Binary Phase Behavior of 1,3-Distearoyl-2-oleoyl-sn-glycerol (SOS) and Trilaurin (LLL)
Abstract
:1. Introduction
2. Results
2.1. Crystallization Kinetics Analysis Using DSC
2.1.1. Cooling Melt-Crystallization Kinetics
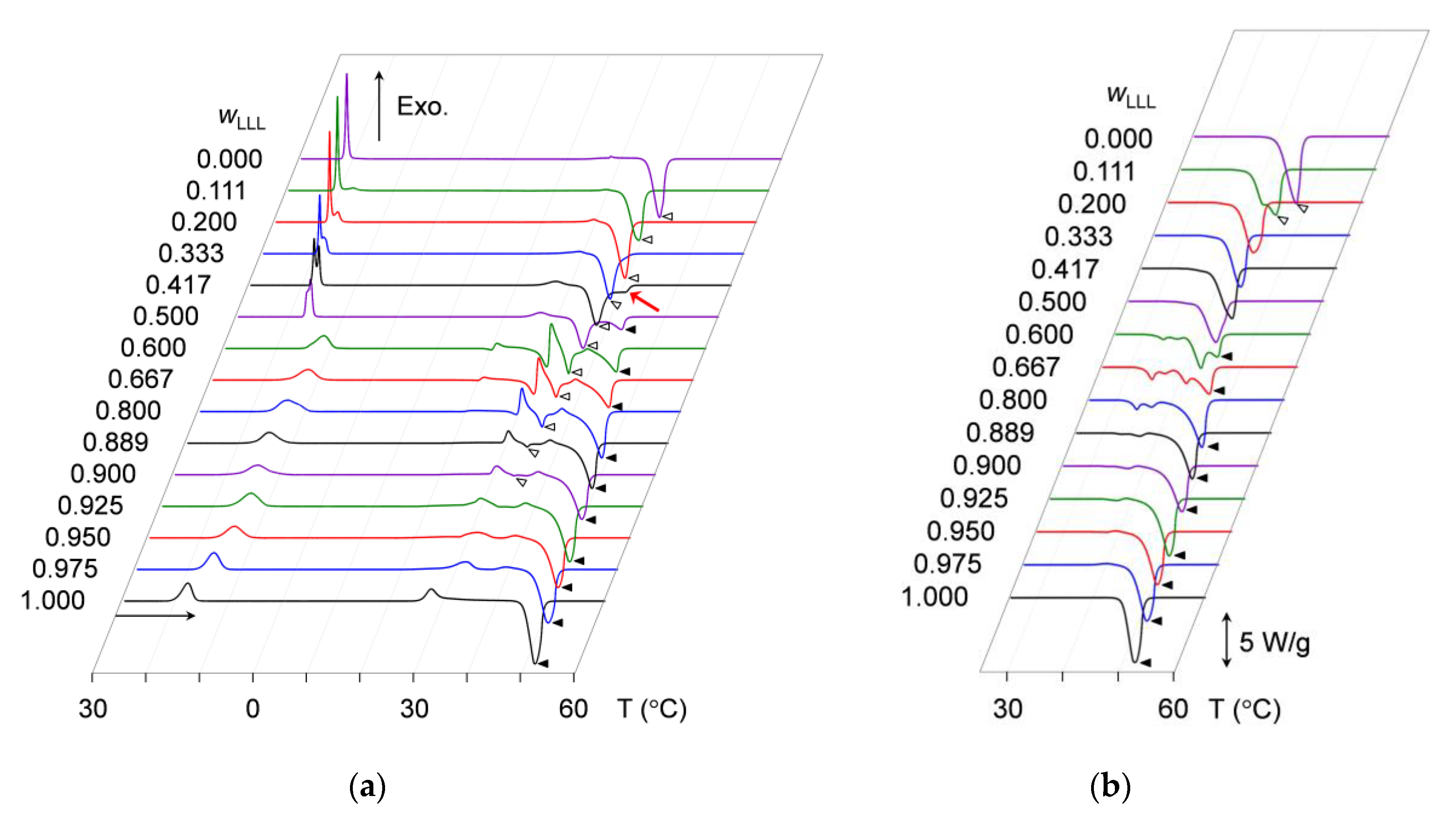
2.1.2. Crystallization-Melting Kinetics during Heating without Aging
2.1.3. Crystallization-Melting Kinetics during Heating after Aging
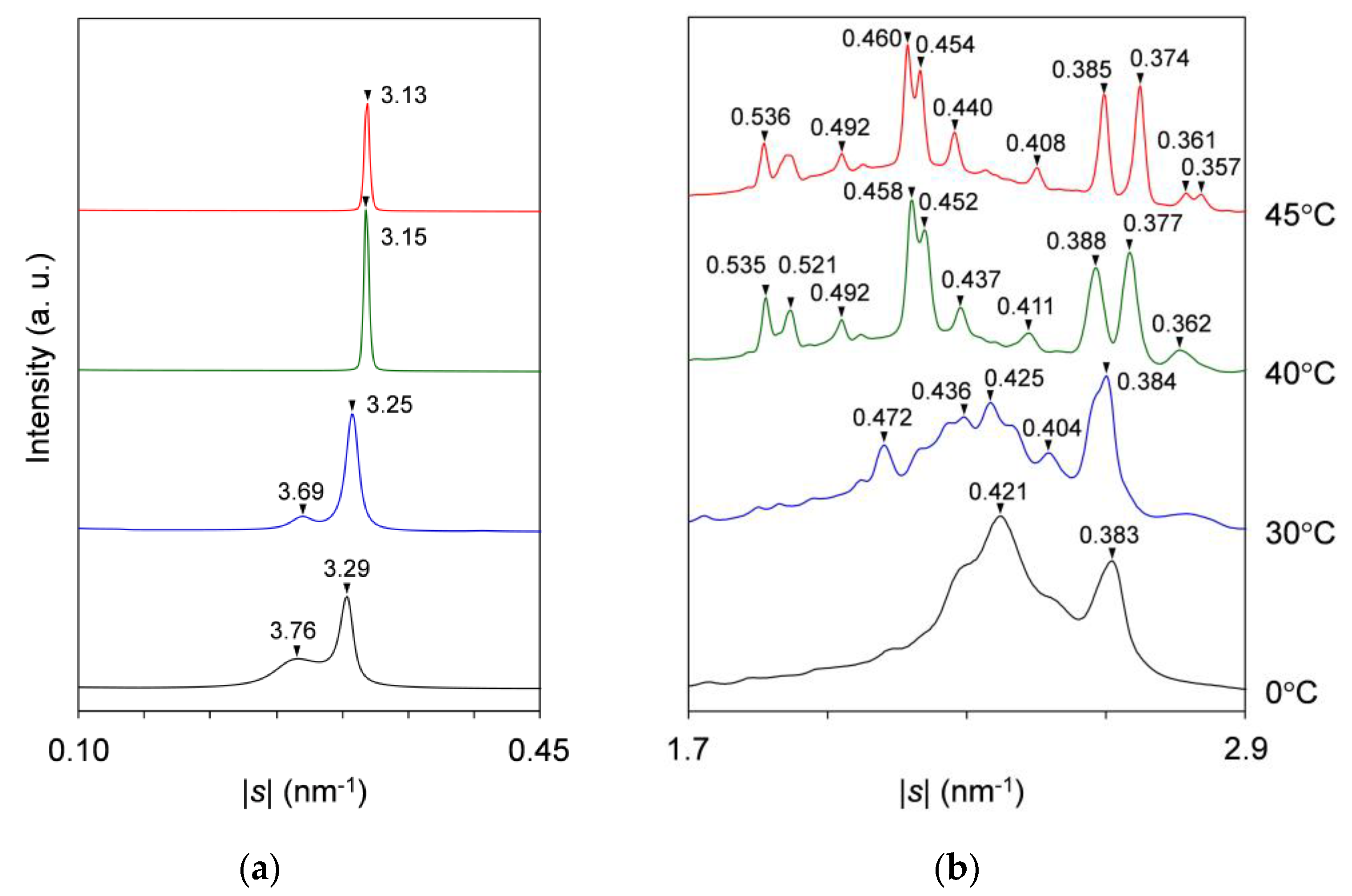
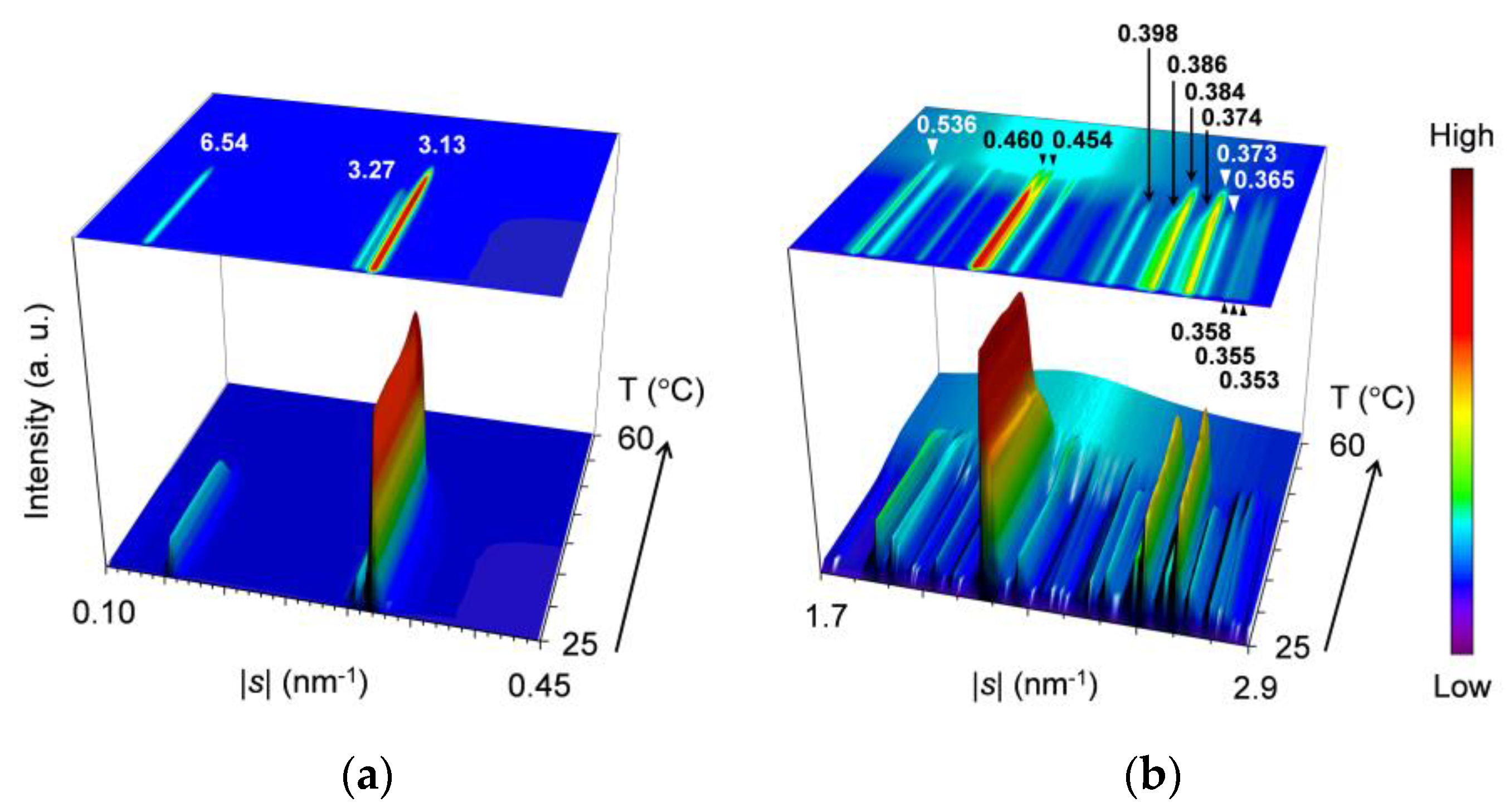
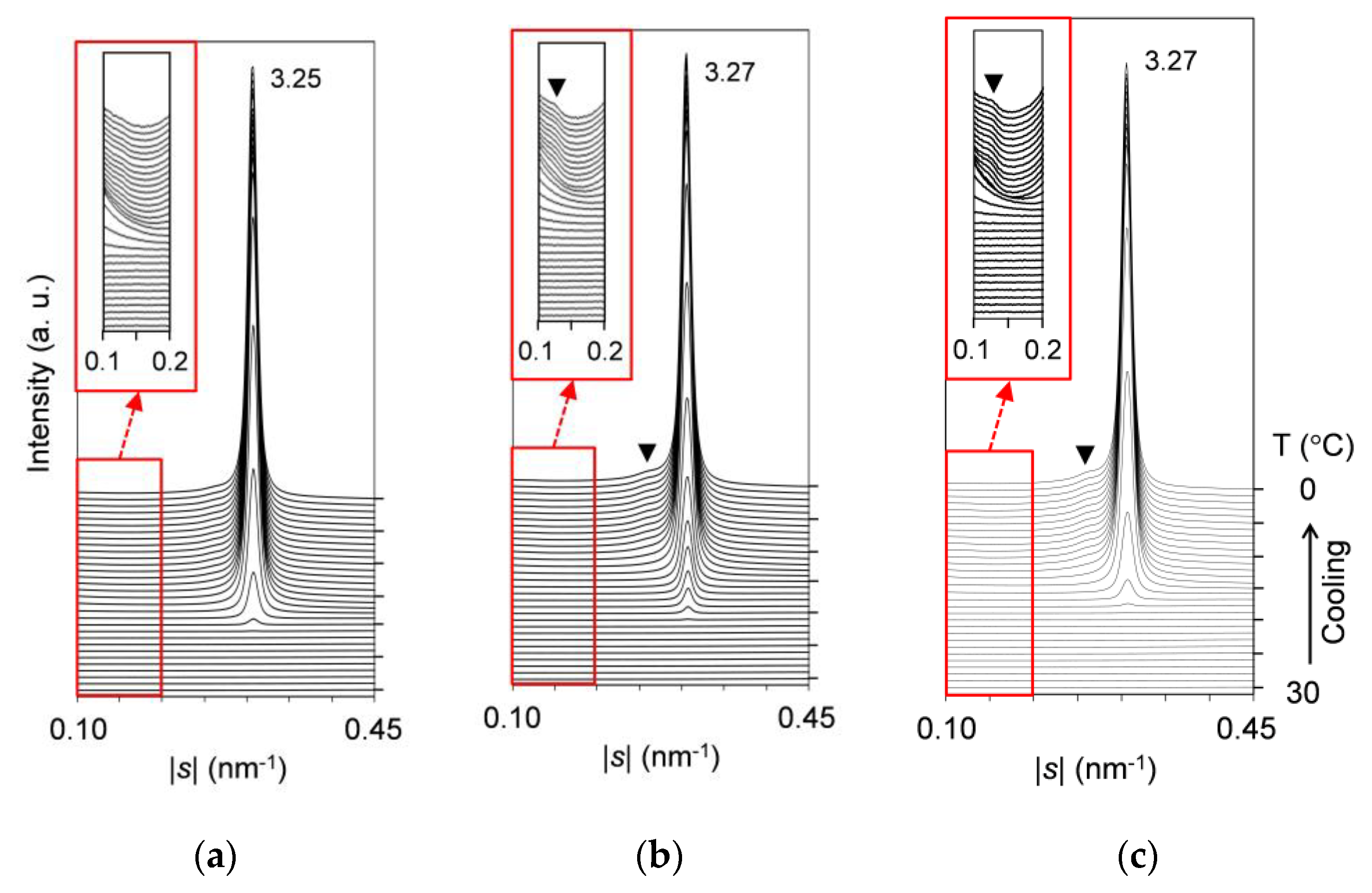
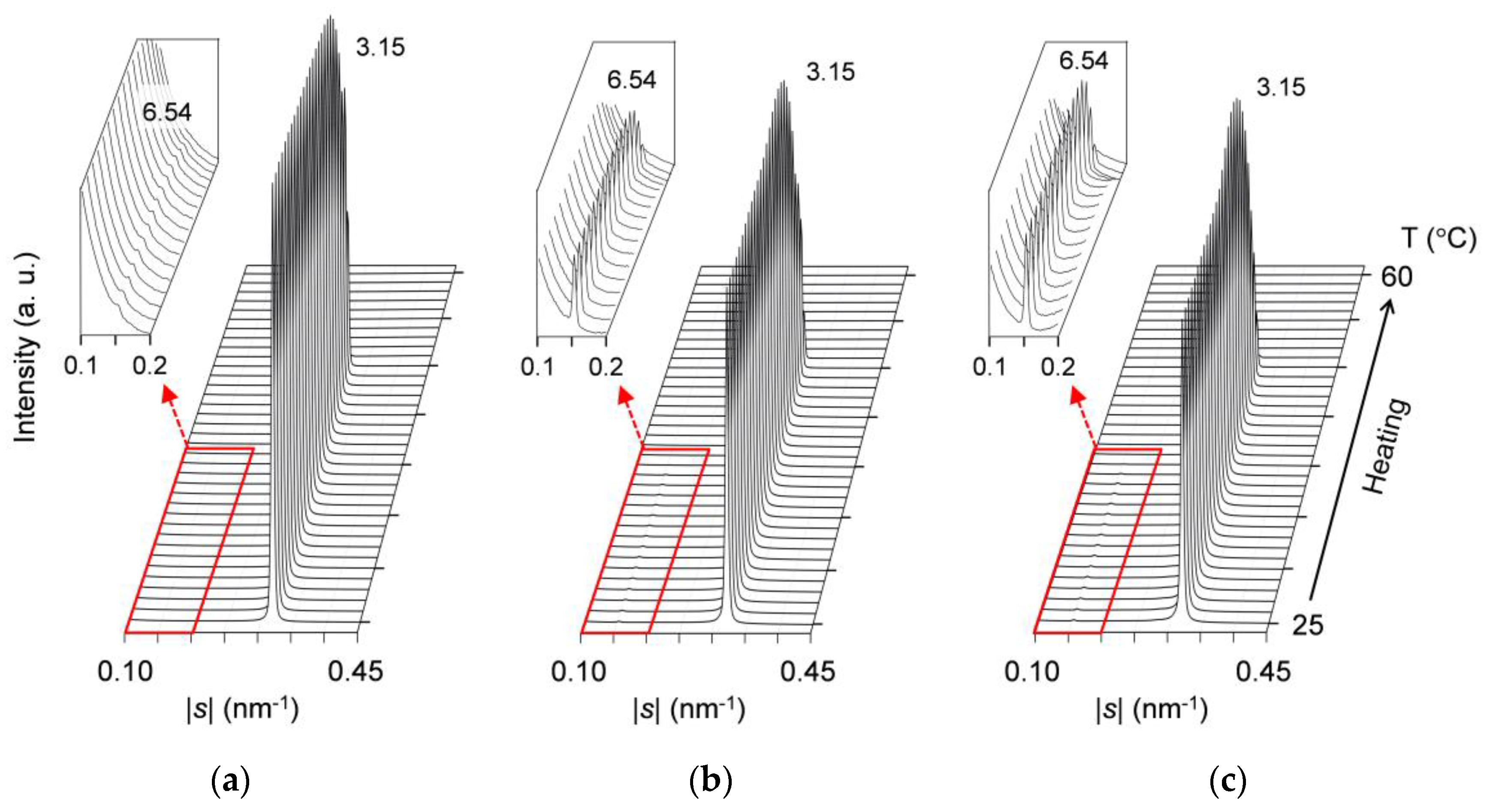
2.2. Polymorphic Phase-Transition Analysis Using SR-XRD
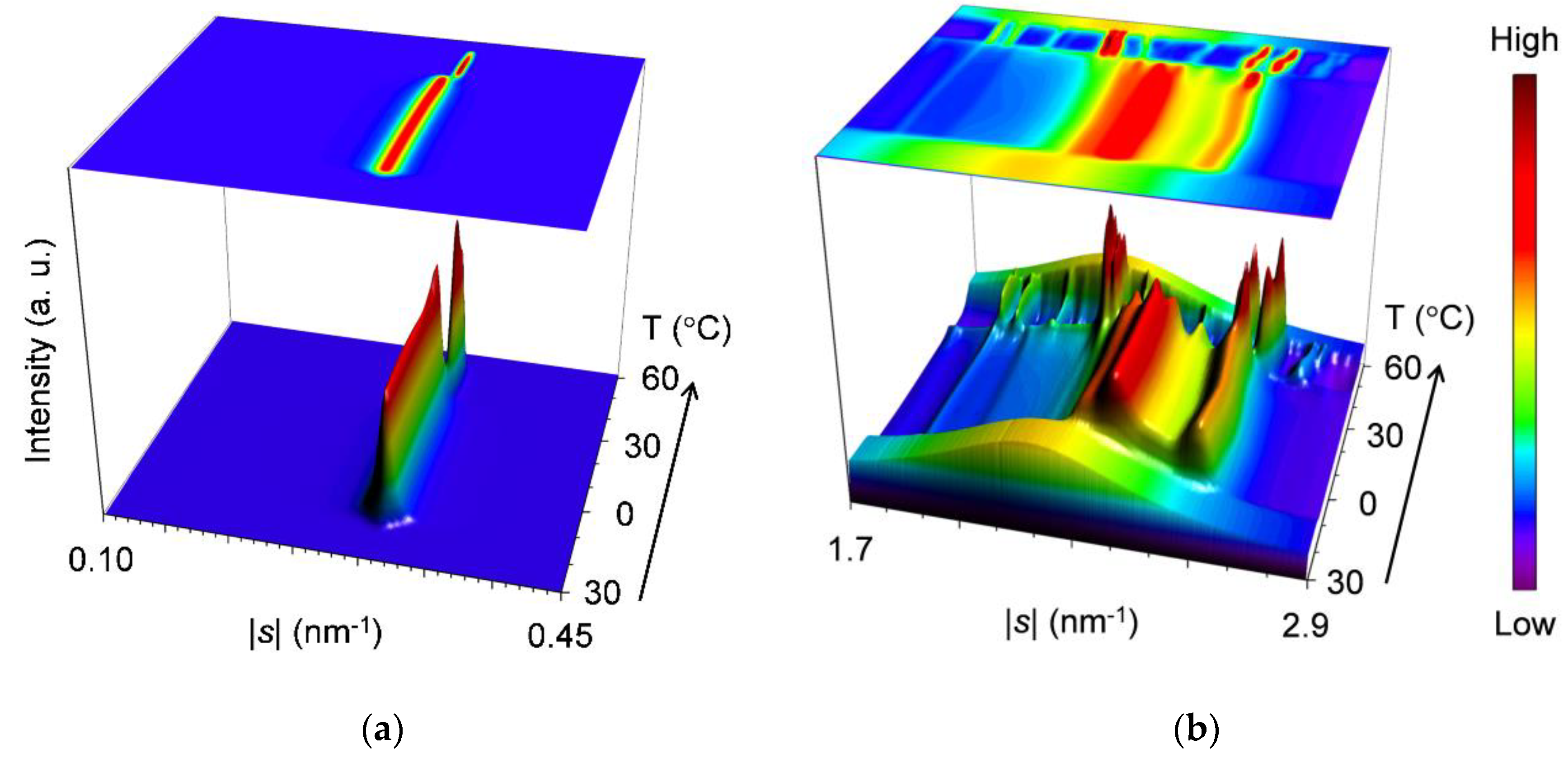
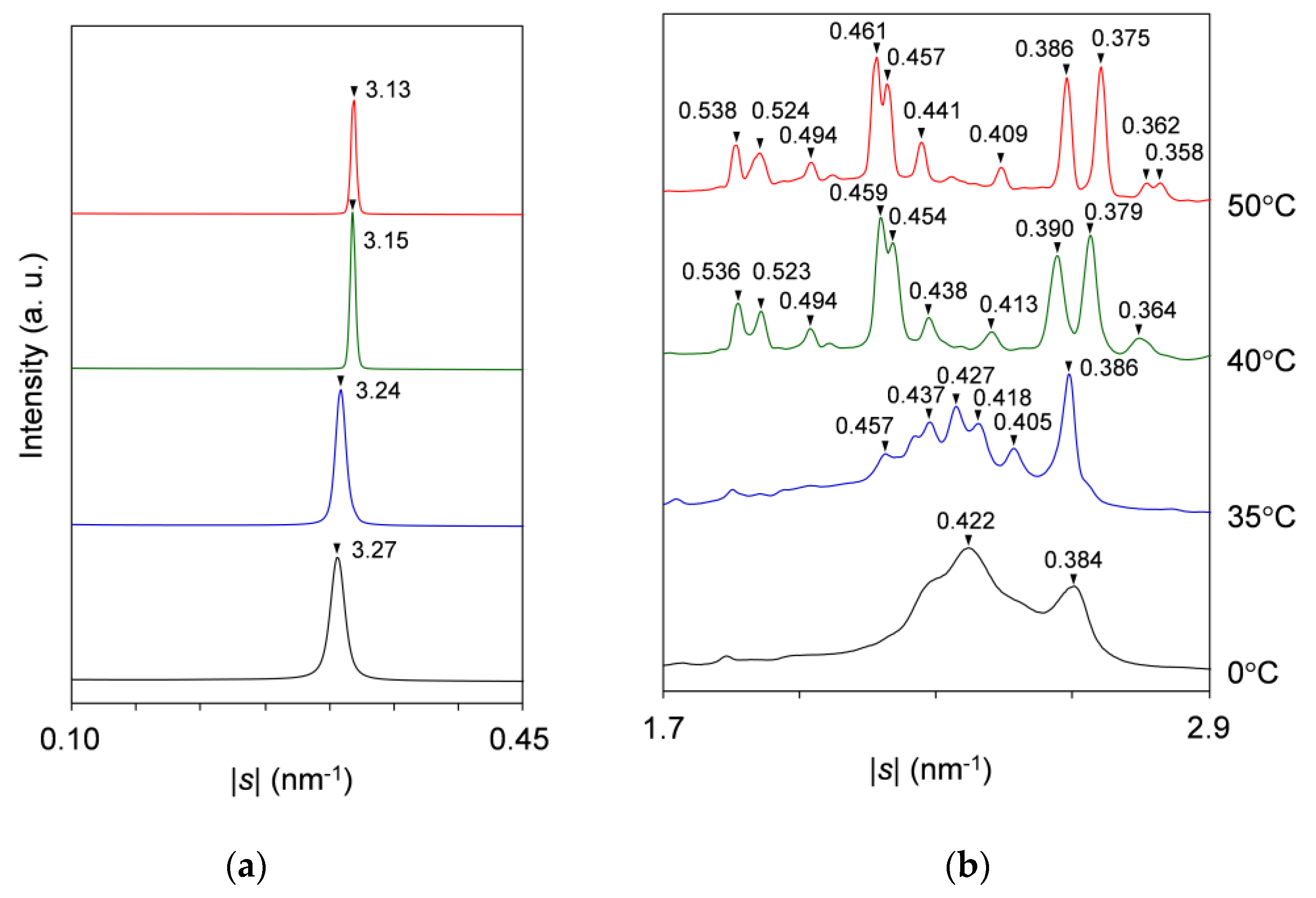
2.2.1. Cooling-and-Heating Phase Transition (wLLL = 0.950)
2.2.2. Cooling-and-Heating Phase Transition (wLLL = 0.667)
2.2.3. Cooling-and-Heating Phase Transition (wLLL = 0.417)
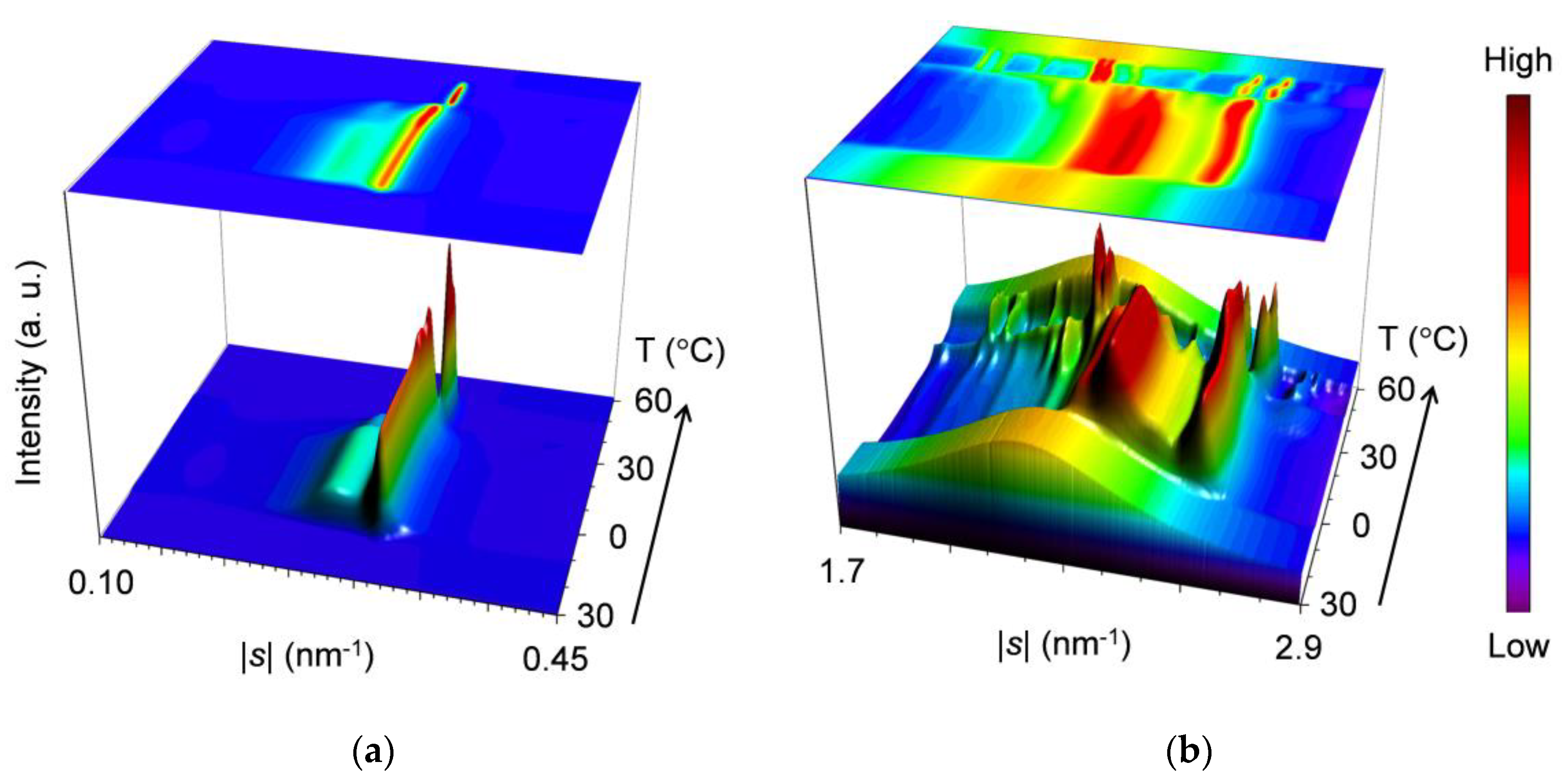
2.2.4. Heating Phase Transition after Aging (wLLL = 0.667)
2.3. Solid-Solubility Limit of SOS in SOS/LLL
2.3.1. Solid-Solubility Limit in Cooling Melt Crystallization
2.3.2. Solid-Solubility Limit after Aging
3. Discussion
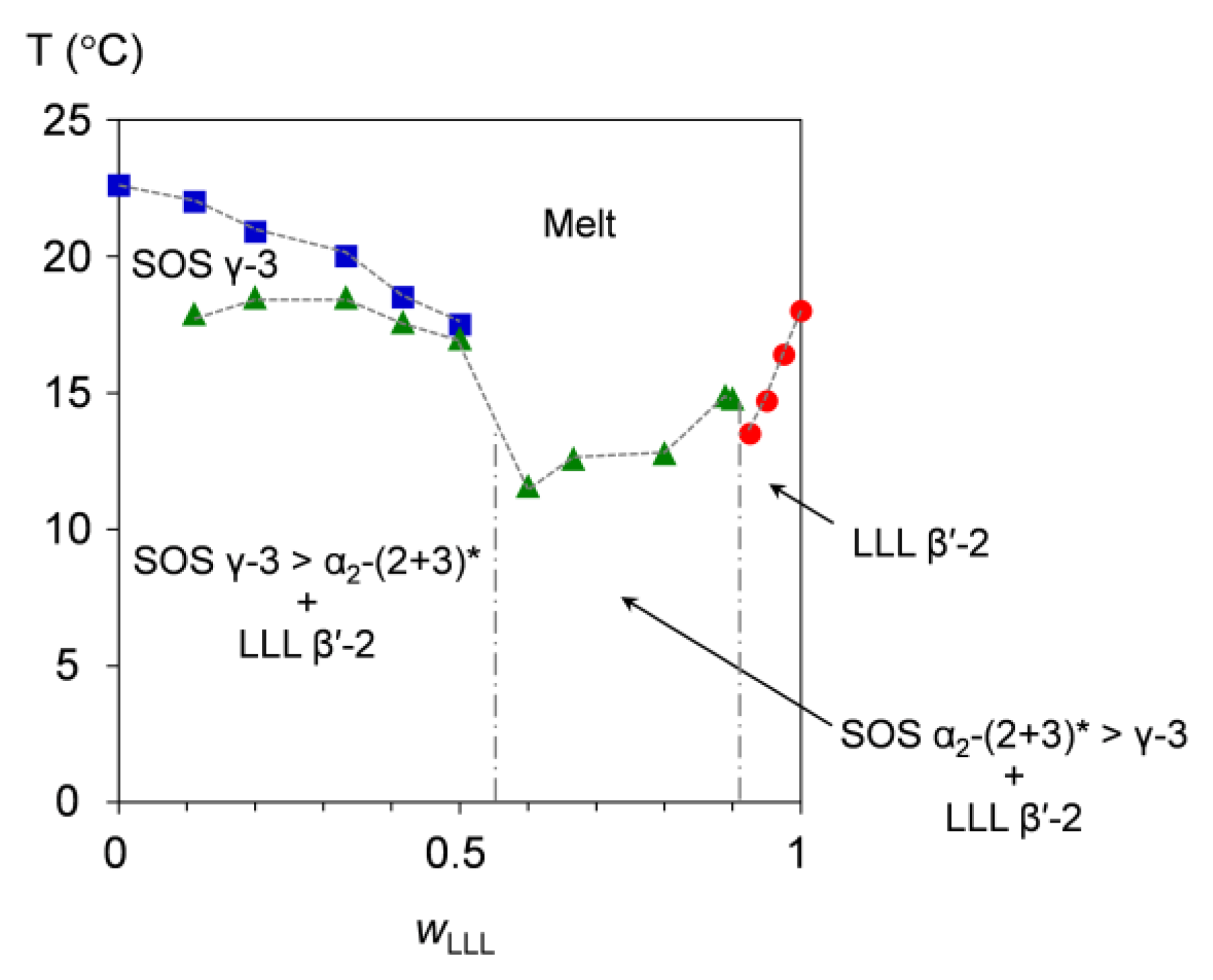
3.1. Binary Phase Behavior of SOS/LLL
3.1.1. Cooling Melt-Crystallization Phase Behavior
3.1.2. Heating Phase Behavior without Aging
3.1.3. Heating Phase Behavior after Aging
3.2. Solubility Limit of SOS in SOS/LLL
3.3. Future Research Directions
4. Materials and Methods
4.1. Materials and Sample Preparation
4.2. Thermal Treatment for Measurements
4.3. DSC Measurements
4.4. SR-XRD Measurements
5. Conclusions
- Under thermodynamically metastable conditions, SOS and LLL form eutectics in their metastable polymorphs, allowing the occurrence of a compatible solid solution;
