Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues
Abstract
:1. Introduction
2. Results and Discussion
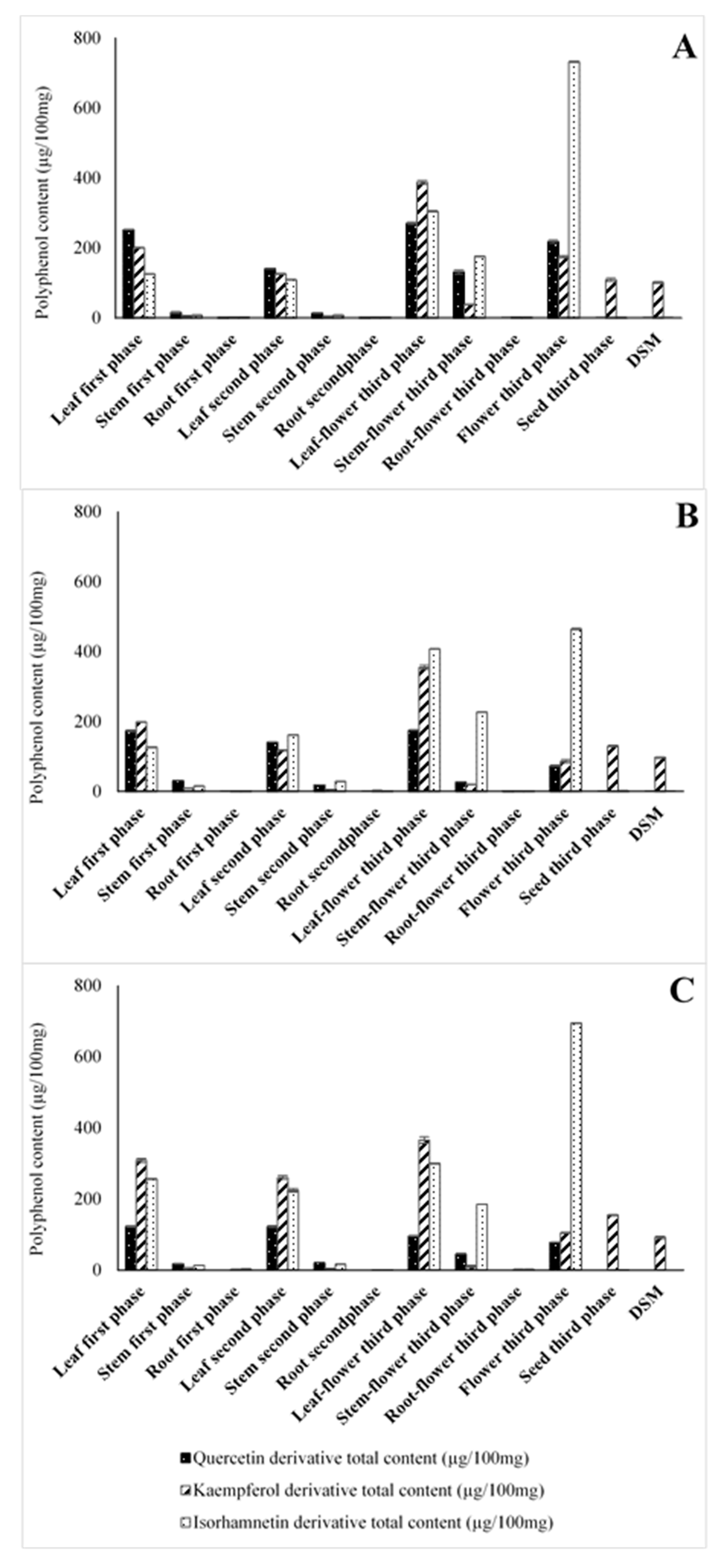
2.1. Identification and Semi-Quantification of the Metabolite Content in B. juncea Cultivars by HPLC-PDA/ESI-MS/MS
2.2. Determination of the Volatile Content of B. juncea Accessions Using GC-FID/MS
2.3. Chemical Characterization of B. juncea DSMs
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Plant Material
3.3. Seed Cake Preparation and Main Characterization
3.4. Sample Preparation
3.4.1. HPLC-PDA-MS
3.4.2. GC-FID/MS
3.5. Instrumentation
3.5.1. HPLC-PDA-MS
3.5.2. GC-FID/MS
3.6. Analytical Conditions
3.6.1. LC-PDA-MS
Construction of Calibration Curves
3.6.2. GC-FID/MS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Podsędek, A. Natural antioxidants and antioxidant capacity of Brassica vegetables: A review. LWT-J. Food Sci. Technol. 2007, 40, 1–11. [Google Scholar] [CrossRef]
- Harbatum, B.; Hubbermann, E.M.; Wolff, C.; Herges, R.; Zhu, Z.; Schwarz, K. Identification of Flavonoids and Hydroxycinnamic Acids in Pak Choi Varieties (Brassica campestris L. ssp. chinensis var. communis) by HPLC–ESI-MSn and NMR and Their Quantification by HPLC–DAD. J. Agric. Food Chem. 2007, 55, 8251–8260. [Google Scholar] [CrossRef] [PubMed]
- Harbatum, B.; Hubbermann, E.M.; Zhu, Z.; Schwarz, K. Impact of Fermentation on Phenolic Compounds in Leaves of Pak Choi (Brassica campestris L. ssp. chinensis var. communis) and Chinese Leaf Mustard (Brassica juncea Coss). J. Agric. Food Chem. 2008, 56, 148–157. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and Quantification of Flavonoids and Hydroxycinnamic Acids in Curly Kale (Brassica oleracea L. Convar. Acephala Var. sabellica) by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Olsen, H.; Aaby, K.; Borge, G.I. Characterization, Quantification, and Yearly Variation of the Naturally Occurring Polyphenols in a Common Red Variety of Curly Kale (Brassica oleracea L. convar. Acephala var. sabellica cv. ‘Redbor’). J. Agric. Food Chem. 2010, 58, 11346–11354. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-Z.; Sun, J.; Chen, P.; Harnly, J. UHPLC-PDA-ESI/HRMS/MSn Analysis of Anthocyanins, Flavonol Glycosides, and Hydroxycinnamic Acid Derivatives in Red Mustard Greens (Brassica juncea Coss Variety). J. Agric. Food Chem. 2011, 59, 12059–12072. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Xiao, Z.; Lin, L.; Lester, G.E.; Wang, Q.; Harnly, J.M.; Chen, P. Profiling Polyphenols in Five Brassica Species Microgreens by UHPLC-PDA-ESI/HRMSn. J. Agric. Food Chem. 2013, 61, 10960–10970. [Google Scholar] [CrossRef] [Green Version]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic Compounds in Brassica Vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef]
- Mageney, V.; Baldermann, S.; Albach, D.C. Intraspecific Variation in Carotenoids of Brassica Oleracea Var. Sabellica, J. Agric. Food Chem. 2016, 64, 3251–3257. [Google Scholar] [CrossRef]
- De Nicola, G.R.; Bagatta, M.; Pagnotta, E.; Donato, A.; Gennari, L.; Ninfali, P.; Rollin, P.; Iori, R. Comparison of bioactive phytochemical content and release of isothiocyanates in selected brassica sprouts. Food Chem. 2013, 141, 297–303. [Google Scholar] [CrossRef]
- Park, S.; Arasu, M.V.; Lee, M.K.; Chun, J.H.; Seo, J.M.; Lee, S.W.; Al-Dhabi, N.A.; Kim, S.J. Quantification of glucosinolates, anthocyanins, free amino acids, and vitamin C in inbred lines of cabbage (Brassica oleracea L.). Food Chem. 2014, 145, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Piccinini, E.; Ferrari, V.; Campanelli, G.; Fusari, F.; Righetti, L.; Pagnotta, E.; Lazzeri, L. Effect of two liquid formulations based on Brassica carinata co-products in containing powdery mildew on melon. Ind. Crops Prod. 2015, 75, 48–53. [Google Scholar] [CrossRef]
