Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review
Abstract
:1. Introduction
2. Results and Discussions
2.1. Study Analysis
2.2. Hydroxychloroquine and Viral Infections
2.2.1. HIV-1
2.2.2. Chikungunya Virus
2.2.3. Flaviviruses
2.2.4. Coronavirus Disease of 2019
2.3. Hydroxychloroquine Biological Activity
2.3.1. Rheumatoid Arthritis
2.3.2. Lupus Erythematosus
2.3.3. Antiphospholipid Syndrome
2.3.4. Sjögren Syndrome
2.3.5. Diabetes
2.3.6. Others (Cancer, Inflammation, Cardiovascular Diseases)
2.4. Hydroxychloroquine and Synergic Effect
2.4.1. Autoimmune Diseases
2.4.2. Cardiovascular Risk Management
2.4.3. Anticancer
2.4.4. Bacterial Infections
3. Materials and Methods
3.1. Search Strategy
3.2. Study Selection
3.3. Data extraction
3.4. Methodological Quality Assessment
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACF | Aceclofenac |
| At | Adenoid tissue |
| AZM | Azithromycin |
| Bcl | β-Cell Lymphoma |
| CHIKV | Chikungunya Virus |
| cpm | Counts Per Minute |
| COVID-19 or 2019-nCoV | new coronavirus delivery in 2019 |
| CQ | Chloroquine |
| DMARDs | Disease-Modifying Antirheumatic Drugs |
| DXC | Doxycycline |
| ECG | Electrocardiogram |
| FGT | Female Genital Tract |
| HAART | Antiretroviral therapy |
| HCQ | Hydroxychloroquine |
| HIV | Human Immunodeficiency Virus |
| HOMA | Homeostatic Model Assessment |
| i.g. | Intragastrically |
| IgG | Immunoglobulin G |
| i.m. | Intramuscular injection |
| i.p. | Intraperitoneal injection |
| ICU | Intensive care units |
| IFN-α | Type I interferon |
| IL | Interleukin |
| LDL | Low-Density Lipoprotein |
| MXT | Methotrexate |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| p.o. | Per oral administration |
| PRD | Prednisolone |
| Q fever. | Query fever |
| RPE | Retinal Pigment Epithelium |
| ROS | Reactive Oxygen Species |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome caused by COVID-19 |
| SOC | Standard-of-care |
| TGF-β | Transforming growth factor-β |
| TLR-4 | Toll-like receptor 4 |
| Tregs | T-regulatory cells |
| TNF-α | Tumor necrosis factors |
| VAS | Visual Analog Scale |
| ZDV | Zidovudine |
| ZIKV | Zika virus |
References
- Wallace, D.J. Antimalarials-the “real” advance in lupus. Lupus 2001, 10, 385–387. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.J. The history of antimalarials. Lupus 1996, 5, 2–3. [Google Scholar] [CrossRef]
- Bezati, E.; Wu, X.-X.; Quinn, A.S.; Taatjes, D.J.; Rand, J.H. A new trick for an ancient drug: Quinine dissociates antiphospholipid immune complexes. Lupus 2015, 24, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Coatney, G.R. Pitfalls in a discovery: The chronicle of chloroquine. Am. J. Trop. Med. Hyg. 1963, 12, 121–128. [Google Scholar] [CrossRef]
- Al-Bari, M.A.A. Chloroquine analogues in drug discovery: New directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J. Antimicrob. Chemoth. 2015, 70, 1608–1621. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of hydroxychloroquine in patients with COVID-19: Results of a randomized clinical trial. MedRxiv 2020. his Preprint. Available online: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3 (accessed on 10 April 2020).
- Xu, C.; Zhu, L.; Chan, T.; Lu, X.; Shen, W.; Madigan, M.C.; Gillies, M.C.; Zhou, F. Chloroquine and hydroxychloroquine are novel inhibitors of human organic anion transporting polypeptide 1A2. J. Pharm. Sci. 2016, 105, 884–890. [Google Scholar] [CrossRef]
- Fantini, J.; Di Scala, C.; Chahinian, H.; Yahi, N. Structural and molecular modeling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob. Agents 2020, 55, 105960. [Google Scholar] [CrossRef]
- McLachlan, A.J.; Tett, S.E.; Cutler, D.J.; Day, R.O. Disposition of the enantiomers of hydroxychloroquine in patients with rheumatoid arthritis following multiple doses of the racemate. Br. J. Clin. Pharmacol. 1993, 36, 78–81. [Google Scholar] [CrossRef] [Green Version]
- Dongre, V.G.; Ghugare, P.D.; Karmuse, P.; Singh, D.; Jadhav, A.; Kumar, A. Identification and characterization of process related impurities in chloroquine and hydroxychloroquine by LC/IT/MS, LC/TOF/MS and NMR. J. Pharm. Biomed. Anal. 2009, 49, 873–879. [Google Scholar] [CrossRef]
- Tett, S.E.; Cutler, D.J.; Day, R.O.; Brown, K.F. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br. J. Clin. Pharmacol. 1989, 27, 771–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Bari, M.A.A.I. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. J. Pharmacol. Res. Perspect. 2017, 5, e20093. [Google Scholar] [CrossRef] [PubMed]
- Plantone, D.; Koudriavtseva, T. Current and future use of chloroquine and hydroxychloroquine in infectious, immune, neoplastic, and neurological diseases: A mini-review. Clin. Drug Investig. 2018, 38, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Samuel, C.E. Antiviral actions of interferons. Clin. Microbiol. Rev. 2001, 14, 778–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperber, K.; Kalb, T.H.; Stecher, V.J.; Banerjee, R.; Mayer, L. Inhibition of human immunodeficiency virus type 1 replication by hydroxychloroquine in T cells and monocytes. AIDS Res. Hum. Retrov. 1993, 9, 91–98. [Google Scholar] [CrossRef]
- Chiang, G.; Sassaroli, M.; Louie, M.; Chen, H.; Stecher, V.J.; Sperber, K. Inhibition of HIV-1 replication by hydroxychloroquine: Mechanism of action and comparison with zidovudine. Clin. Ther. 1996, 18, 1080–1092. [Google Scholar] [CrossRef]
- Sperber, K.; Louie, M.; Kraus, T.; Proner, J.; Sapira, E.; Lin, S.; Stecher, V.; Mayer, L. Hydroxychloroquine treatment of patients with human immunodeficiency virus type 1. Clin. Ther. 1995, 17, 622–636. [Google Scholar] [CrossRef]
- Sperber, K.; Chiang, G.; Chen, H.; Ross, W.; Chusid, E.; Gonchar, M.; Chow, R.; Liriano, O. Comparison of hydroxychloroquine with zidovudine in asymptomatic patients infected with human immunodeficiency virus type 1. Clin Ther 1997, 19, 913–923. [Google Scholar] [CrossRef]
- Paton, N.I.; Aboulhab, J.; Karim, F. Hydroxychloroquine, hydroxycarbamide, and didanosine as economic treatment for HIV-1. The Lancet 2002, 359, 1667–1668. [Google Scholar] [CrossRef]
- Paton, N.; Aboulhab, J.J. Hydroxychloroquine, hydroxyurea and didanosine as initial therapy for HIV-infected patients with low viral load: Safety, efficacy and resistance profile after 144 weeks. HIV Medicine 2005, 6, 13–20. [Google Scholar] [CrossRef]
- Aguirre-Cruz, L.; Torres, K.J.; Jung-Cook, H.; Fortuny, C.; Sánchez, E.; Soda-Mehry, A.; Sotelo, J.; Reyes-Terán, G. Preferential concentration of hydroxychloroquine in adenoid tissue of HIV-infected subjects. AIDS Res. Hum. Retrov. 2010, 26, 339–342. [Google Scholar] [CrossRef] [PubMed]
- González-Hernández, I.; Aguirre-Cruz, L.; Sotelo, J.; López-Arellano, R.; Morales-Hipólito, A.; Jung-Cook, H. Distribution of hydroxychloroquine in lymphoid tissue in a rabbit model for HIV infection. Antimicrob. Agents Chemother. 2014, 58, 584–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piconi, S.; Parisotto, S.; Rizzardini, G.; Passerini, S.; Terzi, R.; Argenteri, B.; Meraviglia, P.; Capetti, A.; Biasin, M.; Trabattoni, D. Hydroxychloroquine drastically reduces immune activation in HIV-infected, antiretroviral therapy–treated immunologic nonresponders. Blood 2011, 118, 3263–3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paton, N.I.; Goodall, R.L.; Dunn, D.T.; Franzen, S.; Collaco-Moraes, Y.; Gazzard, B.G.; Williams, I.G.; Fisher, M.J.; Winston, A.; Fox, J. Effects of hydroxychloroquine on immune activation and disease progression among HIV-infected patients not receiving antiretroviral therapy: A randomized controlled trial. JAMA Ophthalmol. 2012, 308, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Traore, Y.L.; Yang, S.; Lajoie, J.; Fowke, K.R.; Rickey, D.W.; Ho, E.A. Implant delivering hydroxychloroquine attenuates vaginal T lymphocyte activation and inflammation. J. Control. Release 2018, 277, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Padmakumar, B.; Jayan, J.B.; Menon, R.M.; Krishnankutty, B.; Payippallil, R.; Nisha, R. Comparative evaluation of four therapeutic regimes in chikungunya arthritis: A prospective randomized parallel-group study. Indian J. Rheumatol. 2009, 4, 94–101. [Google Scholar] [CrossRef]
- Bouquillard, E.; Fianu, A.; Bangil, M.; Charlette, N.; Ribéra, A.; Michault, A.; Favier, F.; Simon, F.; Flipo, R.-M. Rheumatic manifestations associated with Chikungunya virus infection: A study of 307 patients with 32-month follow-up (RHUMATOCHIK study). Joint Bone Spine. 2018, 85, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, V.; Alias, G. Efficacy of combination DMARD therapy vs. hydroxychloroquine monotherapy in chronic persistent chikungunya arthritis: A 24-week randomized controlled open label study. Clin. Rheumatol. 2017, 36, 1335–1340. [Google Scholar] [CrossRef]
- Pandya, S. Methotrexate and hydroxychloroquine combination therapy in chronic chikungunya arthritis: A 16 week study. Indian J. Rheumatol. 2008, 3, 93–97. [Google Scholar] [CrossRef]
- Helal, G.K.; Gad, M.A.; Abd-Ellah, M.F.; Eid, M.S. Hydroxychloroquine augments early virological response to pegylated interferon plus ribavirin in genotype-4 chronic hepatitis C patients. J. Med. Virol. 2016, 88, 2170–2178. [Google Scholar] [CrossRef]
- Cao, B.; Parnell, L.A.; Diamond, M.S.; Mysorekar, I.U. Inhibition of autophagy limits vertical transmission of Zika virus in pregnant mice. J. Exp. Med. 2017, 214, 2303–2313. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; Dupont, H.T.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Meddeb, L.; Sevestre, J.; Mailhe, M.; Doudier, B.; Aubry, C.; Amrane, S.; Seng, P.; et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID-19 patients with at least a six-day follow up: A pilot observational study. Travel Med. Infect. Di. 2020, 34, 101663. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.M.; Delaugerre, C.; Goff, J.L.; Mela-Lima, B.; Ponscarme, D.; Goldwirt, L.; de Castro, N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020, 50, 384. [Google Scholar] [CrossRef]
- Tang, W.; Cao, Z.; Han, M.; Wang, Z.; Chen, J.; Sun, W.; Wu, Y.; Xiao, W.; Liu, S.; Chen, E. Hydroxychloroquine in patients with COVID-19: An open-label, randomized, controlled trial. MedRxiv 2020. Preprint. Available online: https://www.medrxiv.org/content/10.1101/2020.04.10.20060558v2 (accessed on 7 May 2020).