- The eutectic nature occurring in metastable conditions is retained even after the polymorphic stabilization of SOS and LLL crystals by aging;
- The polymorphic stabilization narrows the solid-solution area of SOS in LLL crystals, indicating that SOS incorporated in LLL crystals as a solid solution separates from the LLL crystals and crystallizes to form a new solid phase.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Asep, E.K.; Jinap, S.; Russly, A.R.; Harcharan, S.; Nazimah, S.A.H. The effects of particle size, fermentation and roasting of cocoa nibs on supercritical fluid extraction of cocoa butter. J. Food Eng. 2008, 85, 450–458. [Google Scholar] [CrossRef]
- Kamphuis, H.J. Production and quality standards of cocoa mass, cocoa butter and cocoa powder. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 121–141. [Google Scholar] [CrossRef]
- Marty-Terrade, S.; Marangoni, A.G. Impact of cocoa butter origin on crystal behavior. In Cocoa Butter and Related Compounds, 1st ed.; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 245–274. [Google Scholar] [CrossRef]
- Loisel, C.; Keller, G.; Lecq, G.; Bourgaux, C.; Ollivon, M. Phase transitions and polymorphism of cocoa butter. J. Am. Oil Chem. Soc. 1998, 75, 425–439. [Google Scholar] [CrossRef]
- Lipp, M.; Simoneau, C.; Ulberth, F.; Anklam, E.; Crews, C.; Brereton, P.; de Greyt, W.; Schwack, W.; Wiedmaier, C. Composition of genuine cocoa butter and cocoa butter equivalents. J. Food Compos. Anal. 2001, 14, 399–408. [Google Scholar] [CrossRef]
- Biehl, B.; Ziegleder, G. Cocoa: Chemistry of processing. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Finglas, P., Toldra, F., Eds.; Academic Press: London, UK, 2003; pp. 1436–1448. [Google Scholar] [CrossRef]
