrate-synthase-catalysed methylcitrate formation. Biochem. Soc. Trans. 1987, 15, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Podebrad, F.; Heil, M.; Scharrer, A.; Feldmer, S.; Schulte-Mater, O.; Mosandl, A.; Sewell, A.C.; Bohles, H.J. Analysis of methylcitric acid by enantioselective multidimensional gas chromatography-mass spectrometry. J. High Resol. Chromatogr. 1999, 22, 604–608. [Google Scholar] [CrossRef]
- Krawczyk, H.; Matyniuk, T. Characterization of the 1H and 13C-NMR spectra of methylcitric acid. Spectrochim. Acta Part A 2007, 67, 298–305. [Google Scholar] [CrossRef]
- Krawczyk, H.; Gradowska, W. 1H-NMR spectra of methylcitric acid in urine. J. Inherit. Metab. Dis. 2007, 30, 263. [Google Scholar] [CrossRef]
- Burns, S.P.; Iles, R.A.; Saudubray, J.-M.; Chalmers, R.A. Propionylcarnitine excretion is not affected by metronidazole administration to patients with disorders of propionate metabolism. Eur. J. Pediatr. 1996, 155, 31–35. [Google Scholar] [CrossRef]
- Snyderman, S.E.; Sansaricq, C.; Norton, P.; Phansalkar, S. The use of neomycin in the treatment of methylmalonic aciduria. Pediatrics 1972, 50, 925–927. [Google Scholar]
- Thompson, G.N.; Chalmers, R.A.; Walter, J.H.; Bresson, J.L.; Lyonnet, S.L.; Reed, P.J.; Saudubray, J.M.; Leonard, J.V.; Haliday, D. The use of metronidazole in management of methylmalonic and propionic acidemias. Eur. J. Pediatr. 1990, 149, 792–796. [Google Scholar] [CrossRef]