- Abd-Elsalam, S.; Esmail, E.S.; Khalaf, M.; Abdo, E.F.; Medhat, M.A.; Abd El Ghafar, M.S.; Ahmed, O.A.; Soliman, S.; Serangawy, G.N.; Alboraie, M. Hydroxychloroquine in the treatment of COVID-19: A multicenter randomized controlled study. Am. J. Trop. Med. Hyg. 2020, 103, 1635–1639. [Google Scholar] [CrossRef]
- Skipper, C.P.; Pastick, K.A.; Engen, N.W.; Bangdiwala, A.S.; Abassi, M.; Lofgren, S.M.; Williams, D.A.; Okafor, E.C.; Pullen, M.F.; Nicol, M.R. Hydroxychloroquine in nonhospitalized adults with early COVID-19: A randomized trial. Ann. Intern. Med. 2020, 173, 623–631. [Google Scholar] [CrossRef]
- Mahevas, M.; Tran, V.-T.; Roumier, M.; Chabrol, A.; Paule, R.; Guillaud, C.; Gallien, S.; Lepeule, R.; Szwebel, T.-A.; Lescure, X.; et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: Results of a study using routinely collected data to emulate a target trial. MedRxiv 2020. Prepint. Available online: https://www.medrxiv.org/content/10.1101/2020.04.10.20060699v1 (accessed on 14 April 2020).
- Mahévas, M.; Tran, V.T.; Roumier, M.; Chabrol, A.; Paule, R.; Guillaud, C.; Fois, E.; Lepeule, R.; Szwebel, T.A.; Lescure, F.X.; et al. Clinical efficacy of hydroxychloroquine in patients with covid-19 pneumonia who require oxygen: Observational comparative study using routine care data. BMJ 2020, 369, m2328. [Google Scholar]
- Lee, S.H.; Son, H.; Peck, K.R. Can post-exposure prophylaxis for COVID-19 be considered as one of outbreak response strategies in long-term care hospitals? Int. J. Antimicrob. Agents 2020, 55, 105988. [Google Scholar] [CrossRef]
- Boulware, D.R.; Pullen, M.F.; Bangdiwala, A.S.; Pastick, K.A.; Lofgren, S.M.; Okafor, E.C.; Skipper, C.P.; Nascene, A.A.; Nicol, M.R.; Abassi, M. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N. Engl. J. Med. 2020, 383, 517–525. [Google Scholar] [CrossRef] [PubMed]
- French, M.A.; King, M.S.; Tschampa, J.M.; da Silva, B.A.; Landay, A.L. Serum immune activation markers are persistently increased in patients with HIV infection after 6 years of antiretroviral therapy despite suppression of viral replication and reconstitution of CD4+ T cells. J. Infect. Dis. 2009, 200, 1212–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ornstein, M.H.; Sperber, K. The antiinflammatory and antiviral effects of hydroxychloroquine in two patients with acquired immunodeficiency syndrome and active inflammatory arthritis. Arthritis Rheum. 1996, 39, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Chaaithanya, I.K.; Muruganandam, N.; Sundaram, S.G.; Kawalekar, O.; Sugunan, A.P.; Manimunda, S.P.; Ghosal, S.R.; Muthumani, K.; Vijayachari, P. Role of proinflammatory cytokines and chemokines in chronic arthropathy in CHIKV infection. Viral Immunol. 2011, 24, 265–271. [Google Scholar] [CrossRef]
- Lin, K.-M.; Cheng, T.-T.; Lin, J.-C.; Chen, C.-J. Tumor necrosis factor-α antagonist therapy for concomitant rheumatoid arthritis and hepatitis C virus infection: A case series study. Clin. Rheumatol. 2015, 34, 1039–1046. [Google Scholar] [CrossRef]
- Marzano, A.; Angelucci, E.; Andreone, P.; Brunetto, M.; Bruno, R.; Burra, P.; Caraceni, P.; Daniele, B.; Di Marco, V.; Fabrizi, F.J. Prophylaxis and treatment of hepatitis B in immunocompromised patients. Dig. Liver Dis. 2007, 39, 397–408. [Google Scholar] [CrossRef]
- Zingarelli, S.; Airò, P.; Frassi, M.; Bazzani, C.; Scarsi, M.; Puoti, M. Prophylaxis and therapy of HBV infection in 20 patients treated with disease modifying antirheumatic drugs or with biological agents for rheumatic diseases. Reumatismo 2008, 22–27. [Google Scholar] [CrossRef] [Green Version]
- Mo, Y.-Q.; Liang, A.-Q.; Ma, J.-D.; Chen, L.-F.; Zheng, D.-H.; Schumacher, H.R.; Dai, L. Discontinuation of antiviral prophylaxis correlates with high prevalence of hepatitis B virus (HBV) reactivation in rheumatoid arthritis patients with HBV carrier state: A real-world clinical practice. BMC Musculoskelet. Disord. 2014, 15, 449. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Coronavirus Disease (COVID-19) Outbreak Situation. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 23 March 2020).
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Million, M.; Lagier, J.-C.; Gautret, P.; Colson, P.; Fournier, P.-E.; Amrane, S.; Hocquart, M.; Mailhe, M.; Esteves-Vieira, V.; Doudier, B.; et al. Full-length title: Early treatment of COVID-19 patients with hydroxychloroquine and azithromycin: A retrospective analysis of 1061 cases in Marseille, France. Travel Med. Infect. Di. 2020, 35, 101738. [Google Scholar] [CrossRef]
- Chorin, E.; Dai, M.; Shulman, E.; Wadhwani, L.; Bar-Cohen, R.; Barbhaiya, C.; Aizer, A.; Holmes, D.; Bernstein, S.; Spinelli, M.; et al. The QT interval in patients with COVID-19 treated with hydroxychloroquine and azithromycin. Nat. Med. 2020, 26, 808–809. [Google Scholar] [CrossRef] [PubMed]
- Chorin, E.; Wadhwani, L.; Magnani, S.; Dai, M.; Shulman, E.; Nadeau-Routhier, C.; Knotts, R.; Bar-Cohen, R.; Kogan, E.; Barbhaiya, C.; et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm 2020, 17, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- Mitra, R.L.; Greenstein, S.A.; Epstein, L.M. An algorithm for managing QT prolongation in Coronavirus Disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: Possible benefits of intravenous lidocaine. HeartRhythm Case Rep. 2020, 6, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi, J.R.; Noseworthy, P.A.; Friedman, P.A.; Ackerman, M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for Coronavirus disease 19 (COVID-19). Mayo Clin. Proc. 2020, 95, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020, 6, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.K.; Kinikar, A.A.; Jadhav, T. Adverse drug reaction profile of prophylactic hydroxychloroquine for COVID-19 among doctors. Med. J. DY Patil Vidyapeeth 2020, 13, 204. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Guedj, J.; Contreras, V.; Behillil, S.; Solas, C.; Marlin, R.; Naninck, T.; Pizzorno, A.; Lemaitre, J.; Gonçalves, A. Hydroxychloroquine in the treatment and prophylaxis of SARS-CoV-2 infection in non-human primates. Res. Square 2020. [Google Scholar] [CrossRef]
- Lahouati, M.; Mériglier, E.; Martin, L.; Bouchet, S.; Desclaux, A.; Bonnet, F. COVID-19 infection also occurs in patients taking hydroxychloroquine. J. Antimicrob. Chemother. 2020, 75, 2014–2015. [Google Scholar] [CrossRef]
- Monti, S.; Balduzzi, S.; Delvino, P.; Bellis, E.; Quadrelli, V.S.; Montecucco, C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann. Rheum. Dis. 2020, 79, 667–668. [Google Scholar] [CrossRef] [Green Version]
- EUA Hydroxychloroquine Sulfate Health Care Provider Fact Sheet. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization (accessed on 31 March 2020).
- Coronavirus (COVID-19) Update: FDA Revokes Emergency Use Authorization for Chloroquine and Hydroxychloroquine. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-COVID-19-update-fda-revokes-emergency-use-authorization-chloroquine-and (accessed on 18 June 2020).
- Alhazzani, W.; Møller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 48, 1. [Google Scholar] [CrossRef]
- Qaseem, A.; Yost, J.; Etxeandia-Ikobaltzeta, I.; Miller, M.C.; Abraham, G.M.; Obley, A.J.; Forciea, M.A.; Jokela, J.A.; Humphrey, L.L. Should clinicians use chloroquine or hydroxychloroquine alone or in combination with azithromycin for the prophylaxis or treatment of COVID-19? Living practice points from the American college of physicians (Version 1). Ann. Intern. Med. 2020, 173. [Google Scholar]
- Treatments for COVID-19: Canadian Arm of the SOLIDARITY Trial (CATCO). Available online: https://clinicaltrials.gov/ct2/show/NCT04330690 (accessed on 26 August 2020).
- Randomised Evaluation of COVID-19 Therapy (RECOVERY). Available online: https://clinicaltrials.gov/ct2/show/NCT04381936 (accessed on 27 August 2020).
- Trial of Treatments for COVID-19 in Hospitalized Adults (DisCoVeRy). Available online: https://clinicaltrials.gov/ct2/show/NCT04315948 (accessed on 22 July 2020).
- World Health Organisation. Q&A: Hydroxychloroquine and COVID-19. Available online: https://www.who.int/news-room/q-a-detail/q-a-hydroxychloroquine-and-covid-19 (accessed on 19 June 2020).
