Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis
Abstract
:1. Introduction
2. Results
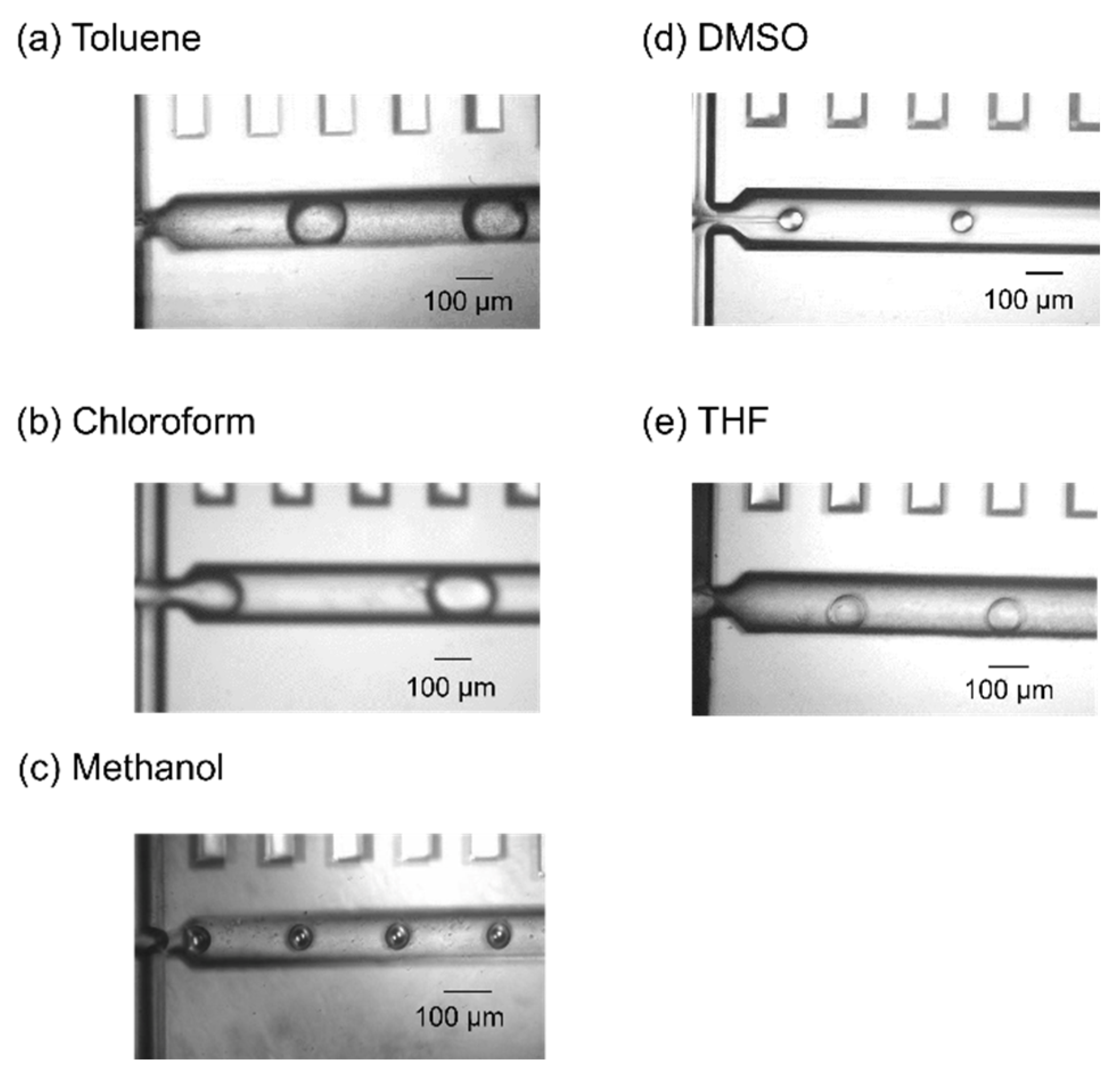
2.1. Droplet Generation of Typical Organic Solvents
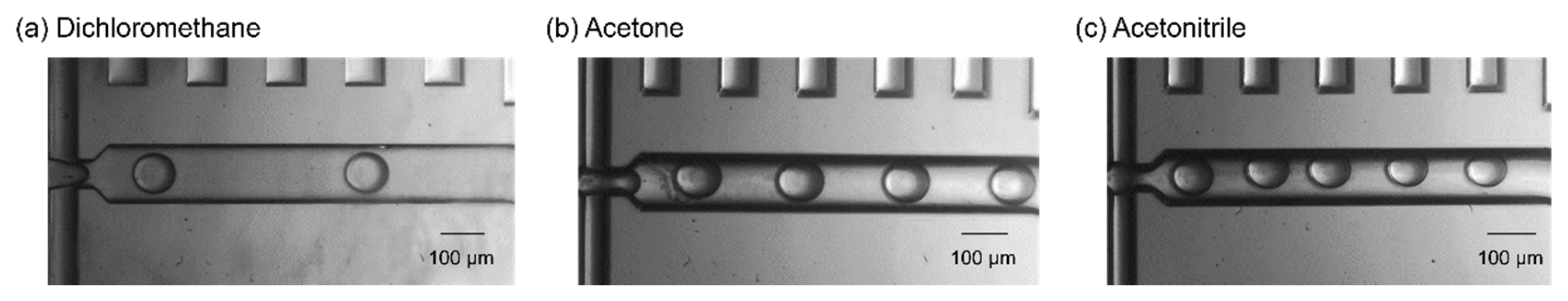
2.2. Droplet Generation of Organic Solvents Incompatible with High Chemical Resistance Resins
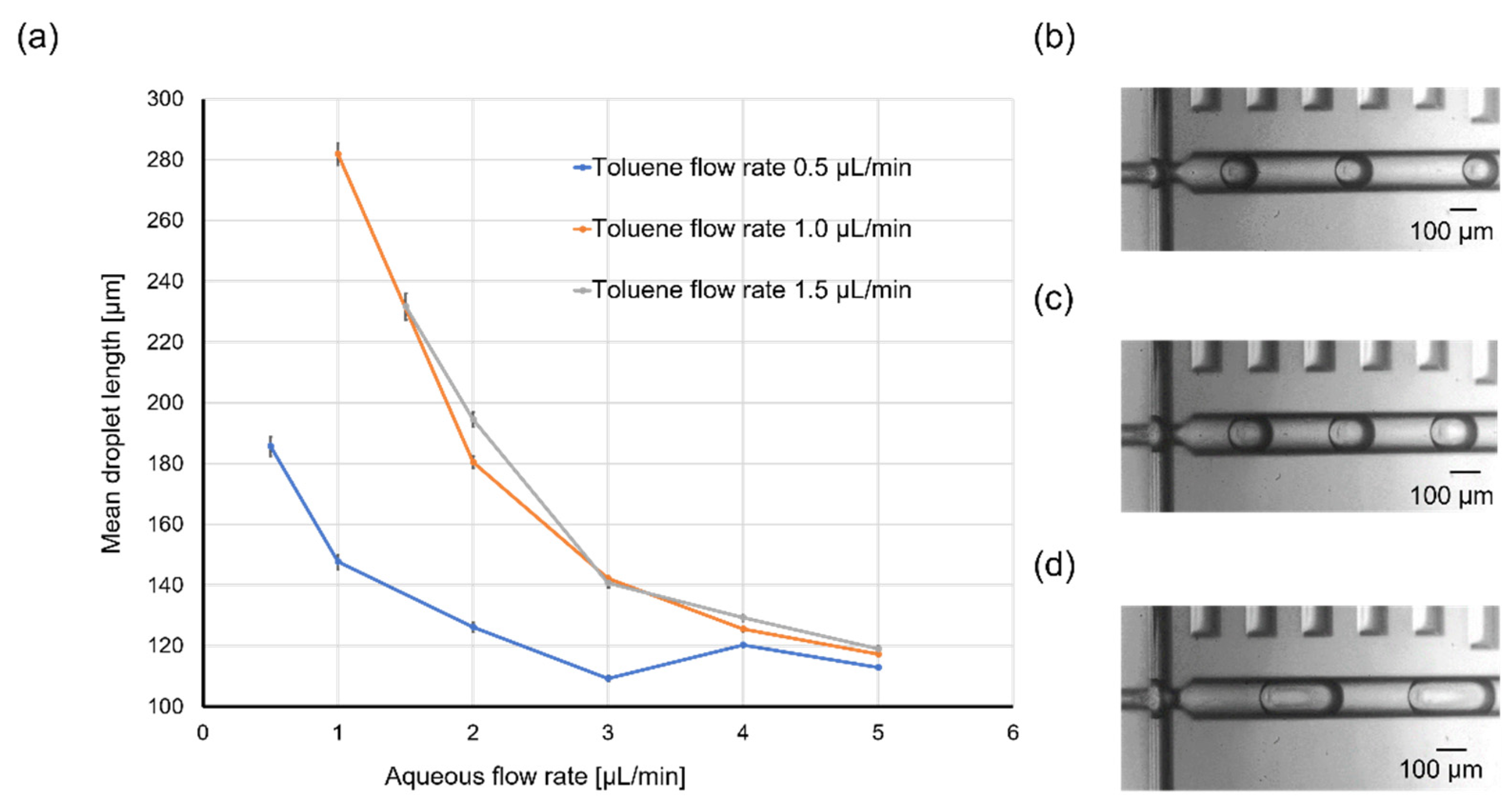
2.3. Droplet Size and Generation Rate Variation
3. Materials and Methods
3.1. Chemicals and Sample Preparation
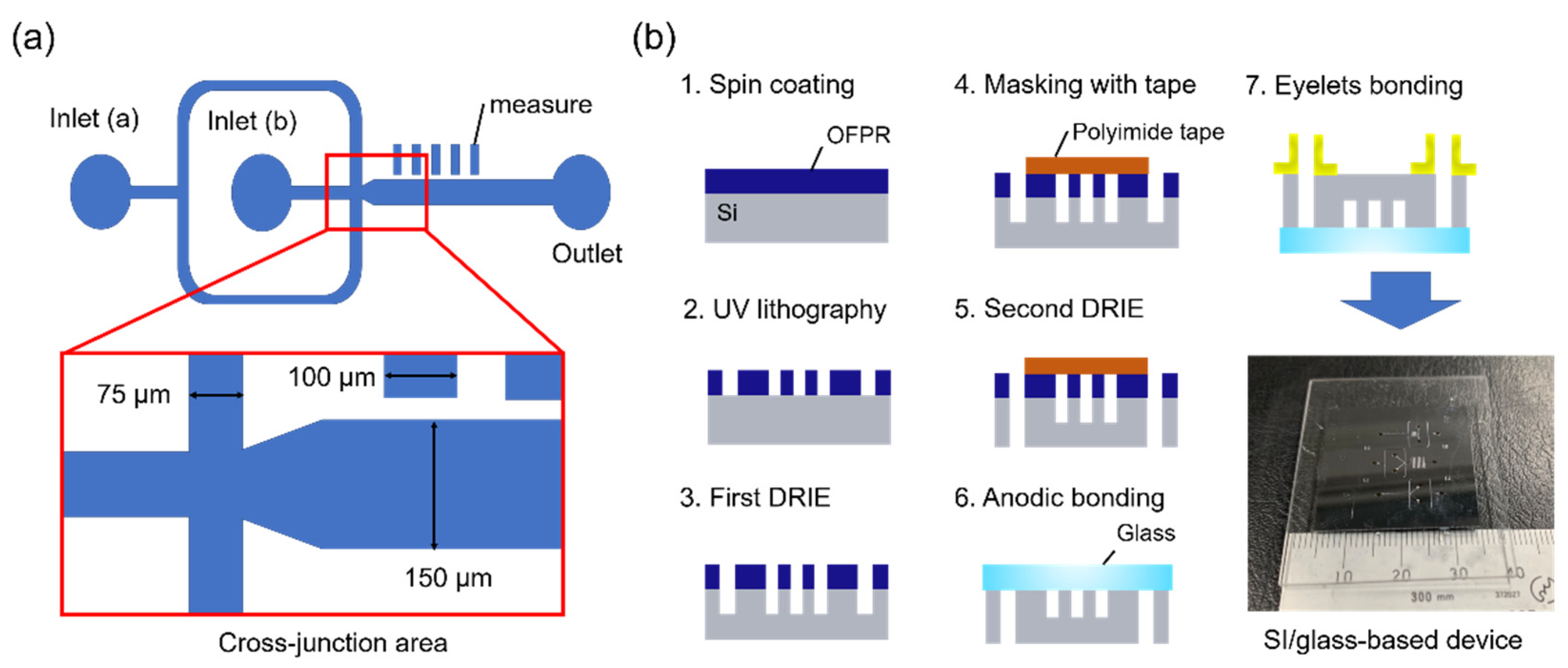
3.2. Device Design and Fabrication
3.3. Channel Surface Treatment
3.4. Microfluidic Experiment Setup
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977–1026. [Google Scholar] [CrossRef] [Green Version]
- Atencia, J.; Beebe, D.J. Controlled microfluidic interfaces. Nat. Cell Biol. 2005, 437, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Darhuber, A.A.; Troian, S.M. Principles of microfluidic actuation by modulation of surface stresses. Annu. Rev. Fluid Mech. 2005, 37, 425–455. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.H.; Tanaka, D.; Sekiguchi, T.; Shoji, S. Microfluidic Stamping on Sheath Flow. Small 2016, 12, 3224–3228. [Google Scholar] [CrossRef] [PubMed]
- McMullen, J.P.; Jensen, K.F. An Automated Microfluidic System for Online Optimization in Chemical Synthesis. Org. Process. Res. Dev. 2010, 14, 1169–1176. [Google Scholar] [CrossRef]
- Demello, A.J. Control and detection of chemical reactions in microfluidic systems. Nat. Cell Biol. 2006, 442, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Craighead, H. Future lab-on-a-chip technologies for interrogating individual molecules. Nanosci. Technol. 2009, 442, 330–336. [Google Scholar] [CrossRef]
- El-Ali, J.; Sorger, P.K.; Jensen, K.F. Cells on chips. Nature 2006, 442, 403–411. [Google Scholar] [CrossRef]