- Thompson, G.N.; Walter, J.H.; Bresson, J.L.; Ford, G.C.; Lyonnet, S.L.; Chalmers, R.A.; Saudubray, J.-M.; Leonard, J.V.; Haliday, D. Sources of propionate in inborn errors of propionate metabolism. Metabolism 1990, 39, 1133–1137. [Google Scholar] [CrossRef]
- Musteata, M.; Nicolescu, A.; Solcan, G.; Deleanu, C. The 1H-NMR profile of healthy dog cerebrospinal fluid. PLoS ONE 2013, 3, e81192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dona, A.C.; Jiménez, B.; Schäfer, H.; Humpfer, E.; Spraul, M.; Lewis, M.R.; Pearce, J.T.M.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Precision high throughput proton NMR spectroscopy of human urine, serum and plasma for large-scale metabolic phenotyping. Anal. Chem. 2014, 86, 9887–9894. [Google Scholar] [CrossRef] [PubMed]
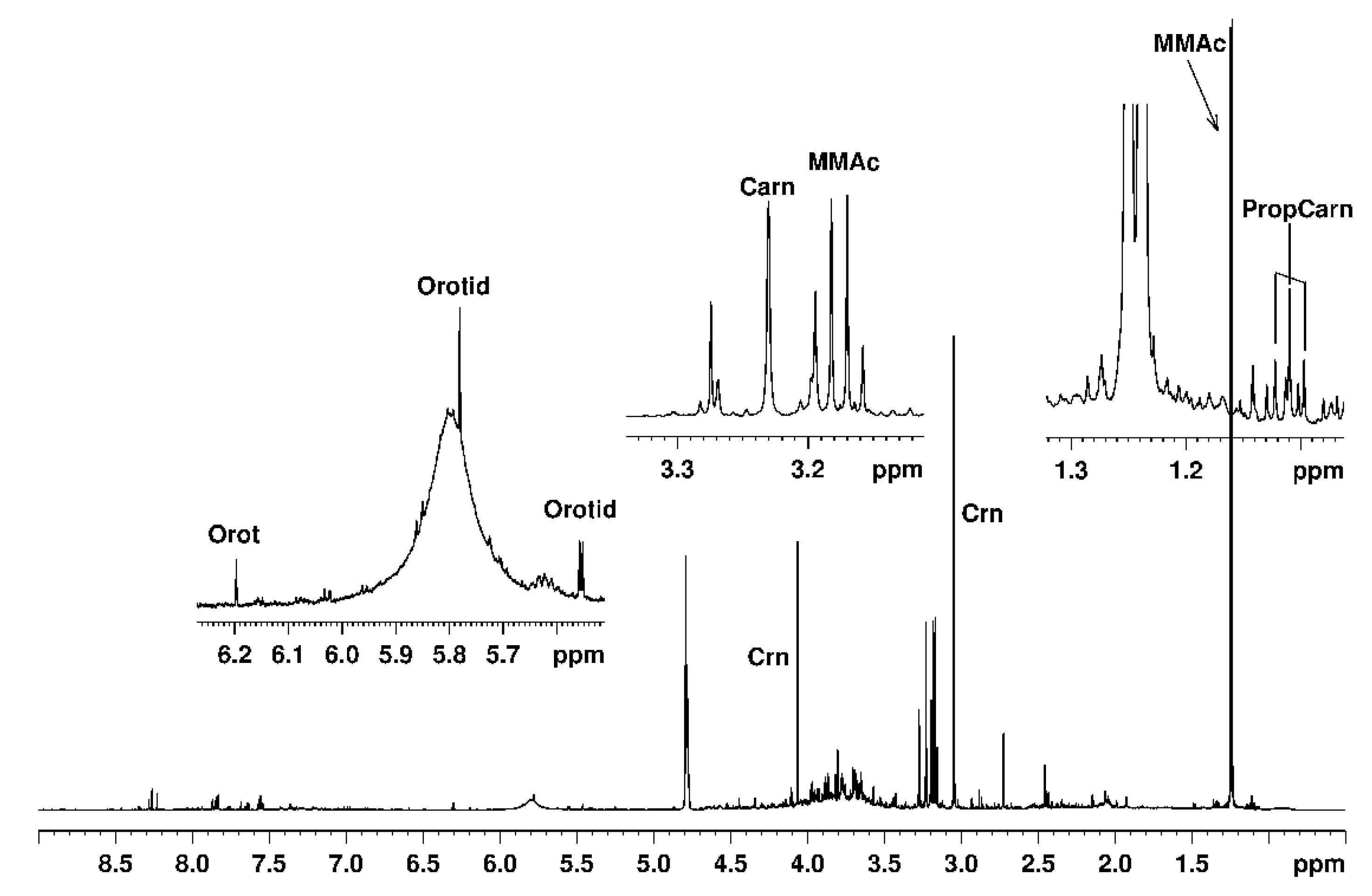
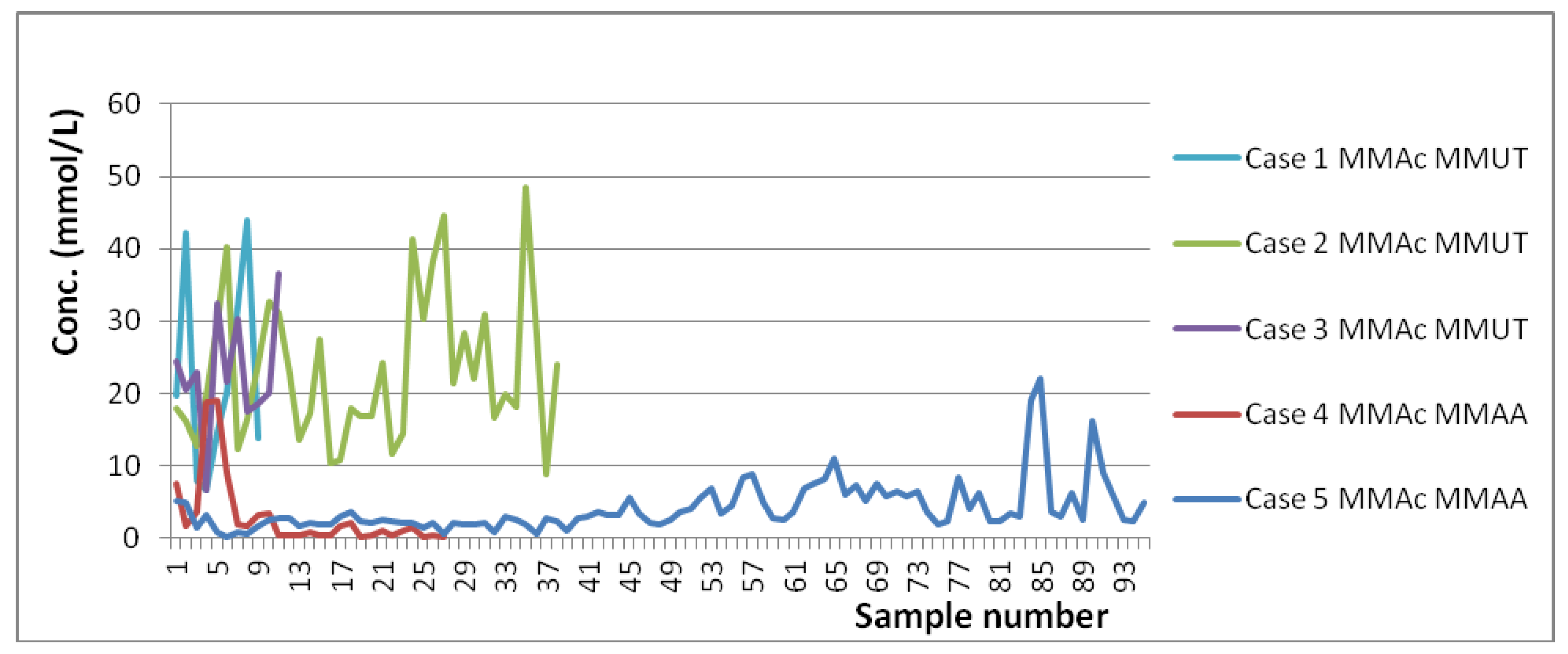

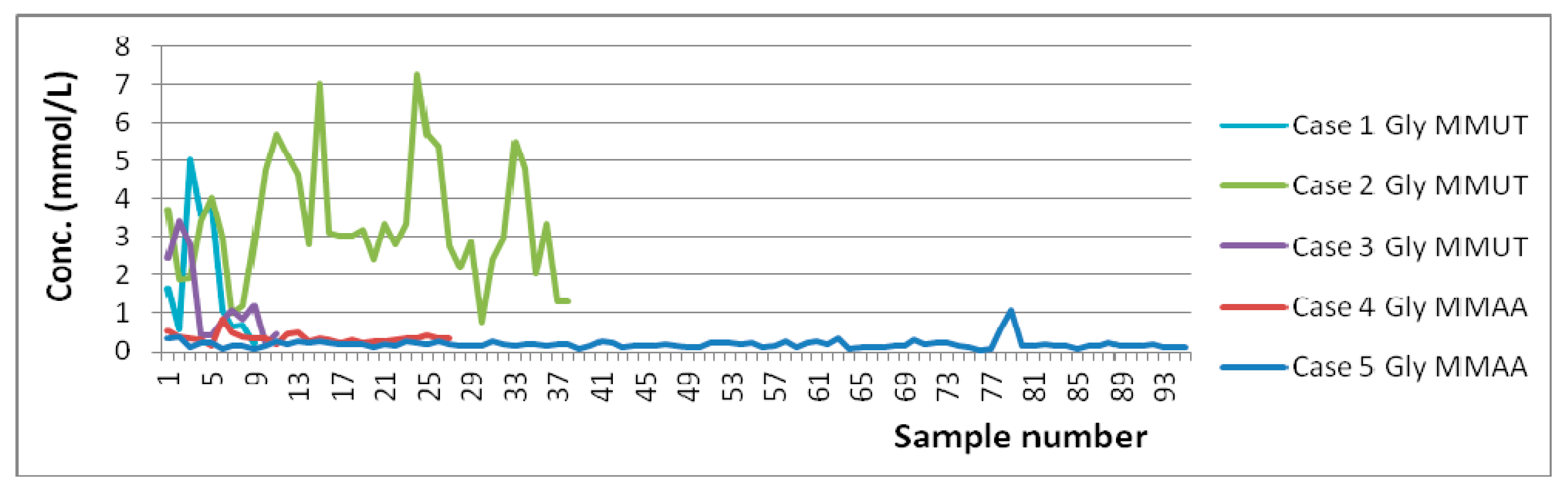
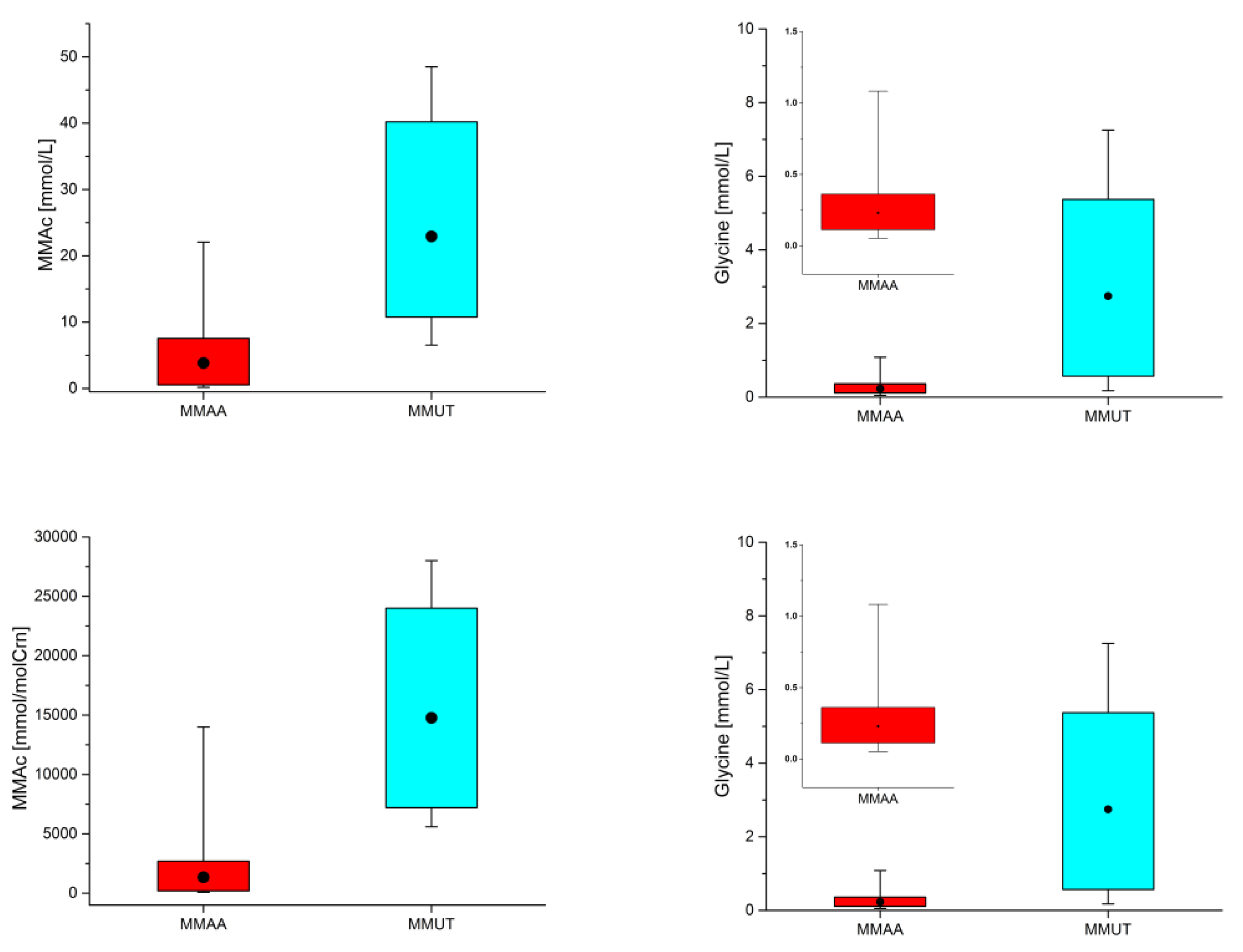
| Averaged Absolute Concentrations and Ranges for MMA Patients (mmol/L) | Concentrations and Ranges for Controls Previously Reported (mmol/L) |
|---|---|
| Glycine (Gly) | |
