Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets
Abstract
:1. Introduction
2. Results
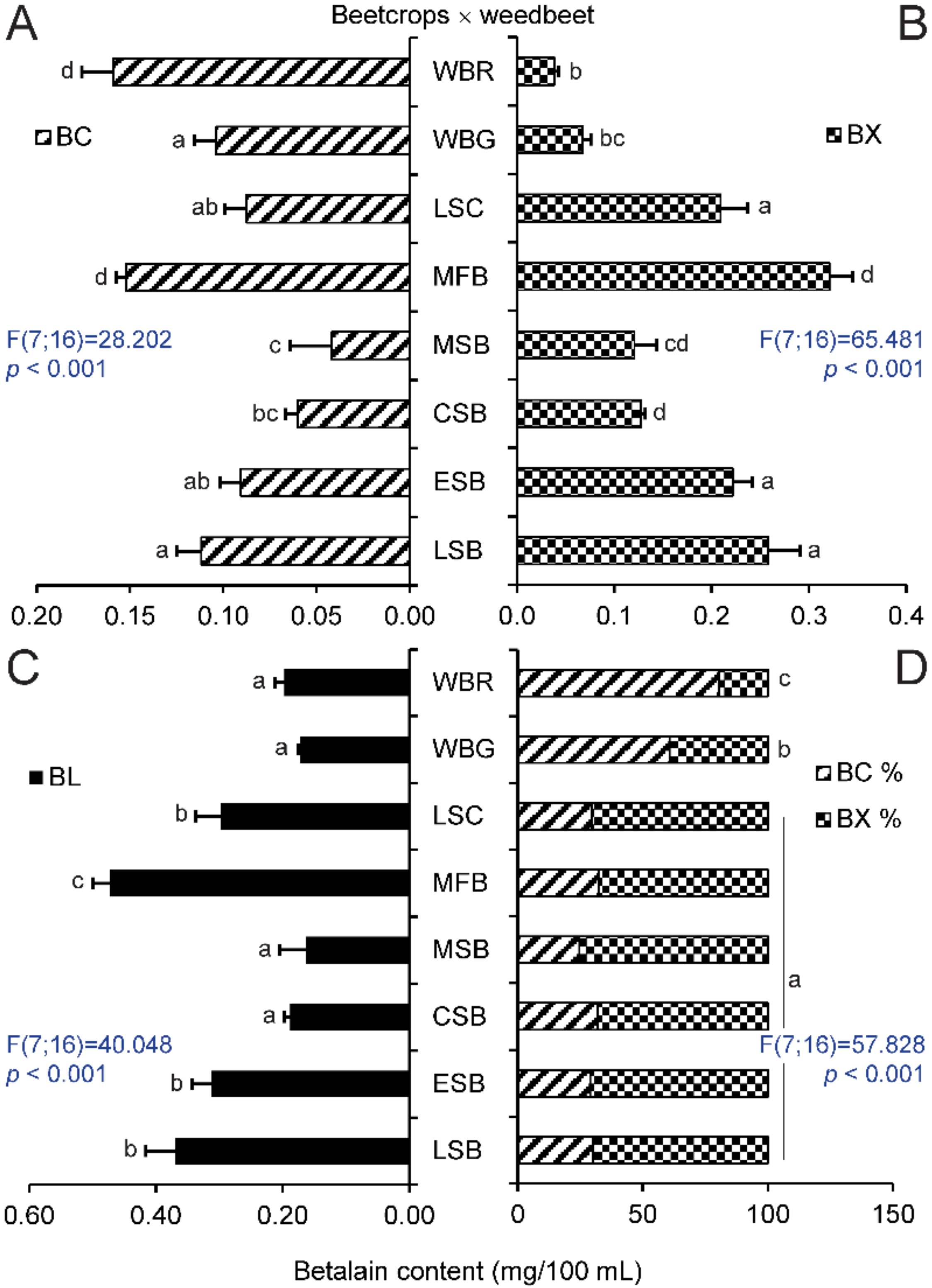
2.1. Betalain Content in Hypocotyls of Different Beet Genotypes
