Possible Participation of Ionotropic Glutamate Receptors and l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate-ATP-Sensitive K+ Channel Pathway in the Antinociceptive Activity of Cardamonin in Acute Pain Animal Models
Abstract
:1. Introduction
2. Results
2.1. Antinociceptive Analysis
2.1.1. Acetic Acid-Induced Abdominal Writhing Test
2.1.2. Involvement of Protein Kinase C
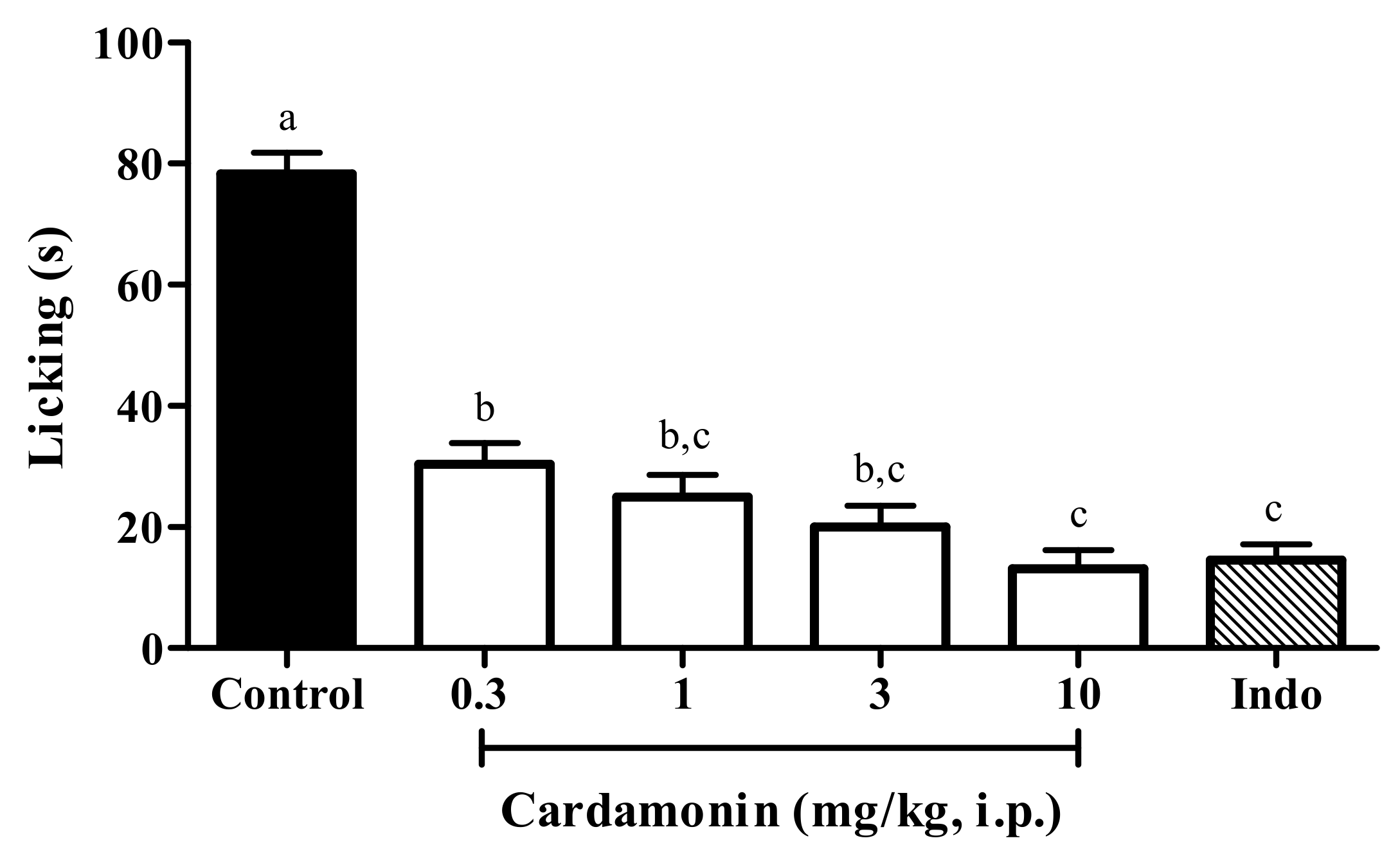
2.1.3. Effect of MK-801 and NBQX on Glutamate-Induced Nociception
2.2. Analysis of the Possible Mechanism of Action of Cardamonin
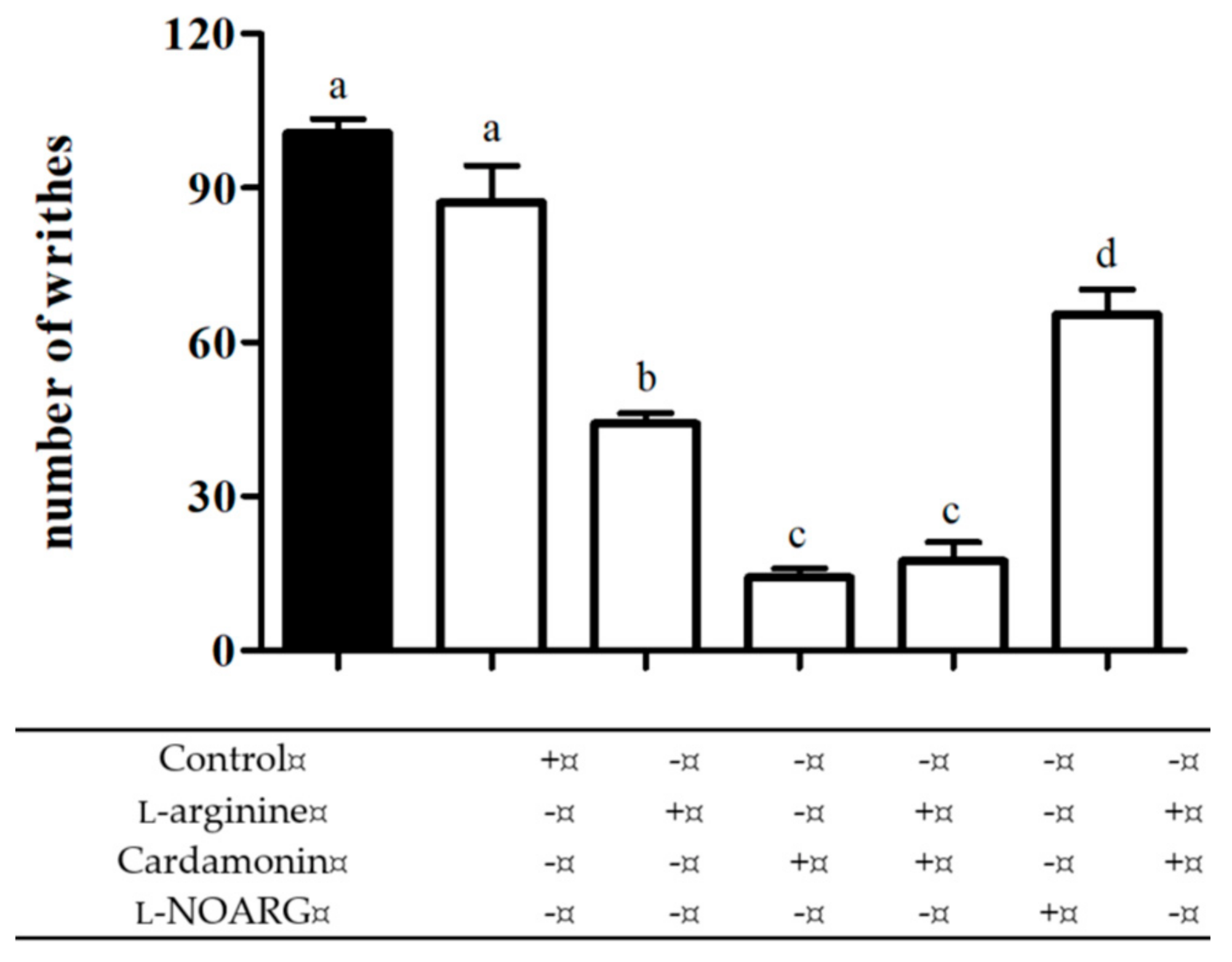
2.2.1. Involvement of l-arginine/Nitric Oxide Pathway
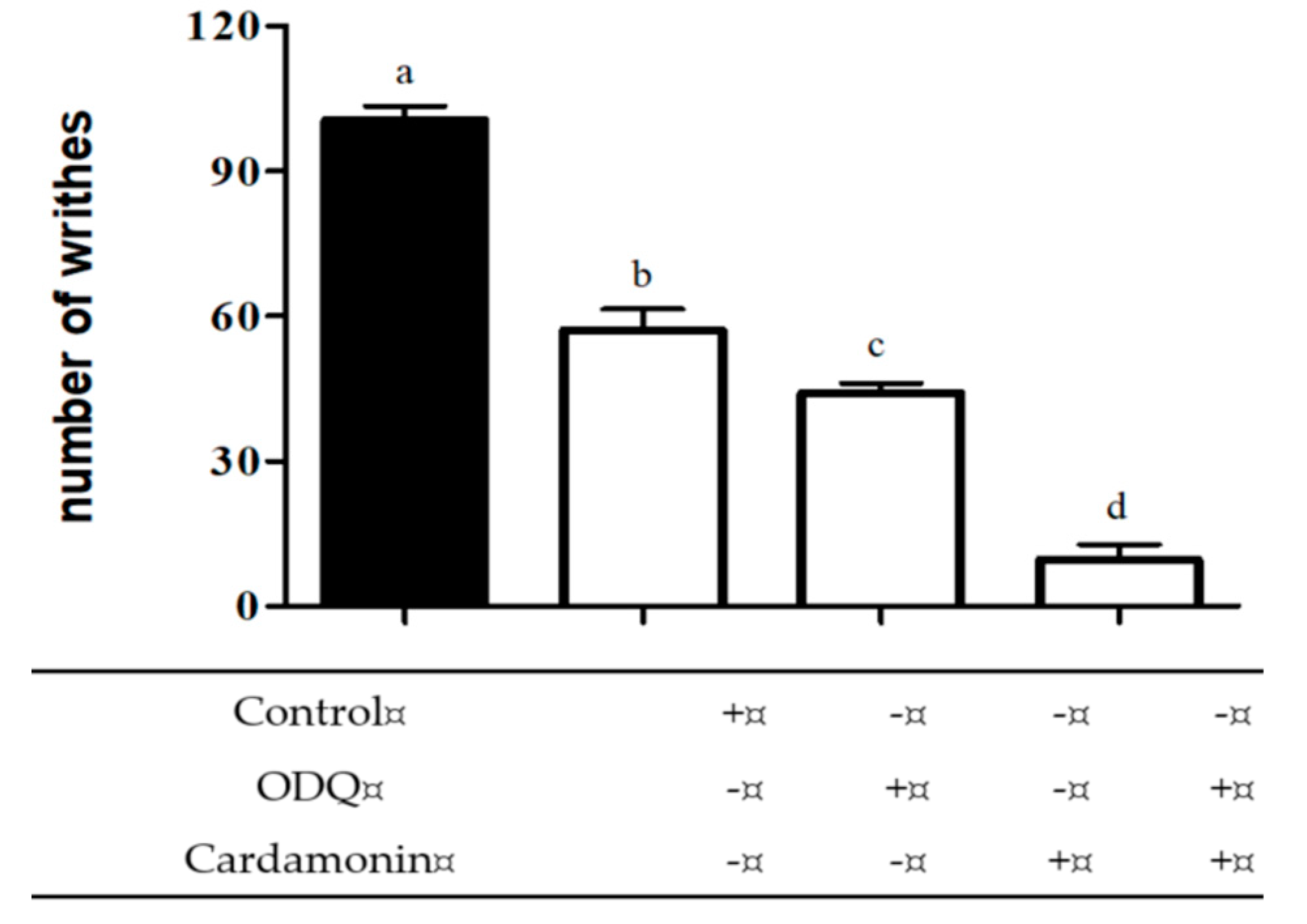
2.2.2. Involvement of Cyclic Guanosine Monophosphate (cGMP)
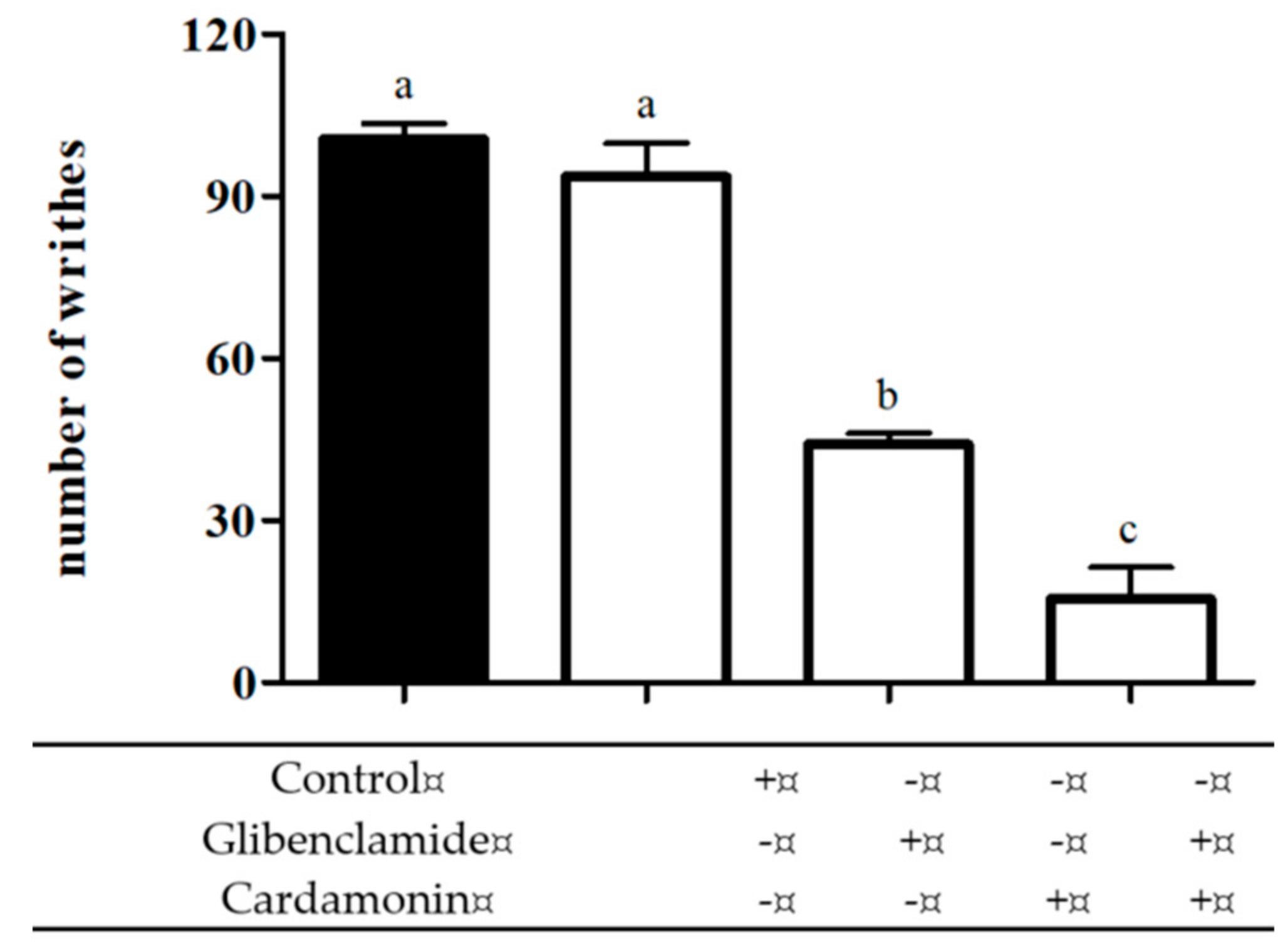
2.2.3. Involvement of ATP-Sensitive K+ Channel
3. Discussion
4. Methodology
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Experimental Animals
4.4. Drugs and Chemicals
4.5. Antinociceptive Analysis
4.5.1. Acetic Acid-Induced Abdominal Writhing Test
4.5.2. Involvement of Protein Kinase C
4.5.3. Effect of MK-801 and NBQX on Glutamate-Induced Nociception
4.6. Analysis of the Possible Mechanism of Action of Cardamonin
4.6.1. Involvement of l-arginine/Nitric Oxide Pathway
4.6.2. Involvement of Cyclic Guanosine Monophosphate (cGMP)
4.6.3. Involvement of ATP-Sensitive K+ Channel
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rao, C.B.; Rao, T.N.; Suryaprakasam, S. Cardamonin and alpinetin from the seeds of Amomum subulatum. Planta Med. 1976, 29, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Israf, D.A.; Lajis, N.H.; Shaari, K.; Mohamed, H.; Wahab, A.A.; Ariffin, K.T.; Hoo, W.Y.; Aziz, N.A.; Kadir, A.A. Cardamonin, inhibits pro-inflammatory mediators in activated RAW 264.7 cells and whole blood. Eur. J. Pharmacol. 2006, 538, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, Y.-L.; Lee, K.-H.; Vidyadaran, S.; Lajis, N.H.; Akhtar, M.N.; Israf, D.A.; Syahida, A. Cardamonin from Alpinia rafflesiana inhibits inflammatory responses in IFN-γ/LPS-stimulated BV2 microglia via NF-ҡB signalling pathway. Int. Immunopharmacol. 2012, 12, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-T.; Lau, C.-W.; Chan, F.L.; Yao, X.; Chen, Z.-Y.; He, Z.-D.; Huang, Y. Vasorelaxant effects of cardamonin and alpinetin from Alpinia henryi K. Schum. J. Cardiovasc. Pharmacol. 2001, 37, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, A.C.R.F.; Ehrenfried, C.A.; Lopez, B.G.