| 1.916 (0.225–5.019) n = 9/9 Case 1 (7 do–9 mo) [MMUT] 1.101 (0.569–1.667) n = 3/3 Case 1—No Diet (7 do–4 mo) 2.324 (0.225–5.019) n = 6/6 Case 1—Diet (19 do–9 mo) 3.367 (0.771–7.253) n = 38/38 Case 2 (6 do–1½ yo) [MMUT] 2.741 (1.871–3.744) n = 4/4 Case 2—No Diet (11 do–1 mo) 3.441 (0.771–7.253) n = 34/34 Case 2—Diet (3 mo–1½ yo) 1.278 (0.175–3.411) n = 11/11 Case 3 (2½–3½ yo) [MMUT] 0.358 (0.152–0.835) n = 27/27 Case 4 (4½–5½ yo) [MMAA] 0.420 (0.152–0.835) n = 9/9 Case 4—No Diet (4y9mo–4y11mo) 0.326 (0.202–0.506) n = 18/18 Case 4—Diet (5yo–5½ yo) 0.194 (0.052–1.083) n = 95/95 Case 5 (2–11 yo) [MMAA] 0.116 (0.075–0.157) n = 5/5 Case 5—No Diet (2–11 yo) 0.198 (0.052–1.083) n = 90/90 Case 5—Diet (2–11 yo) | 0.978 ± 0.790 (<28 do), 1.468 ± 1.185 (28–122 do) [57]; 1.581 ± 0.957 (1 do) [53]. |
| Methylmalonic Acid (MMAc) | |
| 22.349 (6.738–43.860) n = 9/9 Case 1 (7 do–9 mo) [MMUT] 27.317 (19.660–42.150) n = 3/3 Case 1—No Diet (7 do–4 mo) 19.866 (6.738–43.860) n = 6/6 Case 1—Diet (19 do–9 mo) 23.084 (8.872–48.500) n = 38/38 Case 2 (6 do–1½ yo) [MMUT] 16.608 (12.780–19.610) n = 4/4 Case 2—No Diet (11 do–1 mo) 23.846 (8.872–48.500) n = 34/34 Case 2—Diet (3 mo–1½ yo) 22.848 (6.542–36.580) n = 11/11 Case 3 (2½–3½ yo) [MMUT] 2.989 (0.174–18.960) n = 27/27 Case 4 (4½–5½ yo) [MMAA] 7.352 (1.607–18.960) n = 9/9 Case 4—No Diet (4y9mo–4y11mo) 0.807 (0.174–3.437) n = 18/18 Case 4—Diet (5yo–5½ yo) 4.085 (0.201–22.070) n = 95 Case 5 (2–11 yo) [MMAA] 15.253 (8.077–22.070) n = 5/5 Case 5—No Diet (2–11 yo) 3.464 (0.201–9.079) n = 90/90 Case 5—Diet (2–11 yo) | 0.012 ± 0.017 (1 do) [53]. |