- Kashour, T.; Tleyjeh, I.M. It is time to drop hydroxychloroquine from our COVID-19 armamentarium. Med. Hypotheses 2020, 144, 110198. [Google Scholar] [CrossRef]
- Cutler, D. Possible mechanisms of action of antimalarials in rheumatic disease. Agents Actions Suppl. 1993, 44, 139–143. [Google Scholar] [PubMed]
- Garcia-Cremades, M.; Solans, B.P.; Hughes, E.; Ernest, J.P.; Wallender, E.; Aweeka, F.; Luetkemeyer, A.F.; Savic, R.M. Optimizing hydroxychloroquine dosing for patients with COVID-19: An integrative modeling approach for effective drug repurposing. Clin. Pharmacol. Ther. 2020, 108, 253–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naso, J.R.; Wong, D.; Wong, D.R.; Chen, C.-H.; Hoang, L.M. Tropheryma whipplei endocarditis presenting as chronic valvular disease: A case report and review of literature. Hum. Phatol. Case Rep. 2019, 18, 200321. [Google Scholar] [CrossRef]
- Vogl, D.T.; Stadtmauer, E.A.; Tan, K.S.; Heitjan, D.F.; Davis, L.E.; Pontiggia, L.; Rangwala, R.; Piao, S.; Chang, Y.C.; Scott, E.C.; et al. Combined autophagy and proteasome inhibition: A phase 1 trial of hydroxychloroquine and bortezomib in patients with relapsed/refractory myeloma. Autophagy 2014, 10, 1380–1390. [Google Scholar] [CrossRef] [Green Version]
- Kanvinde, S.; Chhonker, Y.S.; Ahmad, R.; Yu, F.; Sleightholm, R.; Tang, W.; Jaramillo, L.; Chen, Y.; Sheinin, Y.; Li, J.; et al. Pharmacokinetics and efficacy of orally administered polymeric chloroquine as macromolecular drug in the treatment of inflammatory bowel disease. Acta Biomater. 2018, 82, 158–170. [Google Scholar] [CrossRef]
- Jancinova, V.; Pazourekova, S.; Lucova, M.; Perecko, T.; Mihalova, D.; Bauerova, K.; Nosal, R.; Drabikova, K. Selective inhibition of extracellular oxidants liberated from human neutrophils-A new mechanism potentially involved in the anti-inflammatory activity of hydroxychloroquine. Int Immunopharmacol 2015, 28, 175–181. [Google Scholar] [CrossRef]
- The HERA Study Group. A-randomized-trial-of-hydroxychloroquine-in-early-rheumatoid. Am. J. Med. 1995, 98, 156–168. [Google Scholar] [CrossRef]
- Nuver-Zwart, I.; Van Riel, P.; Van de Putte, L.; Gribnau, F. A double blind comparative study of sulphasalazine and hydroxychloroquine in rheumatoid arthritis: Evidence of an earlier effect of sulphasalazine. Ann. Rheum. Dis. 1989, 48, 389–395. [Google Scholar] [CrossRef]
- Van Der Heijde, D.; Pl, V.R. Effects of hydroxychloroquine and sulfasalazine on progression of joint damagein RA. Lancet 1989, 1, 1036–1038. [Google Scholar] [CrossRef]
- Batun-Garrido, J.A.J.; Salas-Magana, M.; Juarez-Rojop, I.E. Association between leptin and IL-6 concentrations with cardiovascular risk in patients with rheumatoid arthritis. Clin. Rheumatol. 2018, 37, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.J.; Wasko, M.C.; Antohe, J.L.; Sartorius, J.A.; Kirchner, H.L.; Dancea, S.; Bili, A. Hydroxychloroquine use associated with improvement in lipid profiles in rheumatoid arthritis patients. Arthritis Care Res. (Hoboken) 2011, 63, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Solomon, D.H.; Garg, R.; Lu, B.; Todd, D.J.; Mercer, E.; Norton, T.; Massarotti, E. Effect of hydroxychloroquine on insulin sensitivity and lipid parameters in rheumatoid arthritis patients without diabetes mellitus: A randomized, blinded crossover trial. Arthritis Care Res. (Hoboken) 2014, 66, 1246–1251. [Google Scholar] [CrossRef] [PubMed]
- Penn, S.K.; Kao, A.H.; Schott, L.L.; Elliott, J.R.; Toledo, F.G.; Kuller, L.; Manzi, S.; Wasko, M.C. Hydroxychloroquine and glycemia in women with rheumatoid arthritis and systemic lupus erythematosus. J. Rheumatol 2010, 37, 1136–1142. [Google Scholar] [CrossRef] [Green Version]
- The Canadian Hydroxychloroquine Study Group, T. A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. N. Engl. J. Med. 1991, 324, 150–154. [Google Scholar]
- Tsakonas, E.; Joseph, L.; Esdaile, J.M.; Choquette, D.; Senecal, J.L.; Cividino, A.; Danoff, D.; Osterland, C.K.; Yeadon, C. A long-term study of hydroxychloroquine withdrawal on exacerbations in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. Lupus 1998, 7, 80–85. [Google Scholar]
- Alarcon, G.S.; McGwin, G.; Bertoli, A.M.; Fessler, B.J.; Calvo-Alen, J.; Bastian, H.M.; Vila, L.M.; Reveille, J.D.; Group, L.S. Effect of hydroxychloroquine on the survival of patients with systemic lupus erythematosus: Data from LUMINA, a multiethnic US cohort (LUMINA L). Ann. Rheum. Dis. 2007, 66, 1168–1172. [Google Scholar] [CrossRef] [Green Version]
- An, N.; Chen, Y.; Wang, C.; Yang, C.; Wu, Z.H.; Xue, J.; Ye, L.; Wang, S.; Liu, H.F.; Pan, Q. Chloroquine autophagic nhibition rebalances Th17/Treg-mediated immunity and ameliorates systemic Lupus erythematosus. Cell. Physiol. Biochem. 2017, 44, 412–422. [Google Scholar] [CrossRef]
- Monzavi, S.M.; Alirezaei, A.; Shariati-Sarabi, Z.; Tavakol Afshari, J.; Mahmoudi, M.; Dormanesh, B.; Jahandoost, F.; Khoshdel, A.R.; Etemad Rezaie, A. Efficacy analysis of hydroxychloroquine therapy in systemic Lupus erythematosus: A study on disease activity and immunological biomarkers. Inflammopharmacology 2018, 26, 1175–1182. [Google Scholar] [CrossRef]
- Willis, R.; Seif, A.; McGwin Jr, G.; Martinez-Martinez, L.; Gonzalez, E.; Dang, N.; Papalardo, E.; Liu, J.; Vilá, L.; Reveille, J. Effect of hydroxychloroquine treatment on pro-inflammatory cytokines and disease activity in SLE patients: Data from LUMINA (LXXV), a multiethnic US cohort. Lupus 2012, 21, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Shimomatsu, T.; Li, H.; Ikeda, T.; Kanazawa, N.; Furukawa, F. The effect of hydroxychloroquine on the lupus erythematosus-like skin lesions in MRL/Ipr mice. In Proceedings of the International Investigative Dermatology, Edinburgh, UK, 9 May 2013; p. S34. [Google Scholar]
- Gomez-Guzman, M.; Jimenez, R.; Romero, M.; Sanchez, M.; Zarzuelo, M.J.; Gomez-Morales, M.; O’Valle, F.; Lopez-Farre, A.J.; Algieri, F.; Galvez, J.; et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension 2014, 64, 330–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Virdis, A.; Tani, C.; Duranti, E.; Vagnani, S.; Carli, L.; Kuhl, A.A.; Solini, A.; Baldini, C.; Talarico, R.; Bombardieri, S.; et al. Early treatment with hydroxychloroquine prevents the development of endothelial dysfunction in a murine model of systemic lupus erythematosus. Arthritis Res. Ther. 2015, 17, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wallace, D.J.; Metzger, A.L.; Stecher, V.J.; Turnbull, B.A.; Kern, P.A. Cholesterol-lowering effect of hydroxychloroquine in patients with rheumatic disease reversal of deleterious effects of steroids on lipids. Am. J. Med. 1990, 89, 322–326. [Google Scholar] [CrossRef]
- Levy, R.; Vilela, V.; Cataldo, M.; Ramos, R.; Duarte, J.L.; Tura, B.; Albuquerque, E.M.; Jesus, N. Hydroxychloroquine (HCQ) in lupus pregnancy double-blind and placebo-controlled study. Lupus 2001, 10, 401–404. [Google Scholar] [CrossRef]
- Clowse, M.E.; Magder, L.; Witter, F.; Petri, M. Hydroxychloroquine in lupus pregnancy. Arthritis Rheum. 2006, 54, 3640–3647. [Google Scholar] [CrossRef]
- Bertolaccini, M.L.; Contento, G.; Lennen, R.; Sanna, G.; Blower, P.J.; Ma, M.T.; Sunassee, K.; Girardi, G. Complement inhibition by hydroxychloroquine prevents placental and fetal brain abnormalities in antiphospholipid syndrome. J. Autoimmun 2016, 75, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Syang Pyng Gan, M. Swee Gaik Ong, FRCP, Antithrombotic effects of hydroxychloroquine in a pregnant patient with antiphospholipid syndrome and recurrent venous thromboembolism. Med. J. Malaysia 2016, 72, 124–125. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Oxford, UK, 2019. [Google Scholar]
- Urbanski, G.; Caillon, A.; Poli, C.; Kauffenstein, G.; Begorre, M.A.; Loufrani, L.; Henrion, D.; Belizna, C. Hydroxychloroquine partially prevents endothelial dysfunction induced by anti-beta-2-GPI antibodies in an in vivo mouse model of antiphospholipid syndrome. PLoS ONE 2018, 13, e0206814. [Google Scholar] [CrossRef] [Green Version]
- Schreiber, K.; Breen, K.; Parmar, K.; Rand, J.H.; Wu, X.X.; Hunt, B.J. The effect of hydroxychloroquine on haemostasis, complement, inflammation and angiogenesis in patients with antiphospholipid antibodies. Rheumatology 2018, 57, 120–124. [Google Scholar] [CrossRef] [Green Version]