-C.; de Araujo, T.M.; Pascoal, V.; Gilioli, R.; Anhê, G.F.; Ruiz, A.L.T.G.; Carvalho, J.E.D.; Stefanello, M.L.A. Antiproliferative activity and induction of apoptosis in PC-3 cells by the chalcone cardamonin from Campomanesia adamantium (Myrtaceae) in a bioactivity-guided study. Molecules 2014, 19, 1843–1855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Sun, C.-Y.; Lu, F.-R.; Shu, X.-R.; Yang, D.; Chen, L.; She, X.-M.; Gregg, N.M.; Guo, T.; Hu, Y. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-KB pathway in vitro. Leuk. Res. 2012, 36, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Fang, Q.; Shi, D.; Niu, P.; Chen, Y.; Deng, J. mTOR inhibition of cardamonin on antiproliferation of A549 cells is involved in a FKBP12 independent fashion. Life Sci. 2012, 99, 44–51. [Google Scholar] [CrossRef]
- Wei, Z.; Yang, J.; Xia, Y.e.; Huang, W.h.; Wang, Z.a.; Dai, Y. Cardamonin protects septic mice from acute lung injury by preventing endothelial barrier dysfunction. J. Biochem. Mol. Toxicol. 2012, 26, 282–290. [Google Scholar] [CrossRef]
- Trakoontivakorn, G.; Nakahara, K.; Shinmoto, H.; Takenaka, M.; Onishi-Kameyama, M.; Ono, H.; Yoshida, M.; Nagata, T.; Tsushida, T. Structural analysis of a novel antimutagenic compound, 4-hydroxypanduratin A, and the antimutagenic activity of flavonoids in a Thai spice, fingerroot (Boesenbergia pandurata Schult.) against mutagenic heterocyclic amines. J. Agric. Food Chem. 2001, 49, 3046–3050. [Google Scholar] [CrossRef]
- Liao, Q.; Shi, D.-H.; Zheng, W.; Xu, X.-J.; Yu, Y.-H. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur. J. Pharmacol. 2010, 641, 179–186. [Google Scholar] [CrossRef]
- Cho, M.; Ryu, M.; Jeong, Y.; Chung, Y.-H.; Kim, D.-E.; Cho, H.-S.; Kang, S.; Han, J.-S.; Chang, M.-Y.; Lee, C.-K. Cardamonin suppresses melanogenesis by inhibition of Wnt/β-catenin signaling. Biochem. Biophys. Res. Commun. 2009, 390, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Park, M.K.; Choi, J.K.; Kim, H.J.; Nakahata, N.; Lim, K.M.; Kim, S.Y.; Lee, C.H. Novel inhibitory effects of cardamonin on thromboxane A2-induced scratching response: Blocking of Gh/transglutaminase-2 binding to thromboxane A2 receptor. Pharmacol. Biochem. Behav. 2014, 126, 131–135. [Google Scholar] [CrossRef] [PubMed]
- El-Naga, R.N. Pre-treatment with cardamonin protects against cisplatin-induced nephrotoxicity in rats: Impact on NOX-1, inflammation and apoptosis. Toxicol. Appl. Pharmacol. 2014, 274, 87–95. [Google Scholar] [CrossRef]