- Franco, P.; Spinozzi, S.; Pagnotta, E.; Lazzeri, L.; Ugolini, L.; Camborata, C.; Roda, A. Development of a liquid chromatography-electrospray ionization-tandem mass spectrometry method for the simultaneous analysis of intact glucosinolates and isothiocyanates in Brassicaceae seeds and functional foods. J. Chromatogr. A 2016, 148, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.; Delage, B.; Williams, D.; Dashwood, R. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, Y.; Lee, J.; Ju, J. Anti-cancer activities of Brassica juncea leaves in vitro. EXCLI J. 2016, 15, 699–710. [Google Scholar] [PubMed]
- Kim, H.-Y.; Yokozawa, T.; Cho, E.J.; Cheigh, H.S.; Choi, J.S.; Chung, H.Y. In vitro and in vivo antioxidant effects of mustard leaf (Brassica juncea). Phytother. Res. 2003, 17, 465–471. [Google Scholar]
- Yokozawa, T.; Kim, H.Y.K.; Cho, E.J.; Yamabi, N.; Choi, J.S. Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J. Nutr. Sci. Vitaminol. 2003, 49, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-J.; Kim, H.A.; Lee, J. The effects of Brassica juncea L. leaf extract on obesity and lipid profiles of rats fed a high-fat/high-cholesterol diet. Nutr. Res. Pract. 2018, 12, 298–306. [Google Scholar] [CrossRef]
- Thakur, A.K.; Chatterjee, S.S.; Kumar, V. Beneficial effects of Brassica juncea on cognitive functions in rats. Pharm. Biol. 2013, 51, 1304–1310. [Google Scholar] [CrossRef]
- Harborne, J.B. General Procedures and Measurement of Total Phenolics. Meth. Plant Biochem. 1989, 1, 1–28. [Google Scholar]
- Nicácio, A.E.; Rodrigues, C.A.; Visentainer, J.V.; Maldaner, L. Evaluation of the QuEChERS method for the determination of phenolic compounds in yellow (Brassica alba), brown (Brassica juncea), and black (Brassica nigra) mustard seeds. Food Chem. 2021, 340, 128162. [Google Scholar] [CrossRef] [PubMed]
- Sikora, E.; Cieslik, E.; Leszczynska, T.; Filipiak-Florkiewicz, A.; Pisulewski, P.M. The antioxidant activity of selected cruciferous vegetables subjected to aquathermal processing. Food Chem. 2008, 107, 55–59. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photodiode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, B.; Eaves, D.H.; Shikany, J.M.; Pace, R.D. Phenolic compound profile of selected vegetables frequently consumed by African Americans in the southeast United States. Food Chem. 2007, 103, 1395–1402. [Google Scholar] [CrossRef]
- Zhang, J.; Satterfield, M.B.; Brodbelt, J.S.; Britz, S.J.; Clevidence, B.; Novotny, J.A. Structural characterization and detection of kale flavonoids by electrospray ionization mass spectrometry. Anal. Chem. 2003, 75, 6401–6407. [Google Scholar] [CrossRef]
- Maarse, H. Volatile Compounds in Foods and Beverages; Illustrated Edition CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Kumar, S.; Singh, D.; Dutta, M. Quality characteristics in rapeseed-mustard and role of some anti-nutritional factors in plant defense: Future strategies. J. Oilseed Brassica 2014, 5, 87–95. [Google Scholar]
- Rangkadilok, N.; Nicolas, M.E.; Bennett, R.N.; Premier, R.R.; Eagling, D.R.; Taylor, P.W.J. Developmental changes of sinigrin and glucoraphanin in three Brassica species (Brassica nigra, Brassica juncea and Brassica oleracea var. italica). Sci. Hortic. 2002, 96, 11–26. [Google Scholar] [CrossRef]
- Sharma, A.; Rai, P.K.; Prasad, S. GC–MS detection and determination of major volatile compounds in Brassica juncea L. leaves and seeds. Microchem. J. 2018, 138, 488–493. [Google Scholar] [CrossRef]
- Official Journal of the European Union C 13, Volume 62, 11 January 2019 (European Commission 2019/C 13/01), Common Catalogue of Varieties of Agricultural Plant Species-37th Complete Edition. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:C:2019:013:FULL&from=EN (accessed on 21 October 2020).
- Available online: http://ec.europa.eu/food/plant/plant_propagation_material/plant_variety_catalogues_databases/search/public/index.cfm?event=SearchVariety&ctl_type=A&species_id=237&variety_name=&listed_in=8&show_current=on&show_deleted (accessed on 21 October 2020).