- Yager, P.; Edwards, T.; Fu, E.; Helton, K.; Nelson, K.; Tam, M.R.; Weigl, B.H. Microfluidic diagnostic technologies for global public health. Nat. Cell Biol. 2006, 442, 412–418. [Google Scholar] [CrossRef]
- Psaltis, D.; Quake, S.R.; Yang, C. Developing optofluidic technology through the fusion of microfluidics and optics. Nat. Cell Biol. 2006, 442, 381–386. [Google Scholar] [CrossRef]
- Christopher, G.F.; Anna, S.L. Microfluidic methods for generating continuous droplet streams. J. Phys. D Appl. Phys. 2007, 40, R319–R336. [Google Scholar] [CrossRef]
- Zhu, P.; Wang, L. Passive and active droplet generation with microfluidics: A review. Lab Chip 2017, 17, 34–75. [Google Scholar] [CrossRef] [PubMed]
- Haneoka, M.; Shirasaki, Y.; Sugino, H.; Aoki, T.; Arakawa, T.; Ozaki, K.; Yoon, D.H.; Ishii, N.; Iizuka, R.; Shoji, S.; et al. Microfluidic active sorting of DNA molecules labeled with single quantum dots using flow switching by a hydrogel sol-gel transition. Sens. Actuators B Chem. 2011, 159, 314–320. [Google Scholar] [CrossRef]
- Jamshaid, A.; Igaki, M.; Yoon, D.H.; Sekiguchi, T.; Shoji, S. Controllable Active Micro Droplets Merging Device Using Horizontal Pneumatic Micro Valves. Micromachines 2013, 4, 34–48. [Google Scholar] [CrossRef] [Green Version]
- Link, D.R.; Anna, S.L.; Weitz, D.A.; Stone, H.A. Geometrically Mediated Breakup of Drops in Microfluidic Devices. Phys. Rev. Lett. 2004, 92, 054503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liau, A.; Karnik, R.; Majumdar, A.; Cate, J.H.D. Mixing Crowded Biological Solutions in Milliseconds. Anal. Chem. 2005, 77, 7618–7625. [Google Scholar] [CrossRef] [PubMed]
- Huebner, A.; Bratton, D.; Whyte, G.; Yang, M.; Demello, A.J.; Abell, C.; Hollfelder, F. Static microdroplet arrays: A microfluidic device for droplet trapping, incubation and release for enzymatic and cell-based assays. Lab Chip 2009, 9, 692–698. [Google Scholar] [CrossRef]