- Zhang, T.; Yamamoto, N.; Yamashita, Y.; Ashida, H. The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK. Arch. Biochem. Biophys. 2014, 554, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.A.; Akhtar, M.N.; Biau, F.J.; Tor, Y.S.; Zareen, S.; Binti Shahabudin, S.; Binti Abd Hamid, H.; Ul Haq, Z.; Khalil, R.; Khalaf, R.M. Isolation of Cardamonin and Pinostrobin Chalcone from the Rhizomes of Boesenbergia rotunda (L.) Mansf. and their Cytotoxic Effects on H-29 and MDA-MB-231 Cancer Cell Lines. Nat. Prod. J. 2019, 9, 341–348. [Google Scholar] [CrossRef]
- Niu, P.; Shi, D.; Zhang, S.; Zhu, Y.; Zhou, J. Cardamonin enhances the anti-proliferative effect of cisplatin on ovarian cancer. Oncol. Lett. 2018, 15, 3991–3997. [Google Scholar] [CrossRef] [Green Version]
- Hou, S.; Yuan, Q.; Yu, N.; Liu, B.; Huang, G.; Yuan, X. Cardamonin attenuates chronic inflammation and tumorigenesis in colon. Cell Cycle 2019, 18, 3275–3287. [Google Scholar] [CrossRef]
- Park, M.K.; Jo, S.H.; Lee, H.J.; Kang, J.H.; Kim, Y.R.; Kim, H.J.; Lee, E.J.; Koh, J.Y.; Ahn, K.O.; Jung, K.C.; et al. Novel suppressive effects of cardamonin on the activity and expression of transglutaminase-2 lead to blocking the migration and invasion of cancer cells. Life Sci. 2013, 92, 154–160. [Google Scholar] [CrossRef] [PubMed]
- James, S.; Aparna, J.S.; Paul, A.M.; Lankadasari, M.B.; Mohammed, S.; Binu, V.S.; Santhoshkumar, T.R.; Reshmi, G.; Harikumar, K.B. Cardamonin inhibits colonic neoplasia through modulation of MicroRNA expression. Sci. Rep. 2017, 7, 13945. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Qiu, S.; Wang, P.; Liang, X.; Huang, F.; Wu, H.; Zhang, B.; Zhang, W.; Tian, X.; Xu, R. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J. Exp. Clin. Cancer Res. 2019, 38, 377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hou, G.; Yuan, X.; Li, Y.; Hou, G.; Liu, X. Cardamonin, a natural chalcone, reduces 5-fluorouracil resistance of gastric cancer cells through targeting Wnt/β-catenin signal pathway. Investig. New Drugs 2020, 38, 329–339. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, X.; Wu, X.; Yang, M.; Wang, W.; Wang, L.; Tang, D.; Wang, D. Cardamonin exerts anti-gastric cancer activity via inhibiting LncRNA-PVT1-STAT3 axis. Biosci. Rep. 2019, 39, BSR20190357. [Google Scholar] [CrossRef] [Green Version]