- Joshi, B.L.; Zielbauer, B.I.; Vilgis, T.A. Comparative study on mixing behavior of binary mixtures of cocoa butter/tristearin (CB/TS) and cocoa butter/coconut oil (CB/CO). Foods 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckett, S.T. Traditional chocolate making. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 1–9. [Google Scholar] [CrossRef]
- Da Silva, T.L.T.; Grimaldi, R.; Calligaris, G.A.; Cardoso, L.P.; Gonçalves, L.A.G. Crystallinity properties and crystallization behavior of chocolate fat blends. J. Food Sci. Technol. 2017, 54, 1979–1989. [Google Scholar] [CrossRef] [PubMed]
- Talbot, G. Chocolate temper. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 261–275. [Google Scholar] [CrossRef]
- Bresson, S.; Rousseau, D.; Ghosh, S.; Marssi, M.E.; Faivre, V. Raman spectroscopy of the polymorphic forms and liquid state of cocoa butter. Eur. J. Lipid Sci. Technol. 2011, 113, 992–1004. [Google Scholar] [CrossRef]
- Windhab, E.J. Tempering. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 276–319. [Google Scholar] [CrossRef]
- Delbaere, C.; de Walle, D.V.; Depypere, F.; Gellynck, X.; Dewettinck, K. Relationship between chocolate microstructure, oil migration, and fat bloom in filled chocolates. Eur. J. Lipid Sci. Technol. 2016, 118, 1800–1826. [Google Scholar] [CrossRef]
- Torbica, A.; Jambrec, D.; Tomić, J.; Pajin, B.; Petrović, J.; Kravić, S.; Lončarević, I. Solid fat content, pre-crystallization conditions, and sensory quality of chocolate with addition of cocoa butter analogues. Int. J. Food Prop. 2016, 19, 1029–1043. [Google Scholar] [CrossRef]
- Wiedermann, L.H. Margarine and margarine oil, formulation and control. J. Am. Oil Chem. Soc. 1988, 55, 823–829. [Google Scholar] [CrossRef]
- McClements, D.J.; Decker, E.A. Lipids. In Fennema’s Food Chemistry, 4th ed.; Damodaran, S., Parkin, K.L., Fennema, O.R., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 155–216. [Google Scholar] [CrossRef]