- Fox, R.I.; Chan, E.; Benton, L.; Fong, S.; Friedlaender, M.; Howell, F.V. Treatment of primary Sjögren’s syndrome with hydroxychloroquine. Am. J. Med. 1988, 85, 62–67. [Google Scholar] [CrossRef]
- Cankaya, H.; Alpoz, E.; Karabulut, G.; Guneri, P.; Boyacioglu, H.; Kabasakal, Y. Effects of hydroxychloroquine on salivary flow rates and oral complaints of Sjogren patients: A prospective sample study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2010, 110, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, S.; Asfuroglu, E.; Bicakcigil, M.; Toker, E. Hydroxychloroquine improves dry eye symptoms of patients with primary Sjogren’s syndrome. Rheumatol Int 2011, 31, 1045–1049. [Google Scholar] [CrossRef] [PubMed]
- Li, C.L.; He, J.; Li, Z.G.; Zheng, L.W.; Hua, H. Effects of total glucosides of paeony for delaying onset of Sjogren’s syndrome: An animal study. J. Craniomaxillofac Surg 2013, 41, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Pu, X.; Yu, G.; Li, T. Effects of total glucosides of peony on AQP-5 and its mRNA expression in submandibular glands of NOD mice with Sjogren’s syndrome. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 173–178. [Google Scholar] [PubMed]
- Emami, J.; Gerstein, H.C.; Pasutto, F.M.; Jamali, F. Insulin-sparing effect of hydroxychloroquine in diabetic rats is concentration dependent. Can. J. Physiol. Pharmacol. 1999, 77, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, A.A.; El-Firgany Ael, D. Hydroxychloroquine hindering of diabetic isletopathy carries its signature on the inflammatory cytokines. J. Mol. Histol. 2016, 47, 183–193. [Google Scholar] [CrossRef]
- Gerstein, H.C.; Thorpe, K.E.; Haynes, R.B.; Taylor, D.W. Gerstein_The effectiveness of hydroxychloroquine in patients with type 2 diabetes mellitus who are refractory to sulfonylureas a randomized trial. Diabetes Res. Clin. Pract. 2002, 55, 209–219. [Google Scholar] [CrossRef]
- Mercer, E.; Rekedal, L.; Garg, R.; Lu, B.; Massarotti, E.M.; Solomon, D.H. Hydroxychloroquine improves insulin sensitivity in obese non-diabetic individuals. Arthritis Res. Ther. 2012, 14, R135. [Google Scholar] [CrossRef] [Green Version]
- Sheikhbahaie, F.; Amini, M.; Gharipour, M.; Aminoroaya, A.; Taheri, N. The effect of hydroxychloroquine on glucose control and insulin resistance in the prediabetes condition. Adv. Biomed. Res. 2016, 5, 145. [Google Scholar] [CrossRef]
- Pareek, A.; Chandurkar, N.; Thomas, N.; Viswanathan, V.; Deshpande, A.; Gupta, O.P.; Shah, A.; Kakrani, A.; Bhandari, S.; Thulasidharan, N.K.; et al. Efficacy and safety of hydroxychloroquine in the treatment of type 2 diabetes mellitus: A double blind, randomized comparison with pioglitazone. Curr. Med. Res. Opin. 2014, 30, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Xie, J.; Xie, B.; Li, Y.; Jiang, L.; Sui, X.; Zhou, X.; Pan, H.; Han, W. Therapeutic effect of hydroxychloroquine on colorectal carcinogenesis in experimental murine colitis. Biochem. Pharmacol. 2016, 115, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.; McCormick, J.; Taylor, V.; Pericleous, C.; Blanchet, B.; Costedoat-Chalumeau, N.; Stuckey, D.; Lythgoe, M.F.; Stephanou, A.; Ioannou, Y. Hydroxychloroquine Protects against Cardiac Ischaemia/Reperfusion Injury In Vivo via Enhancement of ERK1/2 Phosphorylation. PLoS ONE 2015, 10, e0143771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Hamid, A.A.M.; Firgany, A.E.L. Favorable outcomes of hydroxychloroquine in insulin resistance may be accomplished by adjustment of the endothelial dysfunction as well as the skewed balance of adipokines. Acta Histochem. 2016, 118, 560–573. [Google Scholar] [CrossRef]
- Shukla, A.M.; Bose, C.; Karaduta, O.K.; Apostolov, E.O.; Kaushal, G.P.; Fahmi, T.; Segal, M.S.; Shah, S.V. Impact of hydroxychloroquine on atherosclerosis and vascular stiffness in the presence of chronic kidney disease. PLoS ONE 2015, 10, e0139226. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Yang, X.; Southwood, M.; Lu, J.; Marciniak, S.J.; Dunmore, B.J.; Morrell, N.W. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ. Res. 2013, 112, 1159–1170. [Google Scholar] [CrossRef]
- Ruiz, A.; Rockfield, S.; Taran, N.; Haller, E.; Engelman, R.W.; Flores, I.; Panina-Bordignon, P.; Nanjundan, M. Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis 2016, 7, e2059. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of rheumatoid arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [Green Version]
- McInnes, I.B.; Schett, G. Pathogenetic insights from the treatment of rheumatoid arthritis. The Lancet 2017, 389, 2328–2337. [Google Scholar] [CrossRef] [Green Version]
- Tam, L.; LiU, E.; Lam, C.; Tomlinsoni, B. Hydroxychloroquine has no significant effect on lipids and apolipoproteins in Chinese systemic lupus erythematosus patients with mild or inactive disease. Lupus 2000, 9, 413–426. [Google Scholar] [CrossRef]
- Moroni, G.; Doria, A.; Giglio, E.; Tani, C.; Zen, M.; Strigini, F.; Zaina, B.; Tincani, A.; de Liso, F.; Matinato, C.; et al. Fetal outcome and recommendations of pregnancies in lupus nephritis in the 21st century. A prospective multicenter study. J. Autoimmun. 2016, 74, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Kruize, A.A.; Hene, R.; Kallenberg, C.G.; van Bijsterveld, P.; van der Heide, A.; Kater, L.; Bijlsma, J. Hydroxychloroquine treatment for primary Sjögren’s syndrome a two year double blind crossover trial. Ann. Rheum. Dis. 1993, 52, 360–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoult, D.; Marrie, T. Q fever. Clin. Infect. Dis. 1995, 20, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Fournier, P.-E.; Carrieri, M.P.; Habib, G.; Messana, T.; Raoult, D. Risks factors and prevention of Q fever endocarditis. Clin. Infect. Dis. 2001, 33, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Kampschreur, L.M.; Dekker, S.; Hagenaars, J.C.; Lestrade, P.J.; Renders, N.H.; de Jager-Leclercq, M.G.; Hermans, M.H.; Groot, C.A.; Groenwold, R.H.; Hoepelman, A.I. Identification of risk factors for chronic Q fever, the Netherlands. Emerg. Infect. Dis. 2012, 18, 563. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.B.; Evavold, C.; Kersh, G.J. The Effect of pH on Antibiotic Efficacy against Coxiella burnetii in Axenic Media. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Maurin, M.; Benoliel, A.M.; Bongrand, P.; Raoult, D. Phagolysosomal alkalinization and the bactericidal effect of antibiotics: The Coxiella burnetii paradigm. J. Infect. Dis. 1992, 166, 1097–1102. [Google Scholar] [CrossRef]
- Brouqui, P.R.D. Treatment of Q fever endocarditis by doxycycline and hydroxychloroquine. Clin. Infect. Dis. 1993, 17, 531. [Google Scholar]
- Lupoglazoff, J.; Brouqui, P.; Magnier, S.; Hvass, U.; Casasoprana, A. Q fever tricuspid valve endocarditis. Arch. Dis. Child. 1997, 77, 448–449. [Google Scholar] [CrossRef]
- Raoult, D.; Houpikian, P.; Tissot Dupont, H.; Riss, J.M.; Arditi-Djiane, J.; Brouqui, P. Treatment of Q fever endocarditis: Comparison of 2 regimens containing doxycycline and ofloxacin or hydroxychloroquine. Arch. Intern. Med. 1999, 159, 167–173. [Google Scholar] [CrossRef]
- Elzein, F.E.; Alsherbeeni, N.; Alnajashi, K.; Alsufyani, E.; Akhtar, M.; Albalawi, R.; Albarrag, A.M.; Kaabia, N.; Mehdi, S.; Alzahrani, A. Ten-year experience of Q fever endocarditis in a tertiary cardiac center in Saudi Arabia. Int. J. Infect. Dis. 2019, 88, 21–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonçalves, M.; Moreira, S.; Gaspar, E.; Santos, L. Rare case of otomastoiditis due to Coxiella burnetii chronic infection. Case Rep. 2018, 2018, bcr-2018–224315. [Google Scholar]
- Allan-Blitz, L.-T.; Sakona, A.; Wallace, W.D.; Klausner, J.D. Coxiella burnetii endocarditis and meningitis, California, USA, 2017. Emerg. Infect. Dis. 2018, 24, 1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, Z.; Duperril, M.; Séguéla, P.-E.; Melenotte, C.; Chabaneix, J.; Raoult, D.; Thambo, J.-B. First case of Q fever endocarditis involving the Melody® transcatheter pulmonary valve in an Afebrile child. Pediatr. Cardiol. 2018, 39, 195–197. [Google Scholar] [CrossRef]
- Stokes, W.; Janvier, J.; Vaughan, S.; Microbiology, M. Chronic Q fever in Alberta: A case of Coxiella burnetii mycotic aneurysm and concomitant vertebral osteomyelitis. Can. J. Infect. Dis. Med. 2016, 2016, 7456157. [Google Scholar]