- Voon, F.-L.; Sulaiman, M.R.; Akhtar, M.N.; Idris, M.F.; Akira, A.; Perimal, E.K.; Israf, D.A.; Ming-Tatt, L. Cardamonin (2′-4′-dihydroxy-6′-methoxychalcone) isolated from Boesenbergia rotunda (L.) Mansf. inhibits CFA-induced rheumatoid arthritis in rats. Eur. J. Pharmacol. 2017, 794, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Sambasevam, Y.; Farouk, A.A.O.; Mohamad, T.A.S.T.; Sulaiman, M.R.; Bharatham, B.H.; Perimal, E.K. Cardamonin attenuates hyperalgesia and allodynia in a mouse model of chronic constriction injury-induced neuropathic pain: Possible involvement of the opioid system. Eur. J. Pharmacol. 2017, 796, 32–38. [Google Scholar] [CrossRef]
- Benchabane, S.; Belguendouz, H.; Behairi, N.; Arroul-Lammali, A.; Boudjelida, A.; Youinou, P.; Touil-boukoffa, C. Cardamonin inhibits pro-inflammatory cytokine production and suppresses NO pathway in PBMCs from patients with primary Sjogren’s syndrome. Immunopharmacol. Immunotoxicol. 2018, 40, 126–133. [Google Scholar] [CrossRef]
- Israf, D.A.; Khaizurin, T.A.; Syahida, A.; Lajis, N.H.; Khozirah, S. Cardamonin inhibits COX and iNOS expression via inhibition of p65NF-ҡB nuclear translocation and ҡ-B phosphorylation in RAW 264.7 macrophage cells. Mol. Immunol. 2007, 44, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Yamamoto, N.; Murakami, A. Cardamonin suppresses nitric oxide production via blocking the IFN-γ/STAT pathway in endotoxin-challenged peritoneal macrophages of ICR mice. Life Sci. 2011, 89, 337–342. [Google Scholar] [CrossRef]
- Park, M.K.; Lee, H.J.; Choi, J.K.; Kim, H.J.; Kang, J.H.; Lee, E.J.; Kim, Y.R.; Kang, J.H.; Yoo, J.K.; Cho, H.Y.; et al. Novel anti-nociceptive effects of cardamonin via blocking expression of cyclooxygenase-2 and transglutaminase-2. Pharmacol. Biochem. Behav. 2014, 118, 10–15. [Google Scholar] [CrossRef]
- Ping, C.P.; Mohamad, T.; Shah, T.A.; Akhtar, M.N.; Perimal, E.K.; Akira, A.; Ali, I.; Ahmad, D.; Sulaiman, M.R. Antinociceptive effects of cardamonin in mice: Possible involvement of TRPV1, glutamate, and opioid receptors. Molecules 2018, 23, 2237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beirith, A.; Santos, A.R.S.; Calixto, J.B. Mechanisms underlying the nociception and paw oedema caused by injection of glutamate into the mouse paw. Brain Res. 2002, 924, 219–228. [Google Scholar] [CrossRef]
- Ferreira, J.; Santos, A.R.S.; Calixto, J.B. The role of systemic, spinal and supraspinal l-arginine-nitric oxide-cGMP pathway in thermal hyperalgesia caused by intrathecal injection of glutamate in mice. Neuropharmacology 1999, 38, 835–842. [Google Scholar] [CrossRef]