- Lipp, M.; Anklam, E. Review of cocoa butter and alternative fats for use in chocolate—Part A. Compositional data. Food Chem. 1998, 62, 73–97. [Google Scholar] [CrossRef]
- Akhter, S.; McDonald, M.A.; Marriott, R. Mangifera sylvatica (wild mango): A new cocoa butter alternative. Sci. Rep. 2016, 6, 32050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Podchong, P.; Inbumrung, P.; Sonwai, S. The effect of hard lauric fats on the crystallization behavior of cocoa butter substitute. J. Oleo Sci. 2020, 69, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Norulaini, N.A.N.; Sahena, F.; Jinap, S.; Azmir, J.; Sharif, K.M.; Omar, A.K.M. Cocoa butter fats and possibilities of substitution in food products concerning cocoa varieties, alternative sources, extraction methods, composition, and characteristics. J. Food Eng. 2013, 117, 467–476. [Google Scholar] [CrossRef]
- Smith, K.W. Confectionery fats. In Cocoa Butter and Related Compounds, 1st ed.; Garti, N., Widlak, N.R., Eds.; AOCS Press: Urbana, IL, USA, 2012; pp. 475–495. [Google Scholar] [CrossRef]
- Lonchampt, P.; Hartel, R.W. Fat bloom in chocolate and compound coatings. Eur. J. Lipid Sci. Technol. 2004, 106, 241–274. [Google Scholar] [CrossRef]
- Talbot, G. Vegetable fats. In Industrial Chocolate Manufacture and Use, 4th ed.; Beckett, S.T., Ed.; Wiley-Blackwell: Oxford, UK, 2008; pp. 415–433. [Google Scholar] [CrossRef]
- Larsson, B.K. The crystal structure of the β-form of trilaurin. Ark. Kemi. 1964, 23, 1–15. [Google Scholar]
- Van Mechelen, J.B.; Peschar, R.; Schenk, H. Structures of mono-unsaturated triacylglycerols. III. The β-2 polymorphs of trans-mono-unsaturated triacylglycerols and related fully saturated triacylglycerols. Acta Cryst. 2008, B64, 240–248. [Google Scholar] [CrossRef]