| Orotic Acid (Orot) | |
| 0.029 (0.007–0.066) n = 18/95 Case 5 (2–11 yo) [MMAA] 0.066 (0.066–0.066) n = 1/5 Case 5—No Diet (2–11 yo) 0.027 (0.007–0.061) n = 17/90 Case 5—Diet (2–11 yo) | |
| Orotidine (Orotid) | |
| 0.082 (0.035–0.047) n = 19/95 Case 5 (2–11 yo) [MMAA] 0.097 (0.097–0.097) n = 1/5 Case 5—No Diet (2–11 yo) 0.081 (0.035–0.132) n = 18/90 Case 5—Diet (2–11 yo) | |
| L-Carnitine (Car) | |
| 0.247 (0.147–0.333) n = 6/9 Case 1 (7 do–9 mo) [MMUT] 0.0 n = 0/3 Case 1—No Diet (7 do–4 mo) 0.247 (0.147–0.333) n = 6/6 Case 1—Diet (19 do–9 mo) 0.538 (0.156–1.334) n = 34/38 (6 do–1½ yo) [MMUT] 0.0 n = 0/4 Case 2—No Diet (11 do–1 mo) 0.538 (0.156–1.334) n = 34/34 Case 2—Diet (3 mo–1½ yo) 0.218 (0.096–0.456) n = 5/11 Case 3 (2½–3½ yo) [MMUT] 0.299 (0.068–0.782) n = 17/27 Case 4 (4½–5½ yo) [MMAA] 0.0 n = 0/9 Case 4—No Diet (4y9mo–4y11mo) 0.299 (0.068–0.782) n = 17/18 Case 4—Diet (5yo–5½ yo) 0.221 (0.024–0.667) n = 13/28 Case 5 (2–11 yo) [MMAA] 0.0 n = 0/5 Case 5—No Diet (2–11 yo) 0.221 (0.024–0.667) n = 13/23 Case 5—Diet (2–11 yo) | 0.009 ± 0.007 (1 do) [53]. |
| Propionylcarnitine (PrCar) | |