- Schneider, T.; Kreutz, J.; Chiu, D.T. The Potential Impact of Droplet Microfluidics in Biology. Anal. Chem. 2013, 85, 3476–3482. [Google Scholar] [CrossRef] [Green Version]
- Mashaghi, S.; Abbaspourrad, A.; Weitz, D.A.; Van Oijen, A.M. Droplet microfluidics: A tool for biology, chemistry and nanotechnology. TrAC Trends Anal. Chem. 2016, 82, 118–125. [Google Scholar] [CrossRef] [Green Version]
- Shembekar, N.; Chaipan, C.; Utharala, R.; Merten, C.A. Droplet-based microfluidics in drug discovery, transcriptomics and high-throughput molecular genetics. Lab Chip 2016, 16, 1314–1331. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Li, W.; Huang, Y.; Zheng, B. Measuring Rapid Enzymatic Kinetics by Electrochemical Method in Droplet-Based Microfluidic Devices with Pneumatic Valves. Anal. Chem. 2009, 81, 5840–5845. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Wang, S.; Song, Y.; Jiang, L. A miniature droplet reactor built on nanoparticle-derived superhydrophobic pedestals. Nano Res. 2010, 4, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Chen, D.L.; Ismagilov, R.F. Reactions in Droplets in Microfluidic Channels. Angew. Chem. Int. Ed. 2006, 45, 7336–7356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theberge, A.B.; Courtois, F.; Schaerli, Y.; Fischlechner, M.; Abell, C.; Hollfelder, F.; Huck, W.T.S. Microdroplets in Microfluidics: An Evolving Platform for Discoveries in Chemistry and Biology. Angew. Chem. Int. Ed. 2010, 49, 5846–5868. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Funaki, N.; Kurabayashi, T.; Suzuki, M.; Yoon, D.H.; Nakahara, A.; Sekiguchi, T.; Shoji, S. Real-time monitoring of chemical reaction in microdroplet using fluorescence spectroscopy. Sens. Actuators B Chem. 2014, 203, 536–542. [Google Scholar] [CrossRef]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koiri, R.K.; Trigun, S.K.; Dubey, S.K.; Singh, S.; Mishra, L. Metal Cu(II) and Zn(II) bipyridyls as inhibitors of lactate dehydrogenase. BioMetals 2007, 21, 117–126. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, S.; Lin, Q.; Cheng, H.; Liu, J. Microdroplets as Microreactors for Fast Synthesis of Ketoximes and Amides. J. Org. Chem. 2018, 84, 851–859. [Google Scholar] [CrossRef]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Cygan, Z.T.; Cabral, J.T.; Beers, K.L.; Amis, E.J. Microfluidic Platform for the Generation of Organic-Phase Microreactors. Langmuir 2005, 21, 3629–3634. [Google Scholar] [CrossRef]
- Geczy, R.; Sticker, D.; Bovet, N.; Häfeli, U.O.; Kutter, J.P. Chloroform compatible, thiol–ene based replica molded micro chemical devices as an alternative to glass microfluidic chips. Lab Chip 2019, 19, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, C.; Li, Z. Thiol–ene-acrylate Ternary Photosensitive Resins for DLP 3D Printing. J. Photopolym. Sci. Technol. 2020, 33, 285–290. [Google Scholar] [CrossRef]
- Anthamatten, M.; O’Neill, S.W.; Liu, D.; Wheler, T.M.; Vallery, R.S.; Gidley, D.W. Tunability of Free Volume and Viscoelastic Damping of Thiol–Ene Networks Deep in the Glassy State. Macromolecules 2018, 51, 2564–2571. [Google Scholar] [CrossRef]
- Piccin, E.; Ferraro, D.; Sartori, P.; Chiarello, E.; Pierno, M.; Mistura, G. Generation of water-in-oil and oil-in-water microdroplets in polyester-toner microfluidic devices. Sens. Actuators B Chem. 2014, 196, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Lashkaripour, A.; Rodriguez, C.; Ortiz, L.; Densmore, D. Performance tuning of microfluidicflow-focusing droplet generators. Lab Chip 2019, 19, 1041–1053. [Google Scholar] [CrossRef]