- Timms, R.E. Physical properties of oils and mixtures of oils. J. Am. Oil Chem. Soc. 1985, 62, 241–249. [Google Scholar] [CrossRef]
- Rossell, J.B. Fractionation of lauric oils. J. Am. Oil Chem. Soc. 1985, 62, 385–390. [Google Scholar] [CrossRef]
- Ribeiro, A.P.B.; Basso, R.C.; dos Santos, A.O.; Andrade, G.C.; Cardoso, L.P.; Kieckbusch, T.G. Hardfats as crystallization modifiers of cocoa butter. Eur. J. Lipid Sci. Technol. 2013, 115, 1462–1473. [Google Scholar] [CrossRef]
- Gordon, M.H.; Padley, F.B.; Timms, R.E. Factors influencing the use of vegetable fats in chocolate. Eur. J. Lipid Sci. Technol. 1979, 81, 116–121. [Google Scholar] [CrossRef]
- Zaliha, O.; Norizzau, A.R. Physico-chemical properties and compatibility study on palm oil products with cocoa butter. J. Oil Palm Res. 2012, 24, 1349–1352. [Google Scholar]
- Hussain, N.; Agus, B.A.P.; Rahim, S.N.F.A.; Halim, H.S.A. Comparison of quality characteristics between compound and pure milk chocolate. MOJ Food Process Technol. 2018, 6, 292–296. [Google Scholar] [CrossRef]
- Williams, S.D.; Ransom-Painter, K.L.; Hartel, R.W. Mixtures of palm kernel oil with cocoa butter and milk fat in compound coatings. J. Am. Oil Chem. Soc. 1997, 74, 357–366. [Google Scholar] [CrossRef]
- Smith, K.W.; Cain, F.W.; Talbot, G. Nature and composition of fat bloom from palm kernel stearin and hydrogenated palm kernel stearin compound chocolates. J. Agric. Food Chem. 2004, 52, 5539–5544. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, Y.; Shan, L.; Jin, Q.; Wang, X.; Li, L. Blooming in cocoa butter substitutes based compound chocolate: Investigations on composition, morphology and melting behavior. J. Am. Oil Chem. Soc. 2010, 87, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Timms, R.E. Confectionery Fats Handbook, 1st ed.; The Oily Press: Bridgwater, UK, 2003; pp. 9–61. [Google Scholar] [CrossRef]