| 0.715 (0.156–1.390) n = 6/9 Case 1 (7 do–9 mo) [MMUT] 0.0 n = 0/3 Case 1—No Diet (7 do–4 mo) 0.715 (0.156–1.390) n = 6/6 Case 1—Diet (19 do–9 mo) 0.003 (0.001–0.008) n = 34/38 Case 2 (6 do–1½ yo) [MMUT] 0.0 n = 0/4 Case 2—No Diet (11 do–1 mo) 0.922 (0.253–2.266) n = 34/34 Case 2—Diet (3 mo–1½ yo) 0.361 (0.182–0.612) n = 5/11 Case 3 (2½–3½ yo) [MMUT] 0.099 (0.036–0.245) n = 17/27 Case 4 (4½–5½ yo) [MMAA] 0.0 n = 0/9 Case 4—No Diet (4y9mo–4y11mo) 0.099 (0.036–0.245) n = 17/18 Case 4—Diet (5yo–5½ yo) 0.128 (0.044–0.199) n = 11/28 Case 5 (2–11 yo) [MMAA] 0.0 n = 0/5 Case 5—NoDiet (2–11 yo) 0.128 (0.044–0.199) n = 11/23 Case 5—Diet (2–11 yo) | <1.20 [8]; 0.002 (1 do) [53]. |
| Averaged Relative Concentrations and Ranges for MMA Patients (mmol/mol Crn) | Concentrations and Ranges for Controls Previously Reported (mmol/mol Crn) |
|---|---|
| Glycine (Gly) | |
| 2322 (120–7300) n = 9/9 Case 1 (7 do–9 mo) [MMUT] 833 (250–1300) n = 3/3 Case 1—No Diet (7 do–4 mo) 3067 (120–7300) n = 6/6 Case 1—Diet (19 do–9 mo) 2277 (680–4400) n = 38/38 Case 2 (6 do–1½ yo) [MMUT] 3100 (2400–3900) n = 4/4 Case 2—No Diet (11 do–1 mo) 2180 (680–4400) n = 34/34 Case 2—Diet (3 mo–1½ yo) 602 (140–1400) n = 11/11 Case 3 (2½–3½ yo) [MMUT] 148 (69–330) n = 27/27 Case 4 (4½–5½ yo) [MMAA] 211 (110–330) n = 9/9 Case 4—No Diet (4y9mo–4y11mo) 117 (69–240) n = 18/18 Case 4—Diet (5yo–5½ yo) 66 (34–240) n = 79/95 Case 5 (2–11 yo) [MMAA] 41 (38–46) n = 3/5 Case 5—No Diet (2–11 yo) 67 (34–240) n = 76/90 Case 5—Diet (2–11 yo) | 106 (44–300) (19–67 yo) [58]; 362.1 (102.6–853.6) (1 do) [53]; 283–1097 (0–1 mo), 210–743 (1–6 mo), 114–445 (6–12 mo), 110–356 (1–2 yo), 11–326 (2–4 yo), 91–246 (4–7 yo), 64–236 (7–13 yo), 43–173 (>13 yo) [8]; 113–1427 (0–1 mo), 147–570 (2–12 mo), 66–453 (1–8 yo), 51–238 (9–18 yo) [51]. |
| Methylmalonic Acid (MMAc) | |
| 16111 (7000–25000) n = 9/9 Case 1 (7 do–9 mo) [MMUT] 17000 (15000–18000) n = 3/3 Case 1—No Diet (7 do–4 mo) 15667 (7000–25000) n = 6/6 Case 1—Diet (19 do–9 mo) 15350 (5600–28000) n = 38/38 Case 2 (6 do–1½ yo) [MMUT] 20000 (14000–28000) n = 4/4 Case 2—No Diet (11 do–1 mo) 14803 (5600–24000) n = 34/34 Case 2—Diet (3 mo–1½ yo) 11645 (5600–25000) n = 11/11 Case 3 (2½–3½ yo) [MMUT] 1624 (76–14000) n = 27/27 Case 4 (4½–5½ yo) [MMAA] 4369 (760–14000) n = 9/9 Case 4—No Diet (4y9mo–4y11mo) 251 (76–690) n = 18/18 Case 4—Diet (5yo–5½ yo) 1259 (130–7900) n = 95/95 Case 5 (2–11 yo) [MMAA] 4980 (3200–7900) n = 5/5 Case 5—No Diet (2–11 yo) 1052 (130–3800) n = 90/90 Case 5—Diet (2–11 yo) | 1–11 (0–4 mo), 2–13 (4–24 mo), 1–4 (2–10 yo), 0–4 (>10 yo) [8]; 0.58–3.56 [54]; 1.9 (0.7–3.5) (19–67 yo) [58]; 2.79 (0.42–19.7) (1 do) [53]. |
| Orotic Acid (Orot) | |