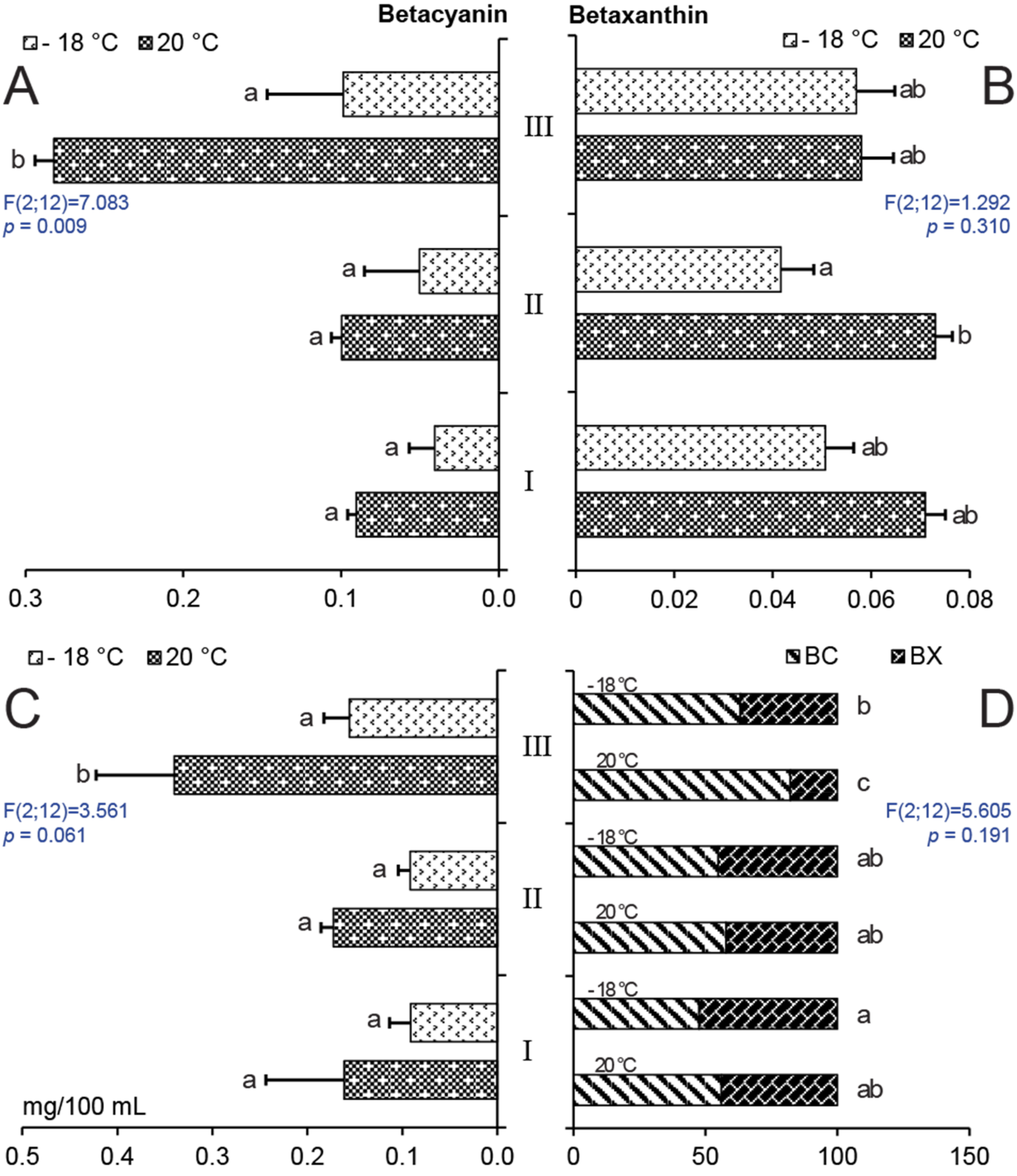
2.2. Effect of Storage on Betalain Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials (Beta Varieties and Weed Beets)
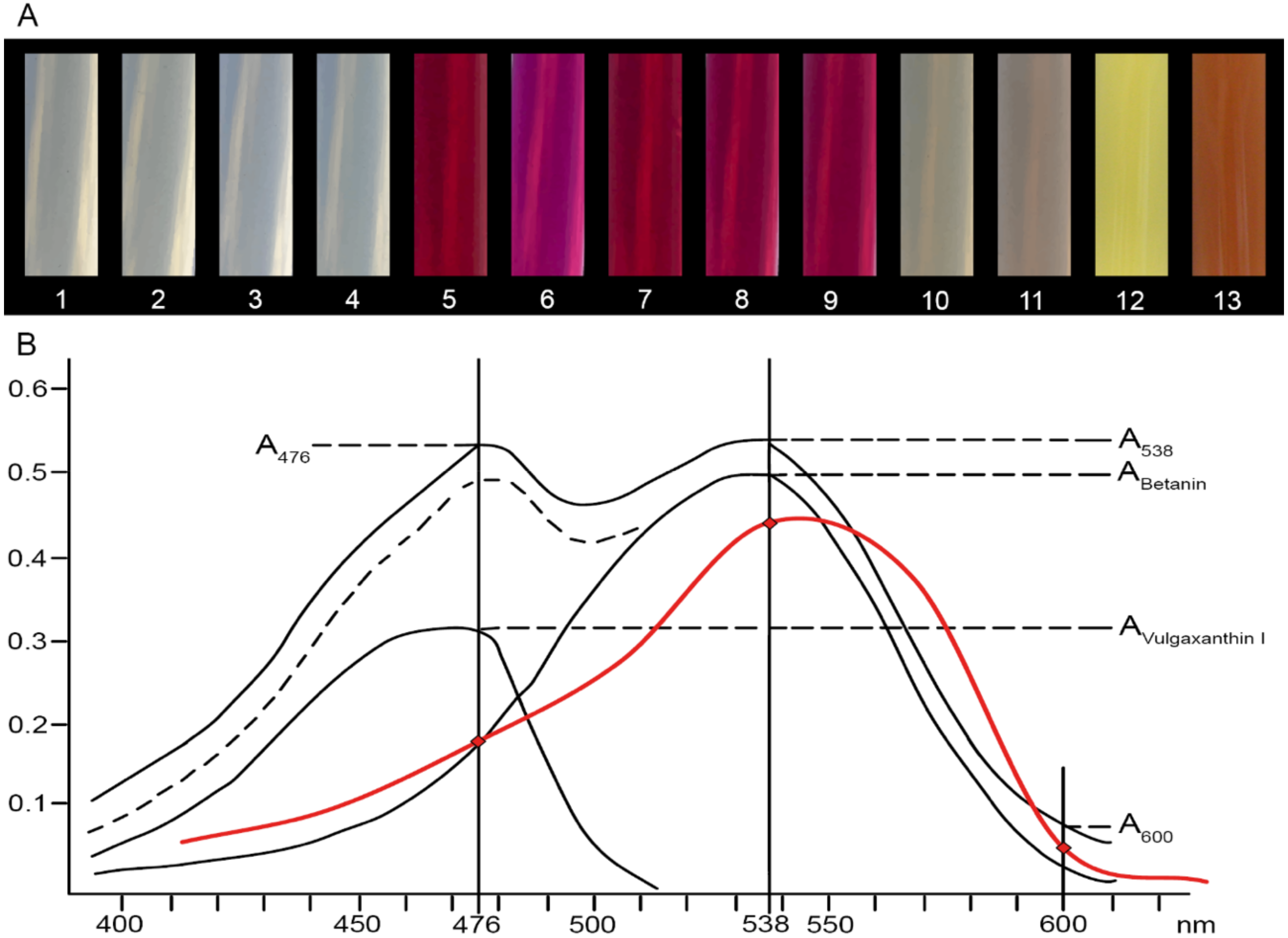
4.2. Extraction and Analysis of Betalains
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO FAOSTAT—Food and Agriculture Organization of the United. 2019. Available online: http://www.fao.org/faostat/en/#home (accessed on 10 April 2020).