- Macridachis-González, J.; Bayés-García, L.; Calvet, T. An insight into the solid-state miscibility of triacylglycerol crystals. Molecules 2020, 25, 4562. [Google Scholar] [CrossRef]
- Timms, R.E. Confectionery Fats Handbook, 1st ed.; The Oily Press: Bridgwater, UK, 2003; pp. 255–294. [Google Scholar] [CrossRef]
- Zhang, S.; Ueno, S.; Sato, K.; Adlof, R.O.; List, G.R. Thermal and structural properties of binary mixtures of 1,3-distearoyl-2-oleoyl-glycerol (SOS) and 1,2-dioleoyl-3stearoyl-sn-glycerol (sn-OOS). J. Therm. Anal Calorim. 2009, 98, 105–111. [Google Scholar] [CrossRef]
- Sato, K.; Arishima, T.; Wang, Z.H.; Ojima, K.; Sagi, N.; Mori, H. Polymorphism of POP and SOS. I. occurrence and polymorphic transformation. J. Am. Oil Chem. Soc. 1989, 66, 664–674. [Google Scholar] [CrossRef]
- Takeuchi, M.; Ueno, S.; Sato, K. Synchrotron radiation SAXS/WAXS study of polymorph-dependent phase behavior of binary mixtures of saturated monoacid triacylglycerols. Cryst. Growth Des. 2003, 3, 369–374. [Google Scholar] [CrossRef]
- Minato, A.; Ueno, S.; Yano, J.; Wang, Z.H.; Seto, H.; Amemiya, Y.; Sato, K. Synchrotron radiation X-ray diffraction study on phase behavior of PPP-POP binary mixtures. J. Am. Oil Chem. Soc. 1996, 73, 1567–1572. [Google Scholar] [CrossRef]
- Wesdorp, L.H.; van Meeteren, J.A.; de Jong, S.; van der Giessen, R.; Overbosch, P.; Grootscholten, P.A.M.; Struik, M.; Royers, E.; Don, A.; de Loos, T.; et al. Liquid-multiple solid phase equilibria in fats: Theory and experiments. In Structure and Properties of Fat Crystal Networks, 2nd ed.; Marangoni, A.G., Wesdorp, L.H., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 241–418. [Google Scholar] [CrossRef]