- Arena, K.; Cacciola, F.; Dugo, L.; Dugo, P.; Mondello, L. Determination of the Metabolite Content of Brassica juncea Cultivars Using Comprehensive Two-Dimensional Liquid Chromatography Coupled with a Photodiode Array and Mass Spectrometry Detection. Molecules 2020, 25, 1235. [Google Scholar] [CrossRef] [Green Version]
- Grover, N.; Patni, V. Phytochemical characterization using various solvent extracts and GC-MS analysis of methanolic extract of Woodfordia fruticosa (L) Kurz. Leaves. Int. J. Pharm. Pharm. Sci. 2013, 5, 291–295. [Google Scholar]
- Sciarrone, D.; Giuffrida, D.; Rotondo, A.; Micalizzi, G.; Zoccali, M.; Pantò, S.; Donato, P.; Rodrigues-das-Dores, R.G.; Mondello, L. Quali-quantitative characterization of the volatile constituents in Cordia verbenacea D.C. essential oil exploiting advanced chromatographic approaches and nuclear magnetic resonance analysis. J. Chromatogr. A 2017, 1524, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Vörös-Horváth, B.; Bencsik, T.; Micalizzi, G.; Mondello, L.; Horváth, G.; Koszegi, T.; Széchenyi, A. Antimicrobial activity of different Artemisia essential oil formulations. Molecules 2020, 25, 2390. [Google Scholar] [CrossRef] [PubMed]
- Valette, L.; Fernandez, X.; Poulain, S.; Loiseau, A.-M.; Lizzani-Cuvelier, L.; Levieil, R.; Restier, L. Volatile constituents from Romanesco cauliflower. Food Chem. 2003, 80, 353–358. [Google Scholar] [CrossRef]
- Kessler, A.; Baldwin, I.T. Plant responses to insect herbivory; the emerging molecular analysis. Annu. Rev. Plant Biol. 2002, 53, 299–328. [Google Scholar] [CrossRef] [PubMed]
- Kesen, S.; Kelebek, H.; Sen, K.; Ulas, M.; Selli, S. GC-MS-olfactometric characterization of the key aroma compounds in Turkish olive oils by application of the aroma extract dilution analysis. Food Res. Int. 2013, 54, 1987–1994. [Google Scholar] [CrossRef]
- Fenwick, G.R.; Heaney, R.K.; Mullin, W.J. Glucosinolates and their breakdown products in food and food plants. Crit. Rev. Food Sci. Nutr. 1983, 18, 123–201. [Google Scholar]
- Gil, V.; MacLeod, A. Benzylglucosinolate degradation in Lepidium sativum: Effect of plant age and time of autolysis. Phytochemistry 1980, 19, 1365–1368. [Google Scholar] [CrossRef]
- Mustakas, G.C. Full-fat and defatted soy flours for human nutrition. J. Am. Oil Chem. Soc. 1971, 48, 815–819. [Google Scholar] [CrossRef]
- Lazzeri, L.; Malaguti, L.; Bagatta, M.; D’Avino, L.; Ugolini, L.; De Nicola, G.R.; Casadei, N.; Cinti, S.; Matteo, R.; Iori, R. Characterization of the main glucosinolate content and fatty acid composition in non-food brassicaceae seeds. Acta Hortic. 2013, 1005, 331–338. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kang, M.S.; Akash, M.W.; Singh, P. Selection indices for improving selection efficiency in Indian mustard. J. Crop Improv. 2019, 33, 25–41. [Google Scholar] [CrossRef]
- ASTM D5373. Standard Test Methods for Determination of Carbon, Hydrogen and Nitrogen in Analysis Samples of Coal and Carbon in Analysis Samples of Coal and Coke; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Smith, A.K.; Circle, S.J. Soybeans: Chemistry and Technology; Avi Publishing Co.: Westport, CT, USA, 1972; Volume 1, pp. 62–63. [Google Scholar]
- ISO 9167-1:1992/Amd 1:2013. Graines de Colza—Dosage des Glucosinolates—Partie 1:Methode par Chromatographie Liquide à Haute Performance, 1992. Available online: https://www.iso.org/fr/standard/72207.html (accessed on 21 October 2020).
- Wathelet, J.P.; Iori, R.; Leoni, O.; Rollin, P.; Quinsac, A.; Palmieri, S. Guidelines for glucosinolates analysis in green tissues used for biofumigation. Agroindustria 2004, 3, 257–266. [Google Scholar]
- Pagnotta, E.; Agerbirk, N.; Olsen, C.E.; Ugolini, L.; Cinti, S.; Lazzeri, L. Hydroxyl and methoxyl derivatives of benzylglucosinolate in Lepidium densiflorum with hydrolysis to isothiocyanates and non-isothiocyanate products: Substitution governs product type and mass spectral fragmentation. J. Agric. Food Chem. 2017, 65, 3167–3178. [Google Scholar] [CrossRef] [PubMed]




| No | Tentative ID | λ max (nm) | tR (min) | [M−H]- | MS2 ions | (µg/100 mg DW) | ||
|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | Sample C | ||||||
| 1 | Malic Acid | 215; 260 | 1.34 | 133.0 | - | * | * | * |