- Pyo, Y.H.; Lee, T.C.; Logendra, L.; Rosen, R.T. Antioxidant activity and phenolic compounds of Swiss chard (Beta vulgaris subspecies cycla) extracts. Food Chem. 2004, 85, 19–26. [Google Scholar] [CrossRef]
- Brockington, S.F.; Walker, R.H.; Glover, B.J.; Soltis, P.S.; Soltis, D.E. Complex pigment evolution in the Caryophyllales. New Phytol. 2011, 190, 854–864. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Corke, H. Characterization and application of betalain pigments from plants of the Amaranthaceae. Trends Food Sci. Technol. 2005, 16, 370–376. [Google Scholar] [CrossRef]
- Sawicki, T.; Bączek, N.; Wiczkowski, W. Betalain profile, content and antioxidant capacity of red beetroot dependent on the genotype and root part. J. Funct. Foods 2016, 27, 249–261. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Miguel, M. Betalains in Some Species of the Amaranthaceae Family: A Review. Antioxidants 2018, 7, 53. [Google Scholar] [CrossRef] [Green Version]
- Polturak, G.; Aharoni, A. “La Vie en Rose”: Biosynthesis, Sources, and Applications of Betalain Pigments. Mol. Plant 2018, 11, 7–22. [Google Scholar] [CrossRef] [Green Version]
- Wybraniec, S.; Jerz, G.; Gebers, N.; Winterhalter, P. Ion-pair high-speed countercurrent chromatography in fractionation of a high-molecular weight variation of acyl-oligosaccharide linked betacyanins from purple bracts of Bougainvillea glabra. J. Chromatogr. B 2010, 878, 538–550. [Google Scholar] [CrossRef]
- Jackman, R.L.; Smith, J.L. Anthocyanins and betalains. In Natural Food Colorants; Hendry, G.A.F., Houghton, J.D., Eds.; Springer US: Boston, MA, USA, 1996; pp. 244–309. ISBN 978-1-4615-2155-6. [Google Scholar]
- Stintzing, F.C.; Schieber, A.; Carle, R. Identification of betalains from yellow beet (Beta vulgaris L.) and cactus pear [Opuntia ficus-indica (L.) Mill.] by high-performance liquid chromatography-electrospray ionization mass spectrometry. J. Agric. Food Chem. 2002, 50, 2302–2307. [Google Scholar] [CrossRef]
- Esquivel, P. Betalains. In Handbook on Natural Pigments in Food and Beverages: Industrial Applications for Improving Food Color; Elsevier: Amsterdam, The Netherlands, 2016; pp. 81–99. ISBN 9780081003923. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Abderrabba, M. Chemical and Antioxidant Properties of Betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef]
- Cai, Y.; Sun, M.; Corke, H. Antioxidant activity of betalains from plants of the Amaranthaceae. J. Agric. Food Chem. 2003, 51, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Czapski, J.; Mikołajczyk, K.; Kaczmarek, M. Relationship between antioxidant capacity of red beet juice and contents of its betalain pigments. Polish J. Food Nutr. Sci. 2009, 59, 119–122. [Google Scholar]
- Pátkai, G.; Barta, J. Decomposition of betacyanins and betaxanthins by heat and pH changes. Nahr. Food 1996, 40, 267–270. [Google Scholar] [CrossRef]
- Huang, A.S.; Elbe, J.H.V. Kinetics of the Degradation and Regeneration of Betanine. J. Food Sci. 1985, 50, 1115–1120. [Google Scholar] [CrossRef]
- Schwartz, S.J.; von Elbe, J.H. Identification of betanin degradation products. Z. Lebensm. Unters. Forsch. 1983, 176, 448–453. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Impact of thermal treatment on color and pigment pattern of red beet (Beta vulgaris L.) preparations. J. Food Sci. 2004, 69, C491–C498. [Google Scholar] [CrossRef]
- Bartsch, D.; Ellstrand, N.C. Genetic evidence for the origin of Californian wild beets (genus Beta). Theor. Appl. Genet. 1999, 99, 1120–1130. [Google Scholar] [CrossRef]