- Wang, S.; Zhai, C.; Zhang, Y.; Yu, Y.; Zhang, Y.; Ma, L.; Li, S.; Qiao, Y. Cardamonin, a novel antagonist of hTRPA1 cation channel, reveals therapeutic mechanism of pathological pain. Molecules 2016, 21, 1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zulazmi, N.A.; Gopalsamy, B.; Farouk, A.A.O.; Sulaiman, M.R.; Bharatham, B.H.; Perimal, E.K. Antiallodynic and antihyperalgesic effects of zerumbone on a mouse model of chronic constriction injury-induced neuropathic pain. Fitoterapia 2015, 105, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Higashi, T.; Togashi, K.; Iida, T.; Segi, E.; Sugimoto, Y.; Tominaga, T.; Narumiya, S.; Tominaga, M. Sensitization of TRPV1 by EP1 and IP reveals peripheral nociceptive mechanism of prostaglandins. Mol. Pain 2005, 1, 3. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, J.; Triches, K.M.; Medeiros, R.; Calixto, J.B. Mechanisms involved in the nociception produced by peripheral protein kinase c activation in mice. Pain 2005, 117, 171–181. [Google Scholar] [CrossRef]
- Velazquez, K.T.; Mohammad, H.; Sweitzer, S.M. Protein kinase C in pain: Involvement of multiple isoforms. Pharmacol. Res. 2007, 55, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellani, V.; Mapplebeck, S.; Moriondo, A.; Davis, J.B.; McNaughton, P.A. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 2001, 534, 813–825. [Google Scholar] [CrossRef]
- Sikand, P.; Premkumar, L.S. Potentiation of glutamatergic synaptic transmission by protein kinase C-mediated sensitization of TRPV1 at the first sensory synapse. J. Physiol. 2007, 581, 631–647. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Wu, Z.; Lin, Q.; Yue, Y.; Fang, L. Regulation of AMPA receptors in spinal nociception. Mol. Pain 2010, 6, 5. [Google Scholar] [CrossRef]
- Alexander, S.P.H. Glutamate. In Intercellular Communication in the Nervous System; Malenka, R., Ed.; Academic Press: Cambridge, MA, USA, 2009. [Google Scholar]
- Petrenko, A.B.; Yamakura, T.; Baba, H.; Shimoji, K. The role of N-methyl-D-aspartate (NMDA) receptors in pain: A review. Anesth. Analg. 2003, 97, 1108–1116. [Google Scholar] [CrossRef]
- Zhou, S.; Bonasera, L.; Carlton, S.M. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport 1996, 7, 895–900. [Google Scholar] [CrossRef]
- Lutfy, K.; Weber, E. Tolerance develops to the antinociceptive and motor impairing effects of ACEA-1416, a NMDA receptor antagonist, in the formalin and rotarod tests in mice. Pharmacol. Res. 1998, 37, 295–302. [Google Scholar] [CrossRef]