| 2 | Citric acid | 215; 260 | 1.53 | 191.0 | - | * | * | * |
| 3 | Km 3-diglucoside-7-glucoside | 265; 345 | 4.20 | 771.2 | 609 | * | * | * |
| 4 | Feruloylglucose | 236; 285 | 13.40 | 355.2 | 193 | Nd | Nd | Nd |
| 5 | Qn 3-sophoroside-7-glucoside | 257; 352 | 13.90 | 787.2 | 625 | 9.73 ± 0.31 | 2.85 ± 0.64 | 3.63 ± 0.07 |
| 6 | Rhamnosyl-ellagic acid | 283; 313 | 14.28 | 447.0 | - | Nd | Nd | Nd |
| 7 | Rhamnosyl-ellagic acid | 283; 313 | 14.55 | 447.0 | - | * | * | * |
| 8 | Qn 3-hydroxyferuloylsophoroside-7-glucoside | 247; 335 | 15.07 | 979.2 | 625 | 65.60 ± 0.50 | 11.17 ± 2.12 | 6.60 ± 0.92 |
| 9 | Km 3-sophoroside-7-glucoside | 266; 343 | 15.33 | 771.2 | 609 | 5.25 ± 0.06 | 6.60 ± 0.86 | 19.05 ± 0.55 |
| 10 | Qn 3-caffeoylsophoroside-7-glucoside | 242; 330 | 15.77 | 949.2 | 625 | 22.04 ± 0.75 | 9.69 ± 2.32 | 5.53 ± 0.17 |
| 11 | Km 3-hydroxyferuloylsophoroside-7-glucoside | 232; 330 | 16.65 | 963.2 | 609 | 35.52 ± 0.07 | 8.16 ± 2.22 | 6.58 ± 0.00 |
| 12 | Feruloylglucose | 236; 326 | 16.88 | 355.2 | 193 | * | * | * |
| 13 | Km 3-caffeoyldiglucoside-7-glucoside | 233; 330 | 17.57 | 933.2 | - | 5.20 ± 0.10 | 4.91 ± 1.21 | 8.57 ± 0.04 |
| 14 | Qn 3-diglucoside | 256; 360 | 17.76 | 625.1 | 463; 301 | 71.75 ± 3.11 | 31.12 ± 8.80 | 40.05 ± 0.60 |
| 15 | Qn 3-sinapoyltriglucoside-7-glucoside | 238; 330 | 17.90 | 1155.3 | 993 | Nd | Nd | 21.86 ± 0.34 |
| 16 | Qn 3-sinapoyltriglucoside-7-glucoside | 245; 340 | 18.03 | 1155.3 | 993 | 49.06 ± 0.89 | 18.83 ± 3.89 | Nd |
| 17 | Km 3-hydroxyferuloylsophoroside-7-glucoside | 254; 338 | 18.51 | 963.2 | 625 | 35.62 ± 0.08 | 9.87 ± 2.66 | 5.93 ± 0.06 |
| 18 | Km 3-hydroxyferuloylsophorotrioside-7-glucoside | 268; 330 | 18.53 | 1125.3 | 963 | Nd | Nd | Nd |
| 19 | Km 3-sinapoylsophorotrioside-7-glucoside | 268; 330 | 18.78 | 1139.3 | 771 | Nd | 14.12 ± 3.33 | 23.17 ± 0.55 |
| 20 | Km 3-sinapoylsophorotrioside-7-glucoside | 268; 330 | 19.29 | 1139.3 | 771 | 1.80 ± 0.06 | 7.85 ± 2.02 | 10.72 ± 0.11 |
| 21 | Km 3-sinapoylsophoroside-7-glucoside | 268; 333 | 19.50 | 977.2 | 609; 815 | 18.69 ± 0.10 | 5.36 ± 1.30 | 5.29 ± 0.00 |
| 22 | Km 3-feruloylsophoroside-7-glucoside | 266; 341 | 20.26 | 947.2 | 609 | 72.18 ± 0.08 | 29.23 ± 8.00 | 25.17 ± 0.50 |
| 23 | Is 3,7-diglucoside | 252; 352 | 21.20 | 639.1 | 477; 315 | 683.62 ± 1.14 | 433.65 ± 2.94 | 644.43 ± 0.63 |
| 24 | Feruloyl malate | 242; 323 | 24.23 | 309.1 | - | * | * | * |
| 25 | Sinapic acid | 270; 326 | 24.27 | 223.1 | 179 | Nd | Nd | Nd |
| 26 | Sinapoyl malic acid | 240; 326 | 25.05 | 339.1 | 223 | * | * | * |
| 27 | Sinapoyl-feruloyl-triglucoside | 280; 325 | 25.21 | 885.3 | 499 | Nd | Nd | Nd |
| 28 | Sinapoyl-hydroxyferuloyl-diglucoside | 244; 330 | 29.46 | 739.2 | 515 | * | * | * |
| 29 | Isorhamnetin glucoside | 256; 351 | 31.94 | 477.1 | - | 48.63 ± 0.10 | 30.37 ± 8.49 | 49.87 ± 0.07 |
| 30 | Km glucoside | 269; 330 | 31.49 | 447.0 | - | Nd | Nd | Nd |
| 31 | Disapoyl-gentiobiose | 240; 330 | 33.32 | 753.2 | 529; 499 | * | * | * |
| 32 | Sinapoyl-feruloyl-gentiobiose | 240; 330 | 34.20 | 723.2 | 529; 499 | * | * | * |
| 33 | Diferuloyldiglucoside | 240; 326 | 34.82 | 693.1 | 499 | * | * | * |
| 34 | Trisinapoylgentiobiose | 240; 326 | 36.53 | 959.3 | 735; 529 | * | * | * |
| 35 | Feruoyl-disapoyl-gentiobiose | 240; 326 | 37.28 | 929.3 | 705; 511 | * | * | * |
| Reference Material | Standard Curve | R2 | LoD (μg/mL) | LoQ (μg/mL) | Precision (RSD, %) |
|---|---|---|---|---|---|
| Qn 3-O-glucoside | y = 13,424x + 898.59 | 0.9939 | 0.013 | 0.043 | 0.41 |
| Is 3-O-glucoside | y = 14,948x − 2966.9 | 0.9963 | 0.048 | 0.159 | 0.34 |
| Km 3-O-glucoside | y = 17,660x – 10,681 | 0.9963 | 0.021 | 0.072 | 0.36 |
| Sample A | Sample B | Sample C | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N. | Compounds Name | Lib. Name | Id. Method | Similarity | LRI Lib | LRI Exp | Area % | Area % | Area % |
| 1 | Ethanoic acid | FFNSC 4.0 | MS, LRI | 98 | 661 | 665 | 4.83 | 4.18 | 4.66 |
| 2 | 2-Butenenitrile | W11N17 | MS, LRI | 90 | 664 | 675 | 0.27 | 0.44 | |
| 3 | Hydroxyacetone | FFNSC 4.0 | MS, LRI | 94 | 682 | 684 | 0.07 | Nd | 0.15 |
| 4 | 3-hydroxy-Pentene | FFNSC 4.0 | MS, LRI | 92 | 691 | 691 | 0.06 | Nd | 0.20 |
| 5 | 3-Pentenone | FFNSC 4.0 | MS, LRI | 93 | 677 | 693 | Nd | 0.45 | Nd |