- LONGDEN, P.C. Effects of increasing weed-beet density on sugar-beet yield and quality. Ann. Appl. Biol. 1989, 114, 527–532. [Google Scholar] [CrossRef]
- Richardson, K.L.; Hellier, B.C.; Sinha, K. Characterization of wild Beta populations in and adjacent to sugar beet fields in the Imperial Valley, California. Genet. Resour. Crop Evol. 2016, 63, 305–314. [Google Scholar] [CrossRef]
- Sester, M.; Dürr, C.; Darmency, H.; Colbach, N. Evolution of weed beet (Beta vulgaris L.) seed bank: Quantification of seed survival, dormancy, germination and pre-emergence growth. Eur. J. Agron. 2006, 24, 19–25. [Google Scholar] [CrossRef]
- Biancardi, E.; Panella, L.W.; Lewellen, R.T.; Biancardi, E.; Panella, L.W.; Lewellen, R.T. Morphology, Physiology, and Ecology. In Beta maritima; Springer: New York, NY, USA, 2012; pp. 85–136. ISBN 978-1-4614-0842-0. [Google Scholar]
- Tricault, Y.; Darmency, H.; Colbach, N. Identifying key components of weed beet management using sensitivity analyses of the GeneSys-Beet model in GM sugar beet. Weed Res. 2009, 49, 581–591. [Google Scholar] [CrossRef]
- Skalický, M.; Tůma, J.; Novák, J.; Pulkrábek, J.; Steklová, J. Phenotype variability of weed beet (Beta vulgaris L.). Cereal Res. Commun. 2007, 35, 1077–1080. [Google Scholar] [CrossRef]
- European Commission European Plant Variety Database. Available online: http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchForm&ctl_type=A (accessed on 10 April 2020).
- Gasztonyi, M.N.; Daood, H.; Hájos, M.T.; Biacs, P. Comparison of red beet (Beta vulgaris var conditiva) varieties on the basis of their pigment components. J. Sci. Food Agric. 2001, 81, 932–933. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Gawęda, M. Selected Indicators of the Root Quality of Fifteen Cultivars of Red Beet (Beta vulgaris L.). J. Hortic. Res. 2015, 23, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Montes-Lora, S.; Rodríguez-Pulido, F.J.; Cejudo-Bastante, M.J.; Heredia, F.J. Implications of the Red Beet Ripening on the Colour and Betalain Composition Relationships. Plant Foods Hum. Nutr. 2018, 73, 216–221. [Google Scholar] [CrossRef]
- Wruss, J.; Waldenberger, G.; Huemer, S.; Uygun, P.; Lanzerstorfer, P.; Müller, U.; Höglinger, O.; Weghuber, J. Compositional characteristics of commercial beetroot products and beetroot juice prepared from seven beetroot varieties grown in Upper Austria. J. Food Compos. Anal. 2015, 42, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Slatnar, A.; Stampar, F.; Veberic, R.; Jakopic, J. HPLC-MSn Identification of Betalain Profile of Different Beetroot (Beta vulgaris L. ssp. vulgaris) Parts and Cultivars. J. Food Sci. 2015, 80, 1952–1958. [Google Scholar] [CrossRef]
- Kugler, F.; Graneis, S.; Stintzing, F.C.; Carle, R. Studies on betaxanthin profiles of vegetables and fruits from the Chenopodiaceae and Cactaceae. Z. Naturforsch. Sect. C J. Biosci. 2007, 62, 311–318. [Google Scholar] [CrossRef] [Green Version]
- Kugler, F.; Stintzing, F.C.; Carle, R. Evaluation of the antioxidant capacity of betalainic fruits and vegetables. J. Appl. Bot. Food Qual. 2007, 81, 69–76. [Google Scholar]
- Stintzing, F.C.; Carle, R. Betalains—Emerging prospects for food scientists. Trends Food Sci. Technol. 2007, 18, 514–525. [Google Scholar] [CrossRef]
- Lombard, K.A.; Geoffriau, E.; Peffley, E. Flavonoid Quantification in Onion by Spectrophotometric and High Performance Liquid Chromatography Analysis. HortScience 2002, 37, 682–685. [Google Scholar] [CrossRef] [Green Version]