- Miclescu, A.; Gordh, T. Nitric oxide and pain:’something old, something new’. Acta Anaesthesiol. Scand. 2009, 53, 1107–1120. [Google Scholar] [CrossRef]
- Esplugues, J.V. NO as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002, 135, 1079–1095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, I.D.G.; Ferreira, S.H. l-NAME causes antinociception by stimulation of the arginine-NO-cGMP pathway. Mediat. Inflamm. 2000, 9, 25–30. [Google Scholar] [CrossRef]
- Sakurada, C.; Sugiyama, A.; Nakayama, M.; Yonezawa, A.; Sakurada, S.; Tan-No, K.; Kisara, K.; Sakurada, T. Antinociceptive effect of spinally injected l-NAME on the acute nociceptive response induced by low concentrations of formalin. Neurochem. Int. 2001, 38, 417–423. [Google Scholar] [CrossRef]
- Moore, P.K.; Oluyomi, A.O.; Babbedge, R.C.; Wallace, P.; Hart, S.L. l-NG-nitro arginine methyl ester exhibits antinociceptive activity in the mouse. Br. J. Pharmacol. 1991, 102, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawamata, T.; Omote, K. Activation of spinal N-methyl-D-aspartate receptors stimulates a nitric oxide/cyclic guanosine 3, 5-monophosphate/glutamate release cascade in nociceptive signaling. Anesthesiology 1999, 91, 1415–1424. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Zhang, H. Activation of ATP-sensitive potassium channels antagonize nociceptive behavior and hyperexcitability of DRG neurons from rats. Mol. Pain 2011, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Gamper, N. Potassium channels in peripheral pain pathways: Expression, function and therapeutic potential. Curr. Neuropharmacol. 2013, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Ocana, M.; Cendan, C.M.; Cobos, E.J.; Entrena, J.M.; Baeyens, J.M. Potassium channels and pain: Present realities and future opportunities. Eur. J. Pharmacol. 2004, 500, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Galeotti, N.; Ghelardini, C.; Bartolini, A. Involvement of potassium channels in amitriptyline and clomipramine analgesia. Neuropharmacology 2001, 40, 75–84. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Amin, B. Effect of glibenclamide on antinociceptive effects of antidepressants of different classes. Clinics 2011, 66, 321–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, A.C.; Leite, R.; Tatsuo, M.A.K.F.; Duarte, I.D.G. Activation of ATP-sensitive K+ channels: Mechanism of peripheral antinociceptive action of the nitric oxide donor, sodium nitroprusside. Eur. J. Pharmacol. 2000, 400, 67–71. [Google Scholar] [CrossRef]