| 6 | n-Pentanal | FFNSC 4.0 | MS, LRI | 94 | 696 | 701 | 0.52 | 0.41 | 0.40 |
| 7 | methyl-Thiocyanate | FFNSC 4.0 | MS, LRI | 91 | 710 | 711 | Nd | 0.54 | Nd |
| 8 | Propionic acid | FFNSC 4.0 | MS, LRI | 91 | 698 | 711 | 0.53 | 0.21 | 0.38 |
| 9 | Acetoin | FFNSC 4.0 | MS, LRI | 90 | 716 | 716 | Nd | Nd | 0.05 |
| 10 | Methyl propenyl ketone | FFNSC 4.0 | MS, LRI | 91 | 733 | 737 | 0.01 | 0.13 | 0.01 |
| 11 | dimethyl-Disulfide | FFNSC 4.0 | MS, LRI | 93 | 722 | 741 | 0.08 | Nd | 0.01 |
| 12 | (E)-2-Pentenal | FFNSC 4.0 | MS, LRI | 92 | 751 | 753 | 0.17 | 0.31 | 0.17 |
| 13 | Isobutyric acid | FFNSC 4.0 | MS, LRI | 91 | 774 | 758 | 0.07 | 0.06 | Nd |
| 14 | Senecionitrile | FFNSC 4.0 | MS, LRI | 93 | 756 | 760 | Nd | Nd | 0.05 |
| 15 | Pentyl alcohol | FFNSC 4.0 | MS, LRI | 96 | 763 | 767 | 0.13 | 0.07 | 0.05 |
| 16 | 2,3-Butadienol | FFNSC 4.0 | MS, LRI | 96 | 788 | 788 | 0.58 | 0.20 | 1.80 |
| 17 | n-Hexanal | FFNSC 4.0 | MS, LRI | 98 | 801 | 802 | 0.36 | 0.37 | 0.34 |
| 18 | 3-Butenoic acid | W11N17 | MS | 91 | - | 806 | Nd | 0.30 | Nd |
| 19 | Butyric acid | FFNSC 4.0 | MS, LRI | 93 | 818 | 808 | 0.10 | Nd | Nd |
| 20 | 2-methyl-Pyrazine | FFNSC 4.0 | MS, LRI | 95 | 820 | 828 | 0.07 | 0.07 | Nd |
| 21 | Furfural | FFNSC 4.0 | MS, LRI | 92 | 845 | 831 | 0.10 | 0.29 | 0.15 |
| 22 | Sclerosol | FFNSC 4.0 | MS, LRI | 96 | 827 | 841 | 0.50 | 0.04 | 0.15 |
| 23 | (E)-2-Hexenal | FFNSC 4.0 | MS, LRI | 95 | 850 | 852 | 0.50 | 1.76 | 0.57 |
| 24 | (Z)-3-Hexenol | FFNSC 4.0 | MS, LRI | 95 | 853 | 854 | 0.61 | 0.51 | 0.33 |
| 25 | Isovaleric acid | FFNSC 4.0 | MS, LRI | 94 | 842 | 860 | 1.02 | 0.07 | 0.17 |
| 26 | 2-methyl-Butyric acid | FFNSC 4.0 | MS, LRI | 89 | 881 | 867 | 0.73 | 0.21 | 0.29 |
| 27 | allyl-Thiocyanate | FFNSC 4.0 | MS, LRI | 94 | 865 | 869 | 0.17 | 0.24 | 0.10 |
| 28 | n-Hexanol | FFNSC 4.0 | MS, LRI | 91 | 867 | 869 | 0.26 | 0.20 | 0.03 |
| 29 | 2-propenyl-Isothiocyanate | FFNSC 4.0 | MS, LRI | 96 | 876 | 880 | 0.74 | 2.07 | 0.61 |
| 30 | 1-(3-methylenecyclopentyl)-Ethanone | W11N17 | MS | 91 | - | 884 | 0.47 | 0.23 | 0.34 |
| 31 | n-Heptanal | FFNSC 4.0 | MS, LRI | 97 | 906 | 902 | Nd | 0.17 | 0.16 |
| 32 | n-Pentanoic acid | FFNSC 4.0 | MS, LRI | 92 | 911 | 903 | 0.90 | 0.20 | 1.27 |
| 33 | 3-methyl-Crotonic acid | FFNSC 4.0 | MS, LRI | 91 | 907 | 905 | 0.12 | Nd | 0.02 |
| 34 | 2-butoxy-Ethanol | W11N17 | MS, LRI | 95 | 906 | 907 | 0.08 | 0.06 | Nd |
| 35 | 2-acetyl-Furan | FFNSC 4.0 | MS, LRI | 94 | 913 | 910 | 0.10 | 0.14 | Nd |
| 36 | 2(5H)-Furanone | FFNSC 4.0 | MS, LRI | 91 | 907 | 911 | Nd | Nd | 0.03 |
| 37 | γ-Butyrolactone | FFNSC 4.0 | MS, LRI | 95 | 910 | 912 | 0.89 | 0.45 | 0.30 |
| 38 | 2,5-dimethyl-Pyrazine | FFNSC 4.0 | MS, LRI | 92 | 912 | 916 | 0.33 | 0.41 | 0.09 |
| 39 | 1,1′-sulfonylbis-Methane | W11N17 | MS, LRI | 95 | 922 | 916 | 0.15 | 0.10 | Nd |
| 40 | methyl-Hexanoate | FFNSC 4.0 | MS, LRI | 92 | 922 | 924 | 0.07 | 0.03 | 0.02 |
| 41 | sec-butyl-Isothiocyanate | FFNSC 4.0 | MS, LRI | 96 | 929 | 929 | 0.10 | 0.03 | 0.05 |
| 42 | 2,7-dimethyl-Oxepine | W11N17 | MS, LRI | 88 | 944 | 931 | 0.03 | 0.02 | 0.01 |
| 43 | 1-butoxy-2-Propanol | W11N17 | MS, LRI | 91 | 945 | 938 | 0.02 | 0.01 | Nd |
| 44 | dihydro-3-methyl-2(3H)-Furanone | W11N17 | MS, LRI | 94 | 941 | 948 | 0.05 | 0.02 | Nd |
| 45 | γ-Pentalactone | FFNSC 4.0 | MS, LRI | 91 | 954 | 953 | 0.16 | 0.02 | 0.06 |
| 46 | (E)-2-Heptenal | FFNSC 4.0 | MS, LRI | 95 | 956 | 956 | 0.05 | 0.11 | 0.41 |
| 47 | Benzaldehyde | FFNSC 4.0 | MS, LRI | 98 | 960 | 963 | 0.54 | 0.66 | 0.23 |
| 48 | N-2-propenyl-Acetamide | W11N17 | MS | 94 | - | 964 | 0.11 | Nd | 0.07 |
| 49 | Dimethyl trisulfide | FFNSC 4.0 | MS, LRI | 97 | 969 | 970 | 0.12 | 0.12 | 0.04 |
| 50 | n-Heptanol | FFNSC 4.0 | MS, LRI | 92 | 970 | 970 | 0.04 | 0.02 | 0.02 |
| 51 | 3,5,5-trimethyl-2-Hexene | W11N17 | MS, LRI | 92 | 985 | 974 | 0.33 | 0.14 | 0.38 |
| 52 | 1-Octen-3-one | FFNSC 4.0 | MS, LRI | 91 | 973 | 977 | 0.03 | 0.03 | 0.12 |
| 53 | Vinyl amyl carbinol | FFNSC 4.0 | MS, LRI | 94 | 978 | 979 | Nd | 0.10 | Nd |
| 54 | 3-butenyl-Isothiocyanate | FFNSC 4.0 | MS, LRI | 91 | 978 | 980 | Nd | Nd | 0.70 |
| 55 | 6-methyl-Hept-5-en-2-one | FFNSC 4.0 | MS, LRI | 95 | 986 | 985 | 0.32 | 0.29 | 0.31 |