- Bąk, A.; Podgórska, W. Interfacial and surface tensions of toluene/water and air/water systems with nonionic surfactants Tween 20 and Tween 80. Colloids Surf. A Physicochem. Eng. Asp. 2016, 504, 414–425. [Google Scholar] [CrossRef]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef]
- Asano, H.; Shiraishi, Y. Development of paper-based microfluidic analytical device for iron assay using photomask printed with 3D printer for fabrication of hydrophilic and hydrophobic zones on paper by photolithography. Anal. Chim. Acta 2015, 883, 55–60. [Google Scholar] [CrossRef]



| Dispersed Phase | Continuous Phase | Example Flow Rate at Which Droplets Were Generated | |||
|---|---|---|---|---|---|
| Surfactant | Dispersed Phase (µL/min) | Continuous Phase (µL/min) | Mean Droplet Length (µm) | Droplet Generation Rate (Hz) | |
| Toluene | DI water | 1 | 3 | 286.3 ± 12.2 | 13.8 |
| Tween 20 (4 wt %) | |||||
| Chloroform | DI water | 1 | 3 | 183.4 ± 2.7 | 9.55 |
| PVA (3 wt %) | |||||
| Methanol | Mineral oil | 0.3 | 3 | 83.9 ± 1.0 | 23.9 |
| Surflon S-656 (1 wt %) | |||||
| DMSO | Diethyl ether | 0.4 | 8 | 56.8 ± 1.5 | 14.7 |
| Span 80 (2 wt %) | |||||
| THF | Saturated saline | 0.5 | 5 | 102.1 ± 0.8 | 17.6 |
| Tween 20 (4 wt %) | |||||
| Dispersed Phase | Continuous Phase | Example Flow Rate at Which Droplets Were Generated | |||
|---|---|---|---|---|---|
| Surfactant | Dispersed Phase (µL/min) | Continuous Phase (µL/min) | Mean Droplet Length (µm) | Droplet Generation Rate (Hz) | |
| Dichloromethane | DI water | 1 | 10 | 109.4 ± 1.75 | 47.6 |
| PVA (1 wt %) | |||||
| Acetone | PFC-based solvents | 1 | 3 | 112.3 ± 2.26 | 27.3 |
| None | |||||
| Acetonitrile | Fluorinated oil | 1 | 3 | 103.8 ± 3.3 | 29.6 |
| None | |||||