- Polo, M.; Mastrandrea, S.; Santoru, L.; Arcadu, A.; Masala, G.; Marras, V.; Bagella, G.; Sechi, M.; Tanda, F.; Pirina, P.J. Pulmonary inflammatory pseudotumor due to Coxiella burnetii. Case report and literature review. Microbes Infect. 2015, 17, 795–798. [Google Scholar] [CrossRef]
- Salvia, A.M.; Cuviello, F.; Coluzzi, S.; Nuccorini, R.; Attolico, I.; Pascale, S.P.; Bisaccia, F.; Pizzuti, M.; Ostuni, A. Expression of some ATP-binding cassette transporters in acute myeloid leukemia. Hematol. Rep. 2017, 9, 7406. [Google Scholar] [CrossRef]
- Miglionico, R.; Ostuni, A.; Armentano, M.F.; Milella, L.; Crescenzi, E.; Carmosino, M.; Bisaccia, F. ABCC6 knockdown in HepG2 cells induces a senescent-like cell phenotype. Cell. Mol. Biol. Lett. 2017, 22, 7. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhang, Z.; Leung, W.T.; Chen, J.; Yi, J.; Ying, C.; Yuan, M.; Wang, M.; Zhang, N.; Qiu, X. Hydroxychloroquine reverses the drug resistance of leukemic K562/ADM cells by inhibiting autophagy. Mol. Med. Rep. 2019, 20, 3883–3892. [Google Scholar] [CrossRef]
- Godinho, I.; Nogueira, E.; Santos, C.; Paulo, S.; Fortes, A.; Guerra, J.; da Costa, A.G. Chronic Q fever in a renal transplant recipient: A case report. Transplant Proc. 2015, 47, 1045–1047. [Google Scholar] [CrossRef]
- Merhej, V.; Tattevin, P.; Revest, M.; Le Touvet, B.; Raoult, D.J. Q fever osteomyelitis: A case report and literature review. Comp. Immunol. Microbiol. Infect. 2012, 35, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Million, M.; Walter, G.; Thuny, F.; Habib, G.; Raoult, D. Evolution from acute Q fever to endocarditis is associated with underlying valvulopathy and age and can be prevented by prolonged antibiotic treatment. Clin. Infect. Dis. 2013, 57, 836–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Million, M.; Thuny, F.; Richet, H.; Raoult, D. Long-term outcome of Q fever endocarditis: A 26-year personal survey. Lancet Infect. Dis. 2010, 10, 527–535. [Google Scholar] [CrossRef]
- Munster, T.; Gibbs, J.P.; Shen, D.; Baethge, B.A.; Botstein, G.R.; Caldwell, J.; Dietz, F.; Ettlinger, R.; Golden, H.E.; Lindsley, H. Hydroxychloroquine concentration–response relationships in patients with rheumatoid arthritis. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2002, 46, 1460–1469. [Google Scholar] [CrossRef]
- Drucker, A.M.; Rosen, C.F. Drug-induced photosensitivity. Drug. Saf. 2011, 34, 821–837. [Google Scholar] [CrossRef]
- Angelakis, E.; Million, M.; Kankoe, S.; Lagier, J.C.; Armougom, F.; Giorgi, R.; Raoult, D. Abnormal weight gain and gut microbiota modifications are side effects of long-term doxycycline and hydroxychloroquine treatment. Antimicrob. Agents Chemother. 2014, 58, 3342–3347. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.; Bijlmer, H.; Fournier, P.-E.; Graves, S.; Hartzell, J.; Kersh, G.J.; Limonard, G.; Marrie, T.J.; Massung, R.F.; McQuiston, J.H. Diagnosis and management of Q fever—United States, 2013: Recommendations from CDC and the Q fever working group. Morb. Mortal. Wkly. Rep. 2013, 62, 1–29. [Google Scholar]
- Fenollar, F.; Puéchal, X.; Raoult, D. Whipple’s disease. N. Engl. J. Med. 2007, 356, 55–66. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Lepidi, H.; Raoult, D.; Fenollar, F. Systemic Tropheryma whipplei: Clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Medicine 2010, 89, 337–345. [Google Scholar] [CrossRef]
- Lagier, J.-C.; Fenollar, F.; Lepidi, H.; Raoult, D. Failure and relapse after treatment with trimethoprim/sulfamethoxazole in classic Whipple’s disease. J. Antimicrob. Chemoth. 2010, 65, 2005–2012. [Google Scholar] [CrossRef] [Green Version]
- Emonet, S.; Wuillemin, T.; Harbarth, S.; Wassilew, N.; Cikirikcioglu, M.; Schrenzel, J.; Lagier, J.-C.; Raoult, D.; Van Delden, C. Relapse of Tropheryma whipplei endocarditis treated by trimethoprim/sulfamethoxazole, cured by hydroxychloroquine plus doxycycline. Int. J. Infect. Dis. 2015, 30, 17–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulos, A.; Rolain, J.; Mallet, M.; Raoult, D. Molecular evaluation of antibiotic susceptibility of Tropheryma whipplei in axenic medium. J. Antimicrob. Chemother. 2005, 55, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, N.; Fenollar, F.; Biswas, S.; Rolain, J.-M.; Raoult, D. Acquired resistance to trimethoprim-sulfamethoxazole during Whipple disease and expression of the causative target gene. J. Infect. Dis. 2008, 198, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Fenollar, F.; Rolain, J.-M.; Alric, L.; Papo, T.; Chauveheid, M.-P.; van de Beek, D.; Raoult, D. Resistance to trimethoprim/sulfamethoxazole and Tropheryma whipplei. Int. J. Antimicrob. Agents 2009, 34, 255–259. [Google Scholar] [CrossRef] [Green Version]
- Boulos, A.; Rolain, J.-M.; Raoult, D. Antibiotic susceptibility of Tropheryma whipplei in MRC5 cells. Antimicrob. Agents Chemother. 2004, 48, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Lagier, J.-C.; Fenollar, F.; Lepidi, H.; Giorgi, R.; Million, M.; Raoult, D. Treatment of classic Whipple’s disease: From in vitro results to clinical outcome. J. Antimicrob. Chemoth. 2014, 69, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Le Goff, M.; Cornec, D.; Guellec, D.; Marhadour, T.; Devauchelle-Pensec, V.; Jousse-Joulin, S.; Herbette, M.; Cauvin, J.M.; Le Guillou, C.; Renaudineau, Y. Peripheral-blood b-cell subset disturbances in inflammatory joint diseases induced by Tropheryma whipplei. PLoS ONE 2019, 14, e0211536. [Google Scholar] [CrossRef]
- Brönnimann, D.; Vareil, M.-O.; Sibon, I.; Lagier, J.-C.; Lepidi, H.; Puges, M.; Haneche, F.; Raoult, D.; Desclaux, A.; Neau, D. Limbic encephalitis as a relapse of Whipple’s disease with digestive involvement and spondylodiscitis. Infection 2019, 47, 637–641. [Google Scholar] [CrossRef]
- Gaudé, M.; Tébib, J.; Puéchal, X. Atypical focal forms of Whipple’s disease seen by rheumatologists. Joint Bone Spine 2015, 82, 56–59. [Google Scholar] [CrossRef]
- Lenfant, M.; Callemeyn, J.; Alaerts, H.; Meersseman, W.; Van, W.M. Whipple’s disease in a man of North African descent: Case report and brief review of the literature. Acta Gastroenterol. Belg. 2019, 82, 83–86. [Google Scholar]
- Vayssade, M.; Tournadre, A.; D’Incan, M.; Soubrier, M.; Dubost, J.-J. Immune reconstitution inflammatory syndrome during treatment of Whipple’s disease. Joint Bone Spine 2015, 82, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, D.; Bär, D.; Cooper, J.; Vogt, T.; Tyndall, A.; Walker, U.A. Multisegmental spondylitis due to Tropheryma whipplei: Case report. Orphanet J. Rare Dis. 2009, 4, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, P.; Hazes, J.; Barendregt, P.; Huisman, M.; van Zeben, D.; Van Der Lubbe, P.; Gerards, A.; de Jager, M.; de Sonnaville, P.; Grillet, B. Induction therapy with a combination of DMARDs is better than methotrexate monotherapy: First results of the tREACH trial. Ann. Rheum. Dis. 2013, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Möttönen, T.; Hannonen, P.; Leirisalo-Repo, M.; Nissilä, M.; Kautiainen, H.; Korpela, M.; Laasonen, L.; Julkunen, H.; Luukkainen, R.; Vuori, K.; et al. Comparison of combination therapy with single-drug therapy in early rheumatoid arthritis: A randomised trial. Lancet 1999, 353, 1568–1573. [Google Scholar]
- Cobankara, V.; Ozatli, D.; Kiraz, S.; Ozturk, M.A.; Ertenli, I.; Turk, T.; Apras, S.; Haznedaroglu, I.C.; Calguneri, M. Successful treatment of rheumatoid arthritis is associated with a reduction in serum sE-selectin and thrombomodulin level. Clin. Rheumatol. 2004, 23, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Kuriachan, M.; Revikumar, K.; Jolly, A. Comparison of treatment outcome in rheumatoid arthritis patients treated with single and two DMARDs in combination with corticosteroids. Int. J. Drug Dev. Res. 2012, 4, 228–235. [Google Scholar]
- Roivainen, A.; Hautaniemi, S.; Mottonen, T.; Nuutila, P.; Oikonen, V.; Parkkola, R.; Pricop, L.; Ress, R.; Seneca, N.; Seppanen, M.; et al. Correlation of 18F-FDG PET/CT assessments with disease activity and markers of inflammation in patients with early rheumatoid arthritis following the initiation of combination therapy with triple oral antirheumatic drugs. Eur J. Nucl Med. Mol Imaging 2013, 40, 403–410. [Google Scholar] [CrossRef]