- Bayés-García, L.; Calvet, T.; Cuevas-Diarte, M.À.; Ueno, S.; Sato, K. In situ synchrotron radiation X-ray diffraction study of crystallization kinetics of polymorphs of 1,3-dioleoyl-2-palmitoyl glycerol (OPO). Cryst. Eng. Comm. 2011, 13, 3592–3599. [Google Scholar] [CrossRef]
- Rigolle, A.; van den Abeele, K.; Foubert, I. Conventional and new technologies to monitor lipid crystallization. In Crystallization of Lipids, 1st ed.; Sato, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 465–492. [Google Scholar] [CrossRef]
- Takeguchi, S.; Hondoh, H.; Satoh, A.; Aoki, M.; Uehara, H.; Ueno, S. Morphology of monosaturated triacylglycerol crystals in different crystallization procedures. Nippon. Shokuhin Kagaku Kogaku Kaishi 2019, 66, 451–458. [Google Scholar] [CrossRef]
- Lee, Y.L.; Ristic, R.I.; de Matos, L.L.; Martin, C.M. Crystallization pathways of polymorphic triacylglycerols induced by mechanical energy. J. Phys. Conf. Ser. 2010, 247, 1–16. [Google Scholar] [CrossRef]
- Kaneko, F.; Yamamoto, Y.; Yoshikawa, S. Structural study on fat crystallization process heterogeneously induced by graphite surfaces. Molecules 2020, 25, 4786. [Google Scholar] [CrossRef]
- Allais, C.; Keller, G.; Lesieur, P.; Ollivon, M.; Artzner, F. X-ray diffraction/calorimetry coupling. J. Therm. Anal. Calorim. 2004, 74, 723–728. [Google Scholar] [CrossRef]
- Mykhaylyk, O.O.; Smith, K.W.; Martin, C.M.; Ryan, A.J. Structural models of metastable phases occurring during the crystallization process of saturated/unsaturated triacylglycerols. J. Appl. Cryst. 2007, 40, s297–s302. [Google Scholar] [CrossRef]
- Timms, R.E. Confectionery Fats Handbook, 1st ed.; The Oily Press: Bridgwater, UK, 2003; pp. 191–254. [Google Scholar] [CrossRef]