- Vale, M.L.; Rolim, D.E.; Cavalcante, I.F.; Ribeiro, R.A.; Souza, M. Role of NO/cGMP/KATP pathway in antinociceptive effect of sildenafil in zymosan writhing response in mice. Inflamm. Res. 2007, 56, 83–88. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Zarpelon, A.C.; Longhi-Balbinot, D.T.; Marchesi, M.; Cunha, T.M.; Alves-Filho, J.C.; Cunha, F.Q.; Ferreira, S.H.; Casagrande, R.; Miranda, K.M. Nitroxyl inhibits overt pain-like behavior in mice: Role of cGMP/PKG/ATP-sensitive potassium channel signaling pathway. Pharmacol. Rep. 2014, 66, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Kawano, T.; Zoga, V.; Kimura, M.; Liang, M.-Y.; Wu, H.-E.; Gemes, G.; McCallum, J.B.; Kwok, W.-M.; Hogan, Q.H.; Sarantopoulos, C.D. Nitric oxide activates ATP-sensitive potassium channels in mammalian sensory neurons: Action by direct S-nitrosylation. Mol. Pain 2009, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, M.R.; Somchit, M.N.; Israf, D.A.; Ahmad, Z.; Moin, S. Antinociceptive effect of Melastoma malabathricum ethanolic extract in mice. Fitoterapia 2004, 75, 667–672. [Google Scholar] [CrossRef]
- Khalid, M.H.; Akhtar, M.N.; Mohamad, A.S.; Perimal, E.K.; Akira, A.; Israf, D.A.; Lajis, N.; Sulaiman, M.R. Antinociceptive effect of the essential oil of Zingiber zerumbet in mice: Possible mechanisms. J. Ethnopharmacol. 2011, 137, 345–351. [Google Scholar] [CrossRef]
- Meotti, F.C.; Luiz, A.P.; Pizzolatti, M.G.; Kassuya, C.A.L.; Calixto, J.B.; Santos, A.R.S. Analysis of the antinociceptive effect of the flavonoid myricitrin: Evidence for a role of the l-arginine-nitric oxide and protein kinase C pathways. J. Pharmacol. Exp. Ther. 2006, 316, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, A.P.; Tort, A.B.; Silveira, P.P.; Bohmer, A.E.; Hansel, G.; Knorr, L.; Schallenberger, C.; Dalmaz, C.; Elisabetsky, E.; Crestana, R.H. The NMDA antagonist MK-801 induces hyperalgesia and increases CSF excitatory amino acids in rats: Reversal by guanosine. Pharmacol. Biochem. Behav. 2009, 91, 549. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.H.; Bae, H.B.; Choi, J.I. Antinociceptive interactions between intrathecal gabapentin and MK801 or NBQX in rat formalin test. J. Korean Med. Sci. 2005, 20, 307–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perimal, E.K.; Akhtar, M.N.; Mohamad, A.S.; Khalid, M.H.; Ming, O.H.; Khalid, S.; Tatt, L.M.; Kamaldin, M.N.; Zakaria, Z.A.; Israf, D.A. Zerumbone-induced antinociception: Involvement of the l-arginine-nitric oxide-cGMP-PKC-K+ ATP channel pathways. Basic Clin. Pharmacol. Toxicol. 