- Vrhovsek, U.; Mattivi, F.; Waterhouse, A.L. Analysis of red wine phenolics: Comparison of HPLC and spectrophotometric methods. Vitis 2001, 40, 87–91. [Google Scholar]
- Güneşer, O. Pigment and color stability of beetroot betalains in cow milk during thermal treatment. Food Chem. 2016, 196, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Sun, M.; Schliemann, W.; Corke, H. Chemical stability and colorant properties of betaxanthin pigments from Celosia argentea. J. Agric. Food Chem. 2001, 49, 4429–4435. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Angosto, J.M.; Giménez, P.J.; León, G. Thermal Stability of Selected Natural Red Extracts Used as Food Colorants. Plant Foods Hum. Nutr. 2013, 68, 11–17. [Google Scholar] [CrossRef]
- Ravichandran, K.; Saw, N.M.M.T.; Mohdaly, A.A.A.; Gabr, A.M.M.; Kastell, A.; Riedel, H.; Cai, Z.; Knorr, D.; Smetanska, I. Impact of processing of red beet on betalain content and antioxidant activity. Food Res. Int. 2013, 50, 670–675. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
- Mikołajczyk-Bator, K.; Czapski, J. Effect of pH Changes on Antioxidant Capacity and the Content of Betalain Pigments during the Heating of a Solution of Red Beet Betalains. Polish J. Food Nutr. Sci. 2017, 67, 123–128. [Google Scholar] [CrossRef] [Green Version]
- Herbach, M.K.; Stintzing, F.C.; Carle, R. Thermal degradation of betacyanins in juices from purple pitaya [Hylocereus polyrhizus (Weber) Brittonv & Rose] monitored by high-performance liquid chromatography-tandem mass spectometric analyses. Eur. Food Res. Technol. 2004, 219, 377–385. [Google Scholar]
- Narkprasom, K.; Wang, S.-P.; Hsiao, S.-M.; Tsai, P.-J. Kinetics of Color Loss of Djulis (Chenopodium formosanum Koidz.) Extracts during Storage in Different Concentrations of Alcohol and Temperature. APCBEE Procedia 2012, 2, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Betalain stability and degradation—Structural and chromatic aspects. J. Food Sci. 2006, 71, R41–R50. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Identification of heat-induced degradation products from purified betanin, phyllocactin and hylocerenin by high-performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2005, 19, 2603–2616. [Google Scholar] [CrossRef] [PubMed]
- Caldas-Cueva, J.P.; Morales, P.; Ludeña, F.; Betalleluz-Pallardel, I.; Chirinos, R.; Noratto, G.; Campos, D. Stability of Betacyanin Pigments and Antioxidants in Ayrampo (Opuntia soehrensii Britton and Rose) Seed Extracts and as a Yogurt Natural Colorant. J. Food Process. Preserv. 2016, 40, 541–549. [Google Scholar] [CrossRef]
- Moßhammer, M.R.; Maier, C.; Stintzing, F.C.; Carle, R. Impact of thermal treatment and storage on color of yellow-orange cactus pear (Opuntia ficus-indica [L.] Mill. cv. ’Gialla’) juices. J. Food Sci. 2006, 71, C400–C406. [Google Scholar] [CrossRef]
- Cardoso-Ugarte, G.A.; Sosa-Morales, M.E.; Ballard, T.; Liceaga, A.; San Martín-González, M.F. Microwave-assisted extraction of betalains from red beet (Beta vulgaris). LWT Food Sci. Technol. 2014, 59, 276–282. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Corke, H. Effect of postharvest treatments on Amaranthus betacyanin degradation evaluated by visible/near-infrared spectroscopy. J. Food Sci. 2001, 66, 1112–1118. [Google Scholar] [CrossRef]
- Woo, K.K.; Ngou, F.H.; Ngo, L.S.; Soong, W.K.; Tang, P.Y. Stability of betalain pigment from red dragon fruit (Hylocereus polyrhizus). Am. J. Food Technol. 2011, 6, 140–148. [Google Scholar] [CrossRef] [Green Version]