- Kawada, T.; Suzuki, S.; Kamata, F.; Matsui, N. Studies on the lauric hard butter. III. Fat bloom (2). J. Jpn. Oil Chem. Soc. 1971, 20, 807–810. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Yoshikawa, S.; Sato, K. Formation and properties of dark chocolate prepared using fat mixtures of cocoa butter and symmetric/asymmetric stearic-oleic mixed-acid triacylglycerols: Impact of molecular compound crystals. Food Chem. 2021, 339, 127808. [Google Scholar] [CrossRef]

| Polymorph 1 | Melting Point (°C) | Long Spacing (nm) | Short Spacing (nm) | |
|---|---|---|---|---|
| SOS 2 | sub α-2 | — | 5.00 | 0.42, 0.41 |
| α-2 | 23.5 | 4.83 | 0.421 | |
| γ-3 | 35.4 | 7.05 | 0.472, 0.450, 0.388, 0.363 | |
| β′-3 | 36.5 | 7.00 | 0.430, 0.415, 0.402, 0.395, 0.383, 0.370 | |
| β2-3 | 41.0 | 6.50 | 0.458, 0.400, 0.390, 0.375, 0.367, 0.357 | |
| β1-3 | 43.0 | 6.50 | 0.458, 0.402, 0.397, 0.385, 0.380, 0.365 | |
| LLL 3 | α-2 | 15.0 | 3.5 | 0.42 |
| β′-2 | 35.0 | 3.2 | 0.42, 0.38 | |
| β-2 | 46.5 | 3.1 | 0.46, 0.39, 0.38 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshikawa, S.; Watanabe, S.; Yamamoto, Y.; Kaneko, F. Binary Phase Behavior of 1,3-Distearoyl-2-oleoyl-sn-glycerol (SOS) and Trilaurin (LLL). Molecules 2020, 25, 5313. https://doi.org/10.3390/molecules25225313
Yoshikawa S, Watanabe S, Yamamoto Y, Kaneko F. Binary Phase Behavior of 1,3-Distearoyl-2-oleoyl-sn-glycerol (SOS) and Trilaurin (LLL). Molecules. 2020; 25(22):5313. https://doi.org/10.3390/molecules25225313
Chicago/Turabian StyleYoshikawa, Shinichi, Shimpei Watanabe, Yoshinori Yamamoto, and Fumitoshi Kaneko. 2020. "Binary Phase Behavior of 1,3-Distearoyl-2-oleoyl-sn-glycerol (SOS) and Trilaurin (LLL)" Molecules 25, no. 22: 5313. https://doi.org/10.3390/molecules25225313