- Tynjala, P.; Vahasalo, P.; Tarkiainen, M.; Kroger, L.; Aalto, K.; Malin, M.; Putto-Laurila, A.; Honkanen, V.; Lahdenne, P. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): A multicentre randomised open-label clinical trial. Ann. Rheum. Dis. 2011, 70, 1605–1612. [Google Scholar] [CrossRef]
- Chafin, C.B.; Regna, N.L.; Hammond, S.E.; Reilly, C.M. Cellular and urinary microRNA alterations in NZB/W mice with hydroxychloroquine or prednisone treatment. Int. Immunopharmacol. 2013, 17, 894–906. [Google Scholar] [CrossRef] [Green Version]
- Fasano, S.; Pierro, L.; Pantano, I.; Iudici, M.; Valentini, G. Longterm hydroxychloroquine therapy and low-dose aspirin may have an additive effectiveness in the primary prevention of cardiovascular events in patients with systemic Lupus erythematosus. J. Rheumatol. 2017, 44, 1032–1038. [Google Scholar] [CrossRef]
- Schultz, K.; Gilman, A.L. Immune suppression by lysosomotropic amines and cyclosporine on T-cell responses to minor and major histocompatibility antigens. Leukemia Lymphoma 1997, 24, 201–220. [Google Scholar] [CrossRef] [PubMed]
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 104689. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; De Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
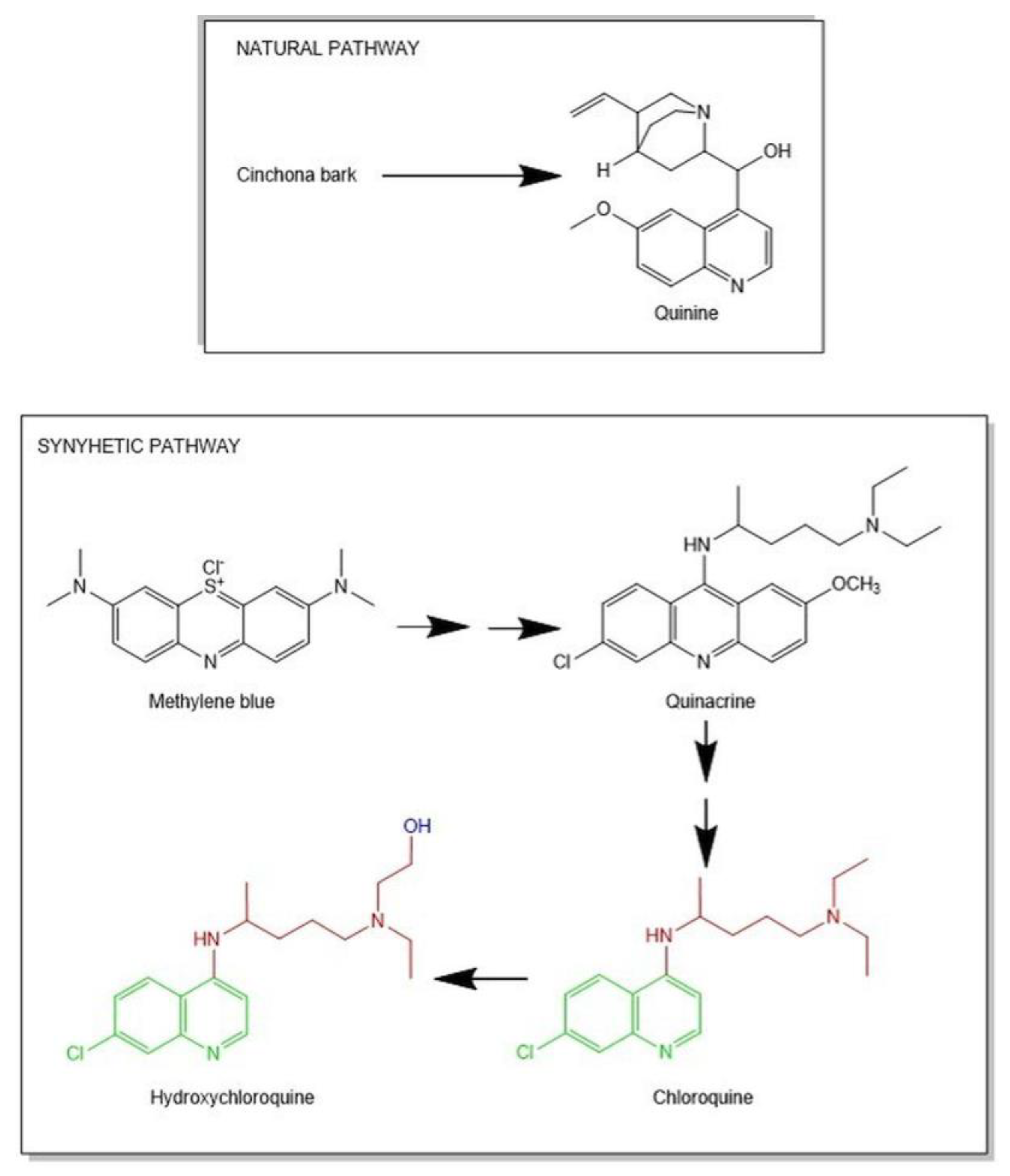

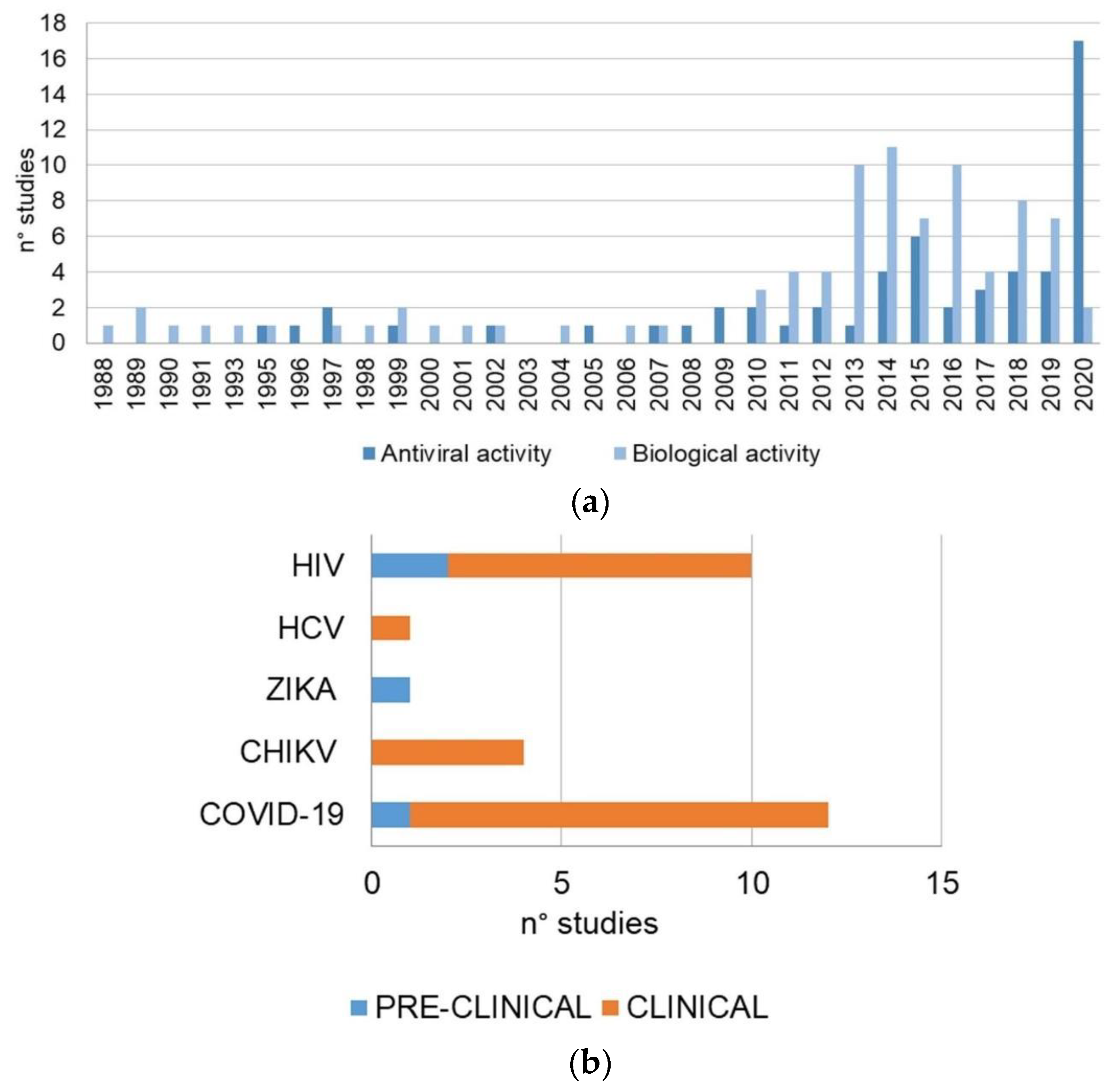
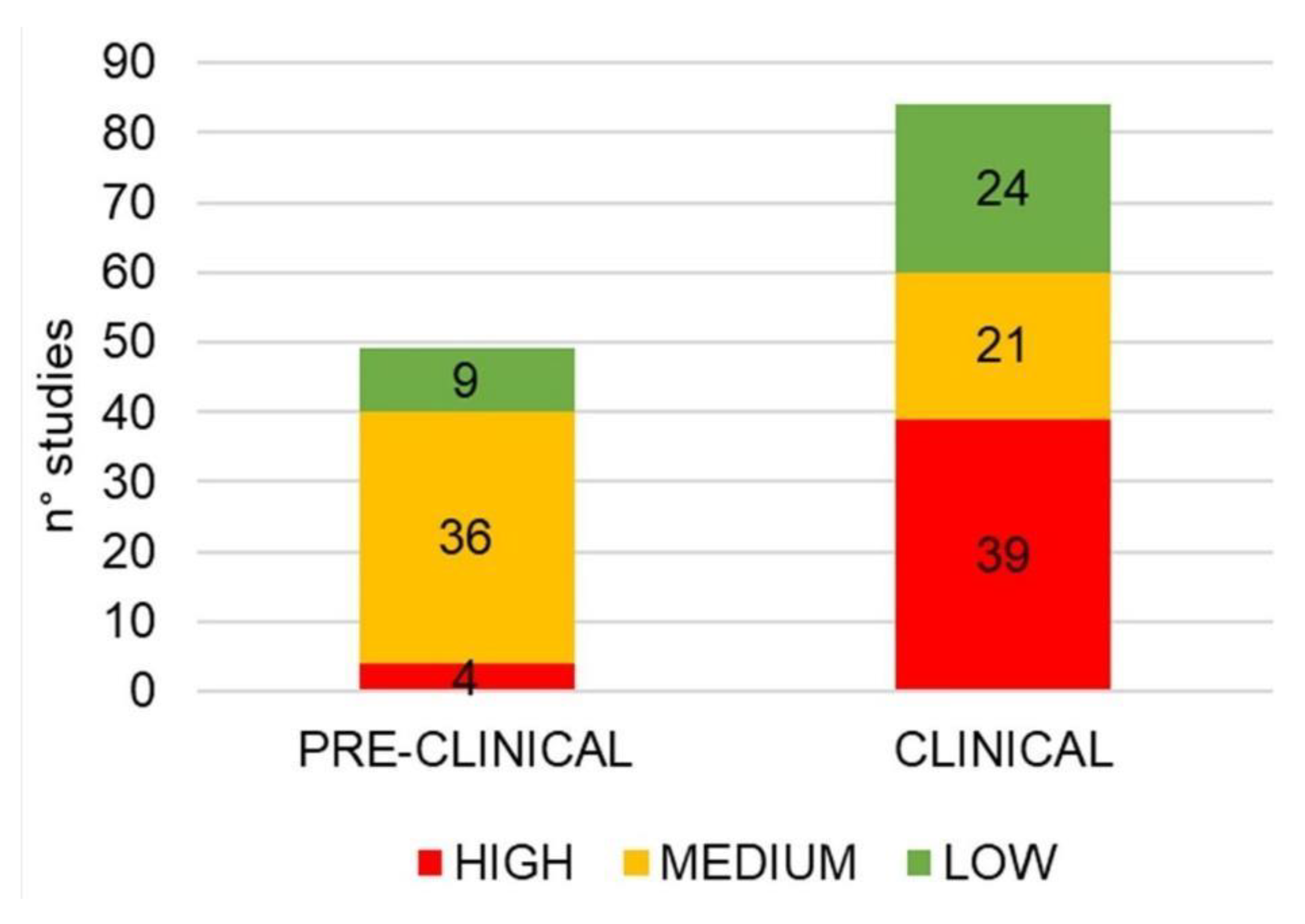


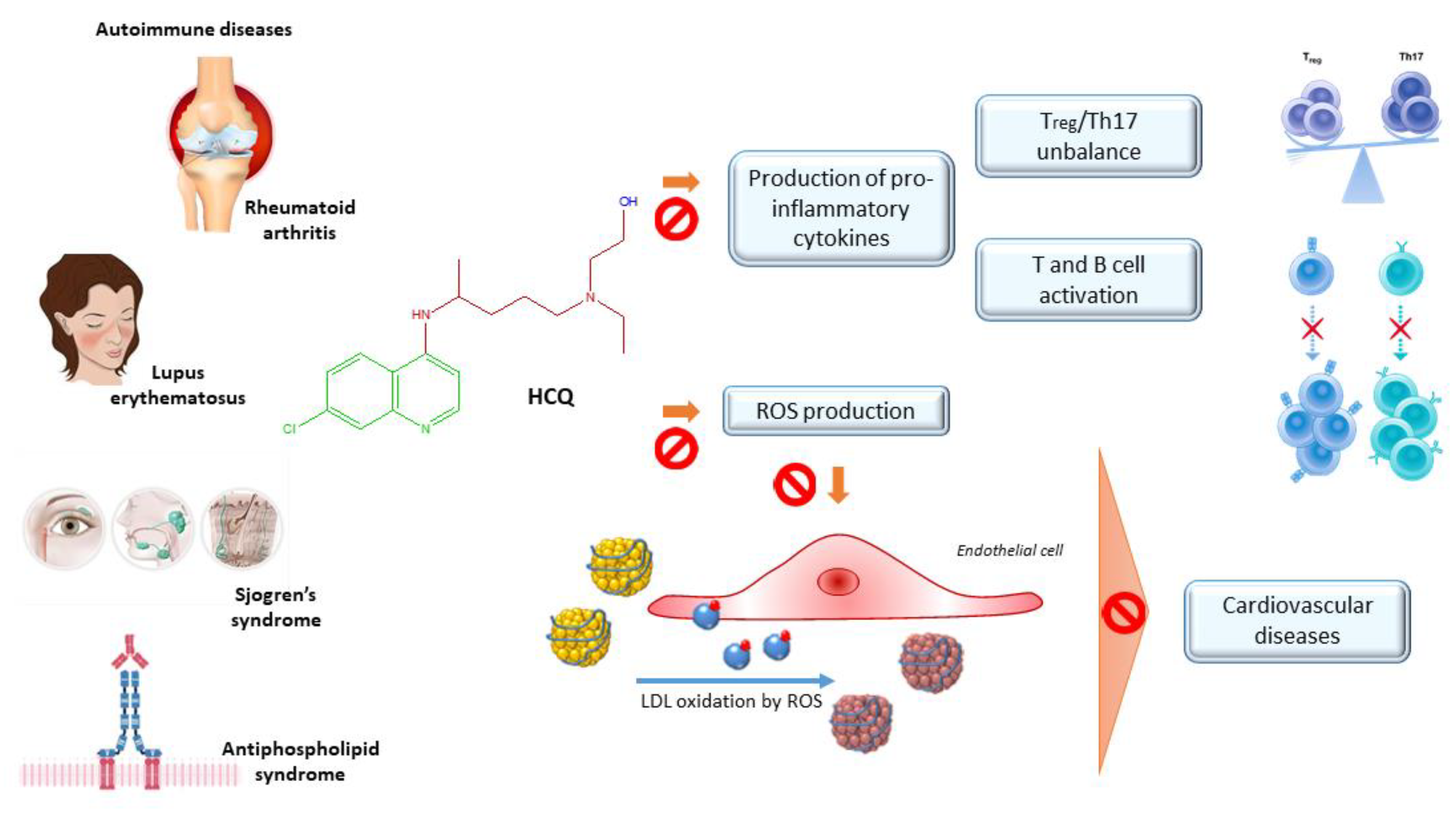

| Author(Year) | Study TypePopulation | DosageTime | Outcomes | Adverse Events Noted | Limitation of the Study |
|---|---|---|---|---|---|
| HIV-1 | |||||
| Sperber, et al. (1995) [17] | Randomized, double-blind, placebo-controlled clinical trial 40 asymptomatic HIV-1 infected patients | HCQ group - > 800 mg/day Control group - > placebo 8 weeks | Total HIV-1 RNA plasma levels significantly decreased in the HCQ group (range, 98 to 2517 cpm; mean, 168 ± 144 cpm vs. 311 ± 331 cpm; p = 0.022). CD4+ T cells percentage remained stable in HCQ group (18.1 ± 9.2% before treatment vs. 18.6 ± 10.5% after treatment) Absolute CD4+ has not reported significant changes in both groups IL-6 and IgG levels decrease in HCQ group (14.3 ± 13.5 U/mL vs. 12.0 f 16.7 U/mL; p = 0.023 and 2563 ± 1352 mg/mL vs. 2307 ± 1372 mg/dL; p = 0.032, respectively) | Not reported. | Small sample-size. All of the patients were asymptomatic with a low viral load. A short period of study time. |
| Sperber, et al. (1997) [18] | Randomized, placebo-controlled clinical trial 72 asymptomatic HIV-1 infected patients | 800 mg/d HCQ (n = 35) 500 mg/d ZDV (n = 37) 16 weeks | After 16 weeks total plasma HIV-1 RNA levels were reduced in both ZDV group (42.709 ± 33.050 vs. 11.228 ± 7459 copies/mL; p = 0.001) and HCQ group (39.456 ± 31.000 vs. 16.434 ± 11.373 copies/mL; p = 0.02). No significant change occurred in CD4+ cells Only in HCQ group it was a reduction in the levels of IL-6 (12.4 ± 12.9 vs. 6.3 ± 5.4 U/nL; p = 0.03) and Ig-G (1453 ± 453 vs. 395 ± 544 mg/dL; p = 0.02) | Not reported. | Small sample-size. All of the patients were asymptomatic. |
| Paton, et al. (2002) [19] | non-comparative clinical study 22 asymptomatic HIV-1 infected patients | HCQ (200 mg) + hydroxyurea (500 mg) + didanosine (125–200 mg), taken twice daily. 48 weeks | In the 12th week there was a significant reduction of 1.3 log10 in viral load and an increase in CD4+ percentage by mean 4.3%. These values were maintained until the 48th week. | Not reported. | Small sample-size. This is a non-comparative design pilot study which not allow determining the contribution made by HCQ alone to the overall decrease in viral load obtained by the combination. |
| Paton, et al. (2005) [20] | open-label, noncomparative stud 17 HIV-1 infected patients | HCQ (200 mg) + hydroxyurea (500 mg) + didanosine (125–200 mg), taken twice daily. 144 weeks | Mean viral load was reduced by 1.6 log10 copies/mL below baseline (p = 0.001) CD4+ cell counts were significantly increased by a mean of 3.3 ± 6.9%, p = 0.095 at 144th week. CD8 cells percentage was reduced by 11.5 ± 14% per 48th week (p = 0.005) and remained around 10% until the 144th week | Not reported. | Small sample-size. Absence of a control group. |
| Aguirre-Cruz, et al. (2010) [21] | Randomized clinical study 8 HIV-infected adults with adenoid hypertrophy were included. | Group A - > 400 mg/day Group B - > 800 mg/day 8 days | HCQ main concentration was significantly higher in at than in plasma | Not reported. | |
| González-Hernández, et al. (2014) [22] | In vivo on rabbit model | Subcutaneous HCQ injection of 15 mg/kg of body weight. | HCQ had a higher affinity for lymphoid tissues than for blood. | Not reported. | |
| Piconi, et al. (2011) [23] | Prospective noncomparative Study 20 HIV-infected immunologic non-responders | 400 mg/day HCQ 6 months | After 6 months, there was an increase in CD4+ T-cells percentage; a reduction of activation/proliferation in CD4+ T-cells (Ki67+) and CD14+ cells (CD69+); a decrease of plasma LPS levels; a downregulation of TLR-7/8 expression. | One patient reported maculopapular exanthema after 10 days of treatment. | Small sample-size. Patients were taking antiretroviral drugs during the treatment with HCQ. |
| Paton, et al. (2015) [24] | Randomized, double-blind, placebo-controlled trial 83 asymptomatic HIV-1 infected patients | 400 mg/day HCQ or placebo 48 weeks | At 48th in HCQ group is revealed a faster decline of CD4+ T-cell counts; no change in activation/proliferation levels in CD8+ and CD4+ T-cells; no change in IL-6 levels; an increase in viral load. | Patients in the HCQ group reported influenza-like illness compared with the placebo group (29% vs. 10%; p = 0.03). | Small sample-size. |
| Chen, et al. (2018) [25] | In vivo on a rabbit model | Intravaginal implant designed to release an HCQ concentration above 4.34 µg/mL but below 21.7 µg/mL 6 days | After 6 days, there was seen an improvement of mucosal epithelial integrity, a reduction in submucosal immune cell recruitment, a decrease of gene expression and T cell activation marker protein, and a minimization of key pro-inflammatory mediators activation. | Not reported. | No clinical study has been designed to test the effectiveness of HCQ in preventing HIV infection |
| Chikungunya Virus | |||||
| Padmakumar, et al. (2009) [26] | Prospective, randomized, parallel-group study 120 patients in the acute phase of CHIKV infection | Group A -> 200 mg/day ACF Group B -> ACF + 400 mg/day HCQ Group C -> ACF + 10 mg/day PRD Group D -> ACF + HCQ + PDR | HCQ did not confer any additional benefit in the treatment of the early stages of chikungunya. | Not reported. | The duration of the study can be considered as a limitation with respect to the efficacy assessment of HCQ, which is a slow-acting drug. |
| Bouquillar, et al. (2018) [27] | Multicenter study 39 patients with chronic CHIKV infection | 400 mg/day HCQ 3 months | After three months of treatment, evidence of synovitis was disappeared in 10 of 20 subjects (50%) with swollen joins while complete remission was verified in 5 patients (19.2%) | In four subjects, the treatment was interrupted due to the onset of side effects such as nausea, stomatitis, rash, and headache. | Small sample-size. |
| Ravindran, et al. (2017) [28] | Randomized controlled open-label study 72 patients with chronic CHIKV infection | 400 mg/day HCQ (n = 35) 15 mg/day MTX, 1g/day sulfasalazine, and 400 mg/day HCQ (n = 37) 34 weeks | At the end of the 24th week, only the combination of drugs improved disease activity (mean ± SD DAS28; 3.39 ± 0.87 vs. 4.74 ± 0.65, p < 0.0001) and reduces disability (mean ± SD HAQ; 1.4 ± 0.31 vs. 1.8 ± 80.47, p < 0.0001) and pain (mean ± SD VAS 46 ± 6.13 vs. 60.8 ± 11.6, p < 0.0001). | In the combination group, one patient withdrew due to nausea. | It is not a blinded study and so the bias in reporting improvement could be present. |
| Pandaya S. (2008) [29] | Uncontrolled clinical study 305 patients with chronic CHIKV infection | 15–20 mg/weekly MTX + 400 mg/day HCQ 16 weeks | At 16th week a reduction in ACR score was shown | Not reported | There is not a control group. Only 114 subjects completed the study. It is not a blinded study and so the bias in reporting improvement could be present. |