- Gengatharan, A.; Dykes, G.A.; Choo, W.S. The effect of pH treatment and refrigerated storage on natural colourant preparations (betacyanins) from red pitahaya and their potential application in yoghurt. LWT Food Sci. Technol. 2017, 80, 437–445. [Google Scholar] [CrossRef]
- Sonar, C.R.; Rasco, B.; Tang, J.; Sablani, S.S. Natural color pigments: Oxidative stability and degradation kinetics during storage in thermally pasteurized vegetable purees. J. Sci. Food Agric. 2019, 99, 5934–5945. [Google Scholar] [CrossRef]
- Strack, D.; Vogt, T.; Schliemann, W. Recent advances in betalain research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Xu, H.; Hao, Q.; Liu, G.; Wang, Q. Inactivation of peroxidase and polyphenol oxidase in red beet (Beta vulgaris L.) extract with continuous high pressure carbon dioxide. Food Chem. 2010, 119, 108–113. [Google Scholar] [CrossRef]
- Paciulli, M.; Medina-Meza, I.G.; Chiavaro, E.; Barbosa-Cánovas, G.V. Impact of thermal and high pressure processing on quality parameters of beetroot (Beta vulgaris L.). LWT Food Sci. Technol. 2016, 68, 98–104. [Google Scholar] [CrossRef]
- Dos Santos, C.D.; Ismail, M.; Cassini, A.S.; Marczak, L.D.F.; Tessaro, I.C.; Farid, M. Effect of thermal and high pressure processing on stability of betalain extracted from red beet stalks. J. Food Sci. Technol. 2018, 55, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Von Elbe, J.H. Betalains. Curr. Protoc. Food Anal. Chem. 2001, 00, F3.1.1–F3.1.7. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Abbr. | Taxon Beta vulgaris L. | Variety Name | Crop | Variety Attributes |
|---|---|---|---|---|
| LSB | Altissima Group | Labonita | Sugar beet | A2011 |2|D| m |
| ESB | Altissima Group | Esperanza | Sugar beet | A2004 |2|D| m |
| CSB | Altissima Group | Caruso | Sugar beet | A2005 |2|D| m |
| MSB | Altissima Group | Merak | Sugar beet | A2003 |2|D| m |
| MBR | Vulgaris Group | Monorubra | Beetroot | A1987 |
| DBR | Vulgaris Group | Detroit Dark Red 2 | Beetroot | A1997 |
| LBR | Vulgaris Group | Libero | Beetroot | A1994 |
| PBR | Vulgaris Group | Pablo | Beetroot | A1994 | hybrid |
| KBR | Vulgaris Group | Kahira | Beetroot | A2003 |
| LSC | Cicla Group | Lucullus | Swiss chard | A1973 |
| MFB | Rapacea Group | Monro | Fodder beet | A1994 |4|-|m |
| WB * | subsp. maritima ** | - | Weed beet | hybridizes withcultivated beets |
| Month | Site | Temperature | Precipitation |
|---|---|---|---|
| April | Campus | 8.3 | 29.7 |
| Commercial | 8.6 | 28 | |
| May | Campus | 13.2 | 33.8 |
| Commercial | 13.7 | 44 | |
| June | Campus | 16.3 | 32.9 |
| Commercial | 16.5 | 58 | |
| July | Campus | 20.9 | 68.4 |
| Commercial | 18.5 | 75 | |
| August | Campus | 22.4 | 8.6 |
| Commercial | 18.0 | 68 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skalicky, M.; Kubes, J.; Shokoofeh, H.; Tahjib-Ul-Arif, M.; Vachova, P.; Hejnak, V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules 2020, 25, 5395. https://doi.org/10.3390/molecules25225395
Skalicky M, Kubes J, Shokoofeh H, Tahjib-Ul-Arif M, Vachova P, Hejnak V. Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules. 2020; 25(22):5395. https://doi.org/10.3390/molecules25225395
Chicago/Turabian StyleSkalicky, Milan, Jan Kubes, Hajihashemi Shokoofeh, Md. Tahjib-Ul-Arif, Pavla Vachova, and Vaclav Hejnak. 2020. "Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets" Molecules 25, no. 22: 5395. https://doi.org/10.3390/molecules25225395
APA StyleSkalicky, M., Kubes, J., Shokoofeh, H., Tahjib-Ul-Arif, M., Vachova, P., & Hejnak, V. (2020). Betacyanins and Betaxanthins in Cultivated Varieties of Beta vulgaris L. Compared to Weed Beets. Molecules, 25(22), 5395. https://doi.org/10.3390/molecules25225395