2011, 108, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, C.A.; Santos, D.S.; Santana, D.G.; Thomazzi, S.M. Evaluation of mechanisms involved in the antinociception of the ethanol extract from the inner bark of Caesalpinia pyramidalis in mice. J. Ethnopharmacol. 2013, 148, 205–209. [Google Scholar] [CrossRef]
- Mohamad, A.S.; Akhtar, M.N.; Khalivulla, S.I.; Perimal, E.K.; Khalid, M.H.; Ong, H.M.; Zareen, S.; Akira, A.; Israf, D.A.; Lajis, N. Possible Participation of Nitric Oxide/Cyclic Guanosine Monophosphate/Protein Kinase C/ATP-Sensitive K+ Channels Pathway in the Systemic Antinociception of Flavokawin B. Basic Clin. Pharmacol. Toxicol. 2011, 108, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Rangel, R.A.S.; Marinho, B.G.; Fernandes, P.D.; Moura, R.S.; Lessa, M.A. Pharmacological mechanisms involved in the antinociceptive effects of dexmedetomidine in mice. Fundam. Clin. Pharmacol. 2014, 28, 104–113. [Google Scholar] [CrossRef] [Green Version]


Sample Availability: Sample of the compoundcardamonin is available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pui Ping, C.; Akhtar, M.N.; Israf, D.A.; Perimal, E.K.; Sulaiman, M.R. Possible Participation of Ionotropic Glutamate Receptors and l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate-ATP-Sensitive K+ Channel Pathway in the Antinociceptive Activity of Cardamonin in Acute Pain Animal Models. Molecules 2020, 25, 5385. https://doi.org/10.3390/molecules25225385
Pui Ping C, Akhtar MN, Israf DA, Perimal EK, Sulaiman MR. Possible Participation of Ionotropic Glutamate Receptors and l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate-ATP-Sensitive K+ Channel Pathway in the Antinociceptive Activity of Cardamonin in Acute Pain Animal Models. Molecules. 2020; 25(22):5385. https://doi.org/10.3390/molecules25225385
Chicago/Turabian StylePui Ping, Chung, Muhammad Nadeem Akhtar, Daud Ahmad Israf, Enoch Kumar Perimal, and Mohd Roslan Sulaiman. 2020. "Possible Participation of Ionotropic Glutamate Receptors and l-Arginine-Nitric Oxide-Cyclic Guanosine Monophosphate-ATP-Sensitive K+ Channel Pathway in the Antinociceptive Activity of Cardamonin in Acute Pain Animal Models" Molecules 25, no. 22: 5385. https://doi.org/10.3390/molecules25225385