| 56 | 2-pentyl-Furan | FFNSC 4.0 | MS, LRI | 92 | 991 | 989 | 0.34 | 0.15 | Nd |
| 57 | 2,3,5-trimethyl-Pyrazine | FFNSC 4.0 | MS, LRI | 90 | 1002 | 1001 | 1.08 | 0.30 | Nd |
| 58 | 2-ethyl-,5-methyl-Pyrazine | FFNSC 4.0 | MS, LRI | 88 | 1005 | 1001 | Nd | 0.19 | Nd |
| 59 | 2-ethyl-, 6-methyl-Pyrazine | FFNSC 4.0 | MS, LRI | 91 | 1000 | 1002 | 0.47 | Nd | Nd |
| 60 | n-Octanal | FFNSC 4.0 | MS, LRI | 91 | 1006 | 1004 | 0.45 | 0.14 | 1.06 |
| 61 | (E,E)-2,4-Heptadienal | FFNSC 4.0 | MS, LRI | 95 | 1013 | 1011 | 0.04 | 0.59 | 0.82 |
| 62 | n-Hexanoic acid | FFNSC 4.0 | MS, LRI | 97 | 997 | 1013 | 2.45 | 0.64 | 3.09 |
| 63 | 2-ethenyl-6-methyl-pyrazine | W11N17 | MS, LRI | 82 | 1031 | 1020 | 0.36 | 0.54 | Nd |
| 64 | (Z)-3-Hexenoic acid | FFNSC 4.0 | MS, LRI | 94 | 996 | 1022 | 0.47 | 0.74 | 1.07 |
| 65 | 2-ethyl-Hexanol | FFNSC 4.0 | MS, LRI | 88 | 1030 | 1030 | 0.33 | 0.20 | Nd |
| 66 | Benzyl alcohol | FFNSC 4.0 | MS, LRI | 90 | 1040 | 1037 | 0.41 | 0.22 | 0.17 |
| 67 | (E)-2-Hexenoic acid | FFNSC 4.0 | MS, LRI | 90 | 1036 | 1039 | 0.34 | 0.32 | 0.05 |
| 68 | Oct-3-en-2-one | FFNSC 4.0 | MS, LRI | 93 | 1036 | 1040 | 0.22 | 0.18 | 0.11 |
| 69 | Phenylacetaldehyde | FFNSC 4.0 | MS, LRI | 97 | 1045 | 1043 | Nd | 0.51 | 0.12 |
| 70 | γ-Hexalactone | FFNSC 4.0 | MS, LRI | 98 | 1060 | 1054 | 0.68 | 0.11 | 0.39 |
| 71 | (E)-2-Octenal | FFNSC 4.0 | MS, LRI | 90 | 1058 | 1061 | 0.22 | 0.11 | 0.20 |
| 72 | α-Phenylethanol | FFNSC 4.0 | MS, LRI | 94 | 1064 | 1063 | 0.10 | 0.05 | 0.07 |
| 73 | Acetophenone | FFNSC 4.0 | MS, LRI | 91 | 1068 | 1065 | Nd | 0.12 | 0.12 |
| 74 | 2-acetyl-Pyrrole | FFNSC 4.0 | MS, LRI | 94 | 1060 | 1068 | 0.30 | 0.15 | 0.46 |
| 75 | (E,E)-3,5-Octadien-2-one | W11N17 | MS, LRI | 90 | 1073 | 1071 | 1.07 | 0.53 | 1.16 |
| 76 | n-Octanol | FFNSC 4.0 | MS, LRI | 92 | 1076 | 1073 | 0.35 | 0.19 | 0.44 |
| 77 | p-Cresol | FFNSC 4.0 | MS, LRI | 89 | 1072 | 1077 | Nd | Nd | 0.12 |
| 78 | 2-Pyrrolidone | FFNSC 4.0 | MS, LRI | 92 | 1070 | 1078 | 0.46 | 0.02 | Nd |
| 79 | 2-ethyl-, 3,6-dimethyl-pyrazine | FFNSC 4.0 | MS, LRI | 87 | 1079 | 1080 | 0.15 | 0.05 | Nd |
| 80 | n-Heptanoic acid | FFNSC 4.0 | MS, LRI | 88 | 1116 | 1092 | 0.90 | 0.28 | 1.06 |
| 81 | 3,5-Octadien-2-one | W11N17 | MS, LRI | 90 | 1091 | 1095 | 1.15 | 0.56 | 0.60 |
| 82 | n-Nonanal | FFNSC 4.0 | MS, LRI | 95 | 1107 | 1104 | 1.13 | 1.24 | 0.93 |
| 83 | 2,6-dimethyl-Cyclohexanol | W11N17 | MS, LRI | 89 | 1112 | 1113 | 2.19 | 0.27 | 0.58 |
| 84 | Phenethyl alcohol | FFNSC 4.0 | MS, LRI | 95 | 1113 | 1117 | 4.16 | 2.39 | 2.68 |
| 85 | methyl-Octanoate | FFNSC 4.0 | MS, LRI | 92 | 1125 | 1124 | 0.20 | Nd | 0.05 |
| 86 | Isophorone | FFNSC 4.0 | MS, LRI | 88 | 1123 | 1126 | 0.41 | 0.08 | 0.11 |
| 87 | 2-Heptenoic acid | W11N17 | MS | 93 | - | 1130 | Nd | Nd | 0.24 |
| 88 | 2-nitro-Phenol | W11N17 | MS, LRI | 96 | 1135 | 1131 | Nd | 0.16 | Nd |
| 89 | Benzene acetonitrile | FFNSC 4.0 | MS, LRI | 91 | 1138 | 1138 | Nd | 0.09 | Nd |
| 90 | Oxophorone | FFNSC 4.0 | MS, LRI | 90 | 1148 | 1147 | 0.48 | 0.18 | 0.43 |
| 91 | 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-Pyran-4-one | W11N17 | MS, LRI | 93 | 1151 | 1148 | 0.20 | 0.16 | 0.49 |
| 92 | (E,Z)-2,6-Nonadienal | FFNSC 4.0 | MS, LRI | 93 | 1153 | 1152 | Nd | 0.15 | 0.48 |
| 93 | γ-Heptalactone | FFNSC 4.0 | MS, LRI | 91 | 1155 | 1153 | 0.05 | Nd | Nd |
| 94 | Menthone | FFNSC 4.0 | MS, LRI | 90 | 1158 | 1156 | Nd | 0.05 | Nd |
| 95 | (E)-2-Nonenal | FFNSC 4.0 | MS, LRI | 90 | 1163 | 1159 | Nd | 0.11 | 0.26 |
| 96 | 2,2,6-trimethyl-1,4-Cyclohexanedione | W11N17 | MS, LRI | 89 | 1183 | 1172 | 0.22 | Nd | Nd |
| 97 | 2,4-dimethyl-Benzaldehyde | FFNSC 4.0 | MS, LRI | 91 | 1190 | 1175 | 0.42 | 0.16 | Nd |
| 98 | Menthol | FFNSC 4.0 | MS, LRI | 97 | 1184 | 1178 | 0.47 | 0.22 | Nd |
| 99 | n-Octanoic acid | FFNSC 4.0 | MS, LRI | 95 | 1192 | 1192 | 2.33 | 1.25 | 6.63 |
| 100 | n-Dodecane | FFNSC 4.0 | MS, LRI | 97 | 1200 | 1200 | 0.10 | 0.13 | Nd |
| 101 | Safranal | FFNSC 4.0 | MS, LRI | 97 | 1201 | 1203 | 0.61 | 1.01 | 1.15 |
| 102 | n-Decanal | FFNSC 4.0 | MS, LRI | 96 | 1208 | 1207 | 0.29 | 0.07 | 1.80 |
| 103 | β-Cyclocitral | FFNSC 4.0 | MS, LRI | 90 | 1223 | 1224 | 0.74 | 0.05 | 0.34 |