| 8 (5–16) n = 18/95 Case 5 (2–11 yo) [MMAA] 22 n = 1/5 Case 5—No Diet (2–11 yo) 12 (2–26) n = 17/90 Case 5—Diet (2–11 yo | 1.85 (0–5) (21 do) [59]. |
| Orotidine (Orotid) | |
| 35 (21–55) n = 19/95 Case 5 (2–11 yo) [MMAA] 32 n = 1/5 Case 5—No Diet (2–11 yo) 36 (21–55) n = 18/90 Case 5—Diet (2–11 yo) | |
| l-Carnitine (Car) | |
| 346 (189–838) n = 6/9 Case 1 (7 do–9 mo) [MMUT] 0 n = 0/3 Case 1—No Diet (7 do–4 mo) 346 (189–838) n = 6/6 Case 1—Diet (19 do–9 mo) 520 (162–1028) n = 34/38 Case 2 (6 do–1½ yo) [MMUT] 0 n = 0/4 Case 2—No Diet (11 do–1 mo) 520 (162–1082) n = 34/34 Case 2—Diet (3 mo–1½ yo) 153 (95–226) n = 5/11 Case 3 (2½–3½ yo) [MMUT] 165 (40–342) n = 17/27 Case 4 (4½–5½ yo) [MMAA] 0 n = 0/9 Case 4—No Diet (4y9mo–4y11mo) 165 (40–342) n = 17/18 Case 4—Diet (5yo–5½ yo) 109 (15–429) n = 13/28 Case 5 (2–11 yo) [MMAA] 0 n = 0/5 Case 5—No Diet (2–11 yo) 109 (15–429) n = 13/23 Case 5—Diet (2–11 yo) | 5.0 (0.7–16.4) (19–67 yo) [58]; 2.01 (0.79–5.67) (1 do) [53]. |
| Propionylcarnitine (PrCar) | |
| 700 (206–1186) n = 6/9 Case 1 (7 do–9 mo) [MMUT] 0 n = 0/3 Case 1—No Diet (7 do–4 mo) 700 (206–1186) n = 6/6 Case 1—Diet (19 do–9 mo) 902 (404–1624) n = 34/38 Case 2 (6 do–1½ yo) [MMUT] 0 n = 0/4 Case 2—No Diet (11 do–1 mo) 902 (404–1624) n = 34/34 Case 2—Diet (3 mo–1½ yo) 262 (187–356) n = 5/11 Case 3 (2½–3½ yo) [MMUT] 55 (21–106) n = 17/27 Case 4 (4½–5½ yo) [MMAA] 0 n = 0/9 Case 4—No Diet (4y9mo–4y11mo) 55 (21–106) n = 17/18 Case 4—Diet (5yo–5½ yo) 65 (40–124) n = 11/28 Case 5 (2–11 yo) [MMAA] 0 n = 0/5 Case 5—No Diet (2–11 yo) 65 (40–124) n = 11/23 Case 5—Diet (2–11 yo) | 0.07 (0.01–0.20) (19–67 yo) [58]; 0.03 (0.01–0.07) (1 do) [53]. |
Sample Availability: All chemicals mentioned in the paper are commercially available compounds. There are no samples available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolescu, A.; Blanita, D.; Boiciuc, C.; Hlistun, V.; Cristea, M.; Rotaru, D.; Pinzari, L.; Oglinda, A.; Stamati, A.; Tarcomnicu, I.; et al. Monitoring Methylmalonic Aciduria by NMR Urinomics. Molecules 2020, 25, 5312. https://doi.org/10.3390/molecules25225312
Nicolescu A, Blanita D, Boiciuc C, Hlistun V, Cristea M, Rotaru D, Pinzari L, Oglinda A, Stamati A, Tarcomnicu I, et al. Monitoring Methylmalonic Aciduria by NMR Urinomics. Molecules. 2020; 25(22):5312. https://doi.org/10.3390/molecules25225312
Chicago/Turabian StyleNicolescu, Alina, Daniela Blanita, Chiril Boiciuc, Victoria Hlistun, Mihaela Cristea, Dorina Rotaru, Ludmila Pinzari, Ana Oglinda, Adela Stamati, Isabela Tarcomnicu, and et al. 2020. "Monitoring Methylmalonic Aciduria by NMR Urinomics" Molecules 25, no. 22: 5312. https://doi.org/10.3390/molecules25225312