| Flavivirus | |||||
| Helal, et al. (2016) [30] | Prospective, randomized, controlled, interventional, single-blind study 120 patients affected by hepatitis C virus | Group 1 -> SOC (160 µg pegylated interferon subcutaneously and 1000–12000 mg/day ribavirin orally) Group 2 -> SOC + 200 mg/day HCQ 12 weeks | HCQ + SOC group showed a high virological response compared to control group [54/60 (90%) vs. 43/60 (71.7%); p = 0.011] and a normalization of ALT levels. | Both groups showed symptoms such as headache, fatigue, influenza-like illness, and gastrointestinal disturbance. | A short period of study time. There was a lack of the rapid virological response (RVR) assessment of defined as HCV RNA negativity at week 4 of treatment. |
| Cao, et al. (2017) [31] | In vivo study on pregnant mice infected with ZIKV | 40 mg/kg/day HCQ | HCQ attenuated placental and fetal ZIKV infection and ameliorated adverse placental and fetal outcomes | Not reported. | No clinical study has been designed to test the effectiveness of HCQ in preventing ZIKV infection. |
| COVID-19 | |||||
| Chen et al. (2020) [6] | Randomized, parallel-group clinical trial 62 patients suffering from COVID-19 | HCQ group -> 400 mg/day HCQ Control group -> SOC Day 5 | Body temperature recovery time in the HCQ group was shorter than the control group (2.2 vs. 3.2 days, p = 0.0008). Cough remission time was significantly decreased in the HCQ group (2.0 vs. 3.1 days, p = 0.0016). Improvement of pneumonia in HCQ group (80.6% vs. 54.8%) Pneumonia absorption in HCQ group (61.3%) | One patient developed a rash. One patient reported a headache. | Small sample-size. Detail about antiviral and antibacterial agents used in the control group are not available. |
| Gautret et al. (2020) [32] | Open-label non-randomized clinical trial 36 patients | HCQ group -> 600 mg/day HCQ (n = 14); 600 mg/day HCQ +500 mg AZM on day 1 followed by 250 mg/day for 4 days (n = 6) Control group (n = 16) Day 10 | On day 6, 70% of HCQ-treated patients were virologically cured comparing to 12.5% in the control group On day 6, 100% of HCQ+AZM treated patients are virologically cured comparing to 57.1% in the HCQ group and 12.5% in the control group. | Gastrointestinal side effects in one patient of HCQ group. One patient of the HCQ group died on day 3 although he was PCR-negative on day 2. | Small sample-size. Dropout of six patients from HCQ group. Data available up to 6 days despite the planned 10 days. Details about control group treatment are not available. |
| Gautret, et al. (2020) [33] | Uncontrolled, non-comparative, observational study 80 mildly infected patients | 600 mg/day HCQ per 10 days + 500 mg AZM on day 1 followed by 250 mg/day for 4 days For patients with pneumonia and NEWS score ≥ 5 ceftriaxone was added to HCQ/AZM treatment | On day 7, nasopharyngeal viral load tested by qPCR was negative for 83% of patients and for 93% of patients at day 8. At day 5 in 97.5% of patients, virus cultures of the respiratory sample were negative. After 10 days only 2 patients were contagious. | One patient died. Six patients had GI side effects (2 nausea or vomiting and 4 diarrhea) One patient had blurred vision. | Six patients from previous trials by Gautret et al. were also included in this study. No analytical approach has been made to take into account possible factors of confusion, including in particular the severity of the disease. |
| Molina et al. (2020) [34] | Prospective, non-comparative study 11 severe COVID-19 infected patients | 600 mg/day HCQ per 10 days + 500 mg AZM on day 1 followed by 250 mg/day for 4 days | On day 5 two patients were transferred to the ICU. At days 5 to 6, after treatment initiation 8 of 10 patients were still positive for SARS-CoV2 RNA. | One patient died. One patient discontinued the treatments due to QT interval prolongation. | Small sample size, 8 of 11 had comorbidities associated with poor outcomes. |
| Tang et al. (2020) [35] | Multicenter, open-label, randomized controlled trial 150 mild/moderate or severe COVID-19 infected patients | HCQ group -> SOC+ HCQ (200 mg daily for three days followed by a maintained dose of 800 mg daily) Control group-> SOC 2 for mild/moderate patients and 3 weeks for severe patients | Within 28 days of treatment, the probability of negative conversion of SARS-CoV-2 was 85.4% (95% CI 73.8% to 93.8%) in the HCQ + SOC group and 81.3% (95% CI 71.2% to 89.6%) in the SOC group. No significant differences in the median time to negative conversion were found between the HCQ + SOC group (8 days, 95% CI 5 to 10 days) and SOC group (7 days, 95% CI 5 to 8 days). No difference in PCR negativity was found between two groups at day 4, 7, 10, 14, or 21. No significant differences in the meantime of clinical symptom alleviation were found between the two groups (19 days for HCQ + SOC vs. 21 days for SOC) | Adverse events noted in 30% of the HCQ group compared to 8.8% of control group The most common adverse effect was diarrhea (10%). One patient had blurred vision. | The study is only based on the virus-negative conversion. |
| Abd-Elsalam, et al. (2020) [36] | Multicenter, randomized controlled trial 194 COVID-19 infected patients | HCQ group -> SOC+ HCQ (400 mg twice daily, on day 1, followed by 200 mg tablets twice daily) Control group -> SOC 4 weeks of treatment | There was no significant difference between the two groups regarding any laboratory parameters or the baseline characteristics. Four patients (4.1%) in the HCQ group and 5 (5.2%) patients in the control group needed mechanical ventilation (p = 0.75). There were no differences in the overall mortality between the two groups, as six patients (6.2%) died in the HCQ group and five (5.2%) died in the control group (p = 0.77). | Not reported. | Small sample size, which was not adequately powered for survival endpoints. Lack of long-term follow-up. |
| Skipper, et al. (2020) [37] | Randomized, double-blind, placebo-controlled trial 491 symptomatic, non-hospitalized adult patients with early or mild COVID-19 | HCQ group -> HCQ 800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg daily for 4 more days Control group-> masked placebo 14 weeks of treatment | HCQ did not reduce symptom severity when compared with placebo in non-hospitalized early/mild COVID-19 patients (difference in symptom severity: relative, 12%; absolute, −0.27 points (95% CI, −0.61 to 0.07 points); p = 0.117) | With HCQ, the most commonly reported adverse effect was related to gastrointestinal symptoms: 31% (66 of 212) of participants reported upset stomach or nausea, and 24% (50 of 212) reported abdominal pain, vomiting, or diarrhea. | Lack of confirmed SARS-CoV-2 infection in all participants. The use of epidemiologic linkage to enroll symptomatic persons. |
| Mahévas, et al. (2020) [38] | No-randomize clinical study 181 COVID-19 infected patients | HCQ group -> 600 mg/day for 5 days (n = 84) within 48 h of admission to hospital Control group (n = 97) | Within day 7: 20.2% infected patients of the HCQ group and 22.1% in the control group died or were transferred to the ICU; 27.4% of the HCQ group and 24.4% of the no-HCQ group shown acute respiratory distress; On day 7 the percentage of death was similar in both HCQ and control group (2.8 vs. 4.8%, 3 vs. 4 events) | 7 patients of the HCQ group showed QT interval prolongation. One patient presented first-degree atrioventricular block after 2 days of HCQ administration. | The study was not randomized. Potential unmeasured confounders may bias the results. |
| Mahévas, et al. (2020) [39] | Observational comparative study 181 severe COVID-19 infected patients | HCQ group -> 600 mg/day (n = 92) Control group -> SOC (n = 89) | On day 21: Overall survival was 89% in the HCQ group and 91% in the control group; survival without acute respiratory distress syndrome was 69% in the HCQ group and 74% in the control group; patients who had been weaned from oxygen was 82% in the HCQ group and 76% in the control group. | 7 patients of HCQ group showed QT interval prolongation One patient presented first-degree atrioventricular block after 2 days of HCQ administration. | Treatment was not randomly assigned and potential unmeasured confounders could bias the results. Patients from previous trials by Mahévas et al. were also included in this study. |