| 104 | 3-ethyl-4-methyl-1H-Pyrrole-2,5-dione | W11N17 | MS, LRI | 92 | 1239 | 1239 | 0.88 | Nd | 1.00 |
| 105 | Benzenepropanenitrile | W11N17 | MS, LRI | 98 | 1244 | 1244 | 8.16 | 34.94 | 6.24 |
| 106 | 2-Phenethyl acetate | FFNSC 4.0 | MS, LRI | 95 | 1257 | 1257 | 0.20 | 0.10 | 0.24 |
| 107 | β-Cyclohomocitral | FFNSC 4.0 | MS, LRI | 90 | 1256 | 1257 | Nd | 0.12 | Nd |
| 108 | γ-Octalactone | FFNSC 4.0 | MS, LRI | 95 | 1263 | 1259 | 0.14 | Nd | Nd |
| 109 | Benzeneacetic acid | W11N17 | MS, LRI | 88 | 1262 | 1259 | Nd | Nd | 0.09 |
| 110 | 2-phenyl-Crotonaldehyde | FFNSC 4.0 | MS, LRI | 88 | 1272 | 1273 | 0.29 | 0.22 | Nd |
| 111 | 3,3-dimethyl-2,7-Octanedione | W11N17 | MS, LRI | 88 | 1290 | 1277 | 1.46 | 0.45 | 0.96 |
| 112 | n-Nonanoic acid | FFNSC 4.0 | MS, LRI | 96 | 1289 | 1280 | 0.48 | 0.31 | 1.18 |
| 113 | Menthyl acetate | FFNSC 4.0 | MS, LRI | 92 | 1290 | 1289 | Nd | 0.05 | Nd |
| 114 | Isobornyl acetate | FFNSC 4.0 | MS, LRI | 95 | 1287 | 1291 | 0.16 | Nd | Nd |
| 115 | (E)-Cinnamonitrile | FFNSC 4.0 | MS, LRI | 96 | 1294 | 1295 | 0.14 | 0.15 | 0.09 |
| 116 | n-Tridecane | FFNSC 4.0 | MS, LRI | 94 | 1300 | 1298 | Nd | 0.07 | Nd |
| 117 | 4-vinyl-Guaiacol | FFNSC 4.0 | MS, LRI | 92 | 1309 | 1314 | 0.09 | 0.31 | 0.43 |
| 118 | γ-Nonalactone | FFNSC 4.0 | MS, LRI | 94 | 1362 | 1364 | 0.36 | 0.10 | 0.41 |
| 119 | n-Decanoic acid | FFNSC 4.0 | MS, LRI | 94 | 1398 | 1368 | 0.45 | 0.24 | 2.12 |
| 120 | 2,6,10-trimethyl-Dodecane | W11N17 | MS, LRI | 91 | 1366 | 1376 | 0.09 | 0.37 | Nd |
| 121 | α-Copaene | FFNSC 4.0 | MS, LRI | 90 | 1375 | 1381 | 0.12 | Nd | Nd |
| 122 | 1-Tetradecene | FFNSC 4.0 | MS, LRI | 94 | 1392 | 1390 | Nd | 0.08 | Nd |
| 123 | n-Tetradecane | FFNSC 4.0 | MS, LRI | 95 | 1400 | 1400 | 0.42 | 0.42 | 0.70 |
| 124 | Vanillin | FFNSC 4.0 | MS, LRI | 88 | 1394 | 1401 | 0.21 | 0.09 | 0.12 |
| 125 | 6,10-dimethyl-2-Undecanone | W11N17 | MS, LRI | 94 | 1408 | 1403 | 0.35 | 0.21 | 0.19 |
| 126 | (E)-,α-Ionone | FFNSC 4.0 | MS, LRI | 90 | 1421 | 1422 | Nd | 0.13 | Nd |
| 127 | (E)-Caryophyllene | FFNSC 4.0 | MS, LRI | 95 | 1424 | 1427 | 0.96 | Nd | Nd |
| 128 | β-Gurjunene | FFNSC 4.0 | MS, LRI | 93 | 1437 | 1439 | 0.57 | Nd. | Nd |
| 129 | (E)-Geranylacetone | FFNSC 4.0 | MS, LRI | 94 | 1450 | 1449 | 0.47 | 0.73 | 0.46 |
| 130 | 2,6,10-Trimethyltridecane | W11N17 | MS, LRI | 93 | 1449 | 1461 | 0.26 | 0.18 | Nd |
| 131 | 2,6-bis(1,1-dimethylethyl)-2,5-Cyclohexadiene-1,4-dione | W11N17 | MS, LRI | 90 | 1471 | 1461 | Nd | 0.17 | Nd |
| 132 | 2,6-Di-tert-butyl-4-hydroxy-4-methylcyclohexa-2,5-dien-1-one | W11N17 | MS, LRI | 91 | 1475 | 1463 | 0.31 | Nd | 0.63 |
| 133 | Phenylethyl isothiocyanate | FFNSC 4.0 | MS, LRI | 95 | 1464 | 1470 | 0.32 | 0.25 | 0.79 |
| 134 | 1-chloro-Dodecane | W11N17 | MS, LRI | 92 | 1469 | 1471 | Nd | 0.10 | Nd |
| 135 | Dodecanol | FFNSC 4.0 | MS, LRI | 94 | 1476 | 1477 | 0.23 | Nd | 0.36 |
| 136 | 4-(2,6,6-Trimethylcyclohexa-1,3-dienyl)but-3-en-2-one | W11N17 | MS, LRI | 93 | 1485 | 1482 | 0.41 | 0.20 | 0.23 |
| 137 | (E)-,β-Ionone | FFNSC 4.0 | MS, LRI | 93 | 1482 | 1485 | 2.53 | 2.44 | 0.89 |
| 138 | Ionone epoxide | FFNSC 4.0 | MS, LRI | 90 | 1483 | 1488 | 1.59 | 1.16 | 0.65 |
| 139 | 1-Pentadecene | W11N17 | MS, LRI | 96 | 1492 | 1493 | 0.91 | 0.19 | 0.56 |
| 140 | β-Selinene | FFNSC 4.0 | MS, LRI | 95 | 1492 | 1498 | 1.10 | Nd | Nd |
| 141 | n-Pentadecane | FFNSC 4.0 | MS, LRI | 96 | 1500 | 1500 | 0.26 | 0.15 | 0.35 |
| 142 | Unknown | - | - | - | - | - | - | - | - |
| 143 | 2,4-bis(1,1-dimethylethyl)-Phenol | W11N17 | MS, LRI | 92 | 1513 | 1507 | Nd | 0.09 | Nd |
| 144 | 5,6,7,7a-tetrahydro-4,4,7a-trimethyl-2(4H)-Benzofuranone | W11N17 | MS, LRI | 97 | 1532 | 1544 | 8.54 | 3.64 | 6.32 |
| 145 | n-Dodecanoic acid | FFNSC 4.0 | MS, LRI | 92 | 1581 | 1564 | Nd | Nd | 0.29 |
| 146 | 3-methyl-Pentadecane | W11N17 | MS, LRI | 90 | 1570 | 1571 | 0.07 | 0.10 | 0.19 |
| 147 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | W11N17 | MS, LRI | 91 | 1588 | 1585 | Nd | 0.17 | Nd |
| 148 | 2-[[[4-(4-hydroxy-4-methylpentyl)-, 3-cyclohexen-1-yl]methylene]amino]-, methyl-Benzoate | FFNSC 4.0 | MS, LRI | 97 | 1589 | 1586 | 0.22 | 0.42 | 0.27 |
| 149 | n-Hexadecene | FFNSC 4.0 | MS, LRI | 86 | 1593 | 1593 | 0.15 | 0.28 | 0.23 |