| Dispersed Phase (Droplets) | Continuous Phase (Carrier) | |||
|---|---|---|---|---|
| Liquid | Type (Dipole Moment) | Liquid | Type | Solubility in the Dispersed-Phase Solution |
| Toluene | Nonpolar sol. (0.36 D) | DI water | Protic polar sol. | 0.045 g/100 mL (20 °C) |
| Chloroform | Nonpolar sol. (1.04 D) | DI water | Protic polar sol. | 0.8 g/100 mL (20 °C) |
| Methanol | Protic polar sol. (1.70 D) | Mineral oil | Nonpolar sol. | Immiscible (No date) |
| DMSO | Aprotic polar sol. (3.96 D) | Diethyl ether | Nonpolar sol. | Immiscible (No date) |
| THF | Aprotic polar sol. (1.75 D) | Saturated saline | Protic polar sol. | 5.49 g/100 mL (25 °C) |
| Acetone | Aprotic polar sol. (2.88 D) | PFC-based solvent | Nonpolar sol. | Immiscible (No date) |
| Acetonitrile | Aprotic polar sol. (3.92 D) | HFE-based oil | Nonpolar sol. | Immiscible (No date) |
| Dichloromethane | Aprotic polar sol. (1.60 D) | DI water | Protic polar sol. | 1.3 g/100 mL (20 °C) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hattori, S.; Tang, C.; Tanaka, D.; Yoon, D.H.; Nozaki, Y.; Fujita, H.; Akitsu, T.; Sekiguchi, T.; Shoji, S. Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis. Molecules 2020, 25, 5360. https://doi.org/10.3390/molecules25225360
Hattori S, Tang C, Tanaka D, Yoon DH, Nozaki Y, Fujita H, Akitsu T, Sekiguchi T, Shoji S. Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis. Molecules. 2020; 25(22):5360. https://doi.org/10.3390/molecules25225360
Chicago/Turabian StyleHattori, Shohei, Chenghe Tang, Daiki Tanaka, Dong Hyun Yoon, Yoshito Nozaki, Hiroyuki Fujita, Takashiro Akitsu, Tetsushi Sekiguchi, and Shuichi Shoji. 2020. "Development of Microdroplet Generation Method for Organic Solvents Used in Chemical Synthesis" Molecules 25, no. 22: 5360. https://doi.org/10.3390/molecules25225360