| Lee, et al. (2020) [40] | Single-center clinical study 211 individuals exposed to COVID-19 | 400 mg day of HCQ as post-exposure prophylaxis 14 days | At the end 14 days of quarantine, there was negative follow-up PRC tests. | The most common side effects were diarrhea or loose stool (9%), skin rash (4.3%), gastrointestinal upset (0.95%) and, bradycardia (0.95%). In 5 patients (2.7%) post-exposure prophylaxis was discontinued due to bradycardia (2), gastrointestinal upset (2), and the need for fasting (1). | There was not a control group and the study was carried out at a single center. |
| Boulware, et al. (2020) [41] | Randomized, double-blind, placebo-controlled clinical trial 821 asymptomatic participants | HCQ group: 800 mg once, followed by 600 mg in 6 to 8 h, then 600 mg daily for 4 additional days Placebo group | The incidence of new illness compatible with Covid-19 did not differ significantly between the HCQ group (49 of 414 (11.8%)) and the placebo group (58 of 407 (14.3%)); the absolute difference was −2.4 percentage points (95% confidence interval, −7.0 to 2.2; p = 0.35). | Nausea, loose stools, and abdominal discomfort were the main side effects. There were no intervention-related severe adverse reactions or cardiac arrhythmias. | Small sample-size |
| Maissonasse, et al. (2020) [40] | In vivo study on macaques | Different strategies of treatment were compared with placebo, including HCQ alone or in combination with AZM, administrated either before or after viral load | When HCQ was administrated as pre-exposure prophylaxis, it did not protect against infection acquisition. Neither HCQ nor HCQ + AZM had beneficial effects in improving viral infection’s symptoms. | Not reported. | |
| Disease | Experimental Model | Dosage | Mechanisms of Action | References |
|---|---|---|---|---|
| Rheumatoid arthritis (RA) | Preclinical | 40 mg/kg/day | ↓neutrophil-derived oxidants ↓inflammation | [75] |
| Clinical (randomized double-blind, placebo-controlled trial) | 7 mg/kg/day | ↓inflammation | [76] | |
| Clinical (comparative randomized double-blind trial) | 200–400 mg/day | ↓inflammation | [77,78] | |
| RA-associated cardiovascular disease | Clinical | n.a. | ↓IL-6 and leptin ↓dyslipidemia | [79] |
| Clinical (cohort study) | 6.5 mg/kg/day | ↓triglycerides and LDL ↓dyslipidemia | [80] | |
| Clinical (randomized double-blind cross-over trial) | 6.5 mg/kg/day | ↓cholesterol and LDL ↓dyslipidemia | [81] | |
| Clinical (cross-sectional observational study) | 200 mg/kg/day | ↓fasting glucose | [82] | |
| Systemic lupus erythematosus (SLE) | Clinical (randomized double-blind placebo-controlled trial) | 100–400 mg/kg/day | ↓inflammation ↓risk of exacerbations | [83] |
| Clinical (long-term randomized study) | 272 mg/day | ↓inflammation ↓risk of exacerbations | [84] | |
| Clinical (case-control study) | 6.5 mg/kg/day | ↓inflammation ↑ survival | [85] | |
| Preclinical | 100 mg/kg/day | ↓Th17 response ↑Treg immunosuppressive effects | [86] | |
| Clinical (prospective cohort study) | 400 mg/day | ↓inflammatory markers | [87] | |
| Clinical (multiethnic US cohort) | n.a. | ↓IFN-α | [88] | |
| Preclinical | 4–40 mg/kg/day | ↓ mast cells ↓ skin lesion | [89] | |
| SLE-associated cardiovascular disease | Preclinical | 10 mg/kg/day | ↓ROS ↓endothelial damage | [90] |
| Preclinical | 3 mg/kg/day | ↓ROS and nitric oxide ↓ endothelial damage | [91] | |
| Clinical | 400 mg/day | ↓triglycerides and LDL | [92] | |
| Clinical (cross-sectional study) | 400 mg/day | ↓ fasting glucose | [82] | |
| SLE-associated pregnancy complications | Clinical (randomized double-blind) | n.a. | ↓inflammation | [93] |
| Clinical (prospective study) | 6.5 mg/kg/day | ↓inflammation ↓risk of exacerbations | [94] | |
| Antiphospholipid syndrome | Preclinical | 200 μg/day | ↓inflammation ↓complement activation ↓placental abnormalities | [95] |
| Clinical (case report) | 400 mg/day | ↓vascular thrombosis | [96] | |
| Preclinical | 12 μg/g/day | ↓endothelial damage ↓nitric oxide synthase | [97] | |
| Preclinical | 20 mg/kg/day | ↓endothelial damage ↓nitric oxide synthase | [98] | |
| Clinical (observational prospective study) | 200 mg/day | ↓thrombotic events in patients ↓soluble tissue factor levels. | [99] | |
| Sjögren syndrome | Clinical | 200 mg/day | ↓inflammation ↓IgG and IgA | [100] |
| Clinical (prospective study) | 400 mg/day | ↓xerostomia | [101] | |
| Clinical (prospective study) | 6.5 mg/kg | ↓eye dryness | [102] | |
| Preclinical | 50 mg/kg/day | ↓ xerostomia ↓ TGF-β↓inflammation | [103] | |
| Preclinical | 60 mg/kg/day | ↓inflammation ↓lymphocytic infiltration | [104] | |
| Diabetes | Preclinical | 80–120–160 mg/kg/day | ↓blood glucose | [105] |
| Preclinical | 200 mg/kg/day | ↓inflammatory markers ↑metabolic profile | [106] | |
| Clinical (randomized, double-blinded study) | 2 × 300 mg/kg | ↓glycated hemoglobin | [107] | |
| Clinical (open-label longitudinal study) | 6.5 mg/kg/day | ↓insulin resistance | [108] | |
| Clinical (randomized, double-blinded controlled trial) | 6.5 mg/kg/day | ↓insulin resistance | [109] | |
| Clinical (randomized, double-blinded trial) | 400 mg/day | ↑glycemic and lipidic profile | [110] | |
| Cancer | Preclinical | 50 mg/kg | ↓tumor size ↓pro-tumorigenic and pro-inflammatory cytokines | [111] |
| Cardiovascular diseases | Preclinical | 200 mg/kg | ↓apoptosis in cardiomyocites | [112] |
| Preclinical | 200 mg/kg/day | ↓triglycerides and LDL | [113] | |
| Preclinical | 10 mg/kg/day | ↓atherosclerosis ↓inflammation | [114] | |
| Inflammatory bowel disease and colitis | Preclinical | 30 mg/kg | ↓inflammation | [74] |
| Pulmonary hypertension | Preclinical | 50 mg/kg/day | ↓inflammation | [115] |
| Endometriosis | Preclinical | 60 mg/kg | ↓inflammation ↓lesion number | [116] |
| Checklist for Assessment of Risk of Bias in Preclinical Studies |
|---|
| Are the hypothesis and objective of the study clearly described? |
| Are the main outcomes to be measured clearly described? |
| Are the main findings of the study clearly described? |
| Are the samples size calculations reported? |
| Are the animals randomly housed during the experiment? |
| Are the investigators blinded from knowledge which treatment used? |
| Are the outcome assessors blinded? |
| Is the dose/route of administration of the HCQ properly reported? |
| Is the dose/route of administration of the drug in co-treatment properly reported? |
| Is the frequency of treatments adequately described? |
| Checklist for Assessment of Risk of Bias in Preclinical Studies |
|---|
| Are the hypothesis and objective of the study clearly described? |
| Are the main outcomes to be measured clearly described? |
| Are the main findings of the study clearly described? |
| Are the samples size calculations reported? |
| Are the animals randomly housed during the experiment? |
| Are the investigators blinded from knowledge which treatment used? |
| Are the outcome assessors blinded? |
| Is the dose/route of administration of the HCQ properly reported? |
| Is the dose/route of administration of the drug in co-treatment properly reported? |
| Is the frequency of treatments adequately described? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faraone, I.; Labanca, F.; Ponticelli, M.; De Tommasi, N.; Milella, L. Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review. Molecules 2020, 25, 5318. https://doi.org/10.3390/molecules25225318
Faraone I, Labanca F, Ponticelli M, De Tommasi N, Milella L. Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review. Molecules. 2020; 25(22):5318. https://doi.org/10.3390/molecules25225318
Chicago/Turabian StyleFaraone, Immacolata, Fabiana Labanca, Maria Ponticelli, Nunziatina De Tommasi, and Luigi Milella. 2020. "Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review" Molecules 25, no. 22: 5318. https://doi.org/10.3390/molecules25225318
APA StyleFaraone, I., Labanca, F., Ponticelli, M., De Tommasi, N., & Milella, L. (2020). Recent Clinical and Preclinical Studies of Hydroxychloroquine on RNA Viruses and Chronic Diseases: A Systematic Review. Molecules, 25(22), 5318. https://doi.org/10.3390/molecules25225318