| 150 | n-Hexadecane | FFNSC 4.0 | MS, LRI | 96 | 1600 | 1601 | 0.79 | 0.38 | 0.72 |
| 151 | 1-butylheptyl-Benzene | W11N17 | MS, LRI | 91 | 1632 | 1633 | 0.09 | 0.06 | 0.16 |
| 152 | Benzophenone | FFNSC 4.0 | MS, LRI | 96 | 1627 | 1639 | 0.10 | 0.02 | 0.26 |
| 153 | 1-propyloctyl-Benzene | W11N17 | MS, LRI | 93 | 1643 | 1640 | 0.10 | 0.12 | 0.19 |
| 154 | 1,1′-oxybis-Octane | W11N17 | MS, LRI | 91 | 1659 | 1660 | Nd | 0.27 | 0.19 |
| 155 | β-Eudesmol | FFNSC 4.0 | MS, LRI | 89 | 1656 | 1666 | 0.58 | Nd | Nd |
| 156 | (Z,Z,Z)-1,8,11,14-Heptadecatetraene | W11N17 | MS, LRI | 93 | 1664 | 1667 | 0.43 | 0.19 | 0.41 |
| 157 | n-Heptadecane | FFNSC 4.0 | MS, LRI | 94 | 1700 | 1700 | 0.10 | 0.08 | 0.32 |
| 158 | Tetradecanoic acid | FFNSC 4.0 | MS, LRI | 93 | 1773 | 1761 | Nd | Nd | 0.55 |
| 159 | 1-ethyldecyl-Benzene | W11N17 | MS, LRI | 90 | 1766 | 1763 | 0.02 | 0.04 | Nd |
| 160 | 3-methyl-Heptadecane | W11N17 | MS, LRI | 92 | 1771 | 1769 | Nd | 0.13 | 0.15 |
| 161 | 6-Hydroxy-4,4,7a-trimethyl-5,6,7,7a-tetrahydrobenzofuran-2(4H)-one | W11N17 | MS, LRI | 90 | 1784 | 1778 | 0.02 | Nd | 0.18 |
| 162 | 1-Octadecene | FFNSC 4.0 | MS, LRI | 95 | 1793 | 1793 | 0.04 | 0.19 | 0.03 |
| 163 | n-Octadecane | FFNSC 4.0 | MS, LRI | 95 | 1800 | 1800 | 0.04 | 0.09 | 0.04 |
| 164 | Neophytadiene | FFNSC 4.0 | MS, LRI | 90 | 1836 | 1837 | 0.03 | Nd | 0.04 |
| 165 | Phytone | FFNSC 4.0 | MS, LRI | 91 | 1841 | 1839 | 2.85 | 3.77 | 1.01 |
| 166 | n-Nonadecane | FFNSC 4.0 | MS, LRI | 90 | 1900 | 1897 | Nd | 0.01 | Nd |
| 167 | methyl-7,10,13-hexadecatrienoate | W11N17 | MS | 91 | - | 1897 | 0.09 | Nd | 0.27 |
| 168 | 3-Methyl-2-(3,7,11-trimethyldodecyl) furan | W11N17 | MS | 92 | - | 1913 | 0.05 | 0.72 | Nd |
| 169 | methyl-Hexadecanoate | FFNSC 4.0 | MS, LRI | 94 | 1925 | 1926 | 0.09 | 0.05 | 0.06 |
| 170 | (Z,Z,Z)-7,10,13-Hexadecatrienoic acid | W11N17 | MS, LRI | 92 | 1945 | 1938 | 0.11 | Nd | Nd |
| 171 | n-Hexadecanoic acid | FFNSC 4.0 | MS, LRI | 91 | 1977 | 1963 | 0.41 | 0.86 | 1.16 |
| 172 | n-Eicosane | FFNSC 4.0 | MS, LRI | 94 | 2000 | 1997 | Nd | 0.01 | Nd |
| 173 | methyl-Linoleate | FFNSC 4.0 | MS, LRI | 91 | 2093 | 2094 | 0.02 | Nd | 0.01 |
| 174 | n-Heneicosane | FFNSC 4.0 | MS, LRI | 90 | 2100 | 2097 | Nd | 0.02 | Nd |
| 175 | methyl-Linolenate | FFNSC 4.0 | MS, LRI | 93 | 2098 | 2100 | 0.07 | 0.00 | 0.05 |
| 176 | (Z,Z)-9,12-Octadecadienoic acid | W11N17 | MS, LRI | 91 | 2133 | 2135 | 0.01 | Nd | 0.10 |
| 177 | (Z,Z,Z)-9,12,15-Octadecatrienoic acid | W11N17 | MS, LRI | 93 | 2139 | 2140 | 0.11 | Nd | 0.51 |
| 178 | n-Tetracosane | FFNSC 4.0 | MS, LRI | 89 | 2400 | 2397 | Nd | 0.00 | Nd |
| 179 | n-Pentacosane | FFNSC 4.0 | MS, LRI | 92 | 2500 | 2496 | Nd | 0.01 | 0.01 |
| 180 | n-Heptacosane | FFNSC 4.0 | MS, LRI | 92 | 2700 | 2700 | 0.01 | 0.02 | 0.03 |
| Total Identified | 83.15 | 86.37 | 75.53 |
| DSM | Moisture | Oil Content | Proteins | Glucosinolates | ||
|---|---|---|---|---|---|---|
| but-3enyl GSL | 2-propenyl GSL | 4-hydroxy-3-indolylmethylGSL | ||||
| (% DW) | (% DW) | (% DW) | (µmol /g) | µmol /g) | (µmol /g) | |
| Sample A | 8.3 ± 0.1 | 11.1 ± 0.1 | 44.0 ± 0.5 | 2.4 ± 0.2 | 200 ± 3 | 3.0 ± 0.7 |
| Sample B | 8.8 ± 0.5 | 15.7 ± 0.2 | 37.4 ± 0.2 | 2.1 ± 0.1 | 137 ± 3 | 2.10 ± 0.04 |
| Sample C | 8.3 ± 0.3 | 16.9 ± 0.1 | 36.8 ± 0.2 | 4.9 ± 0.2 | 148 ± 2 | 2.2 ± 0.2 |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oulad El Majdoub, Y.; Alibrando, F.; Cacciola, F.; Arena, K.; Pagnotta, E.; Matteo, R.; Micalizzi, G.; Dugo, L.; Dugo, P.; Mondello, L. Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues. Molecules 2020, 25, 5421. https://doi.org/10.3390/molecules25225421
Oulad El Majdoub Y, Alibrando F, Cacciola F, Arena K, Pagnotta E, Matteo R, Micalizzi G, Dugo L, Dugo P, Mondello L. Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues. Molecules. 2020; 25(22):5421. https://doi.org/10.3390/molecules25225421
Chicago/Turabian StyleOulad El Majdoub, Yassine, Filippo Alibrando, Francesco Cacciola, Katia Arena, Eleonora Pagnotta, Roberto Matteo, Giuseppe Micalizzi, Laura Dugo, Paola Dugo, and Luigi Mondello. 2020. "Chemical Characterization of Three Accessions of Brassica juncea L. Extracts from Different Plant Tissues" Molecules 25, no. 22: 5421. https://doi.org/10.3390/molecules25225421






