A Comparison between Different Agro-wastes and Carbon Nanotubes for Removal of Sarafloxacin from Wastewater: Kinetics and Equilibrium Studies
Abstract
:1. Introduction
2. Results
2.1. Assessment of the Adsorptive Power of the Tested Adsorbents
2.2. Factorial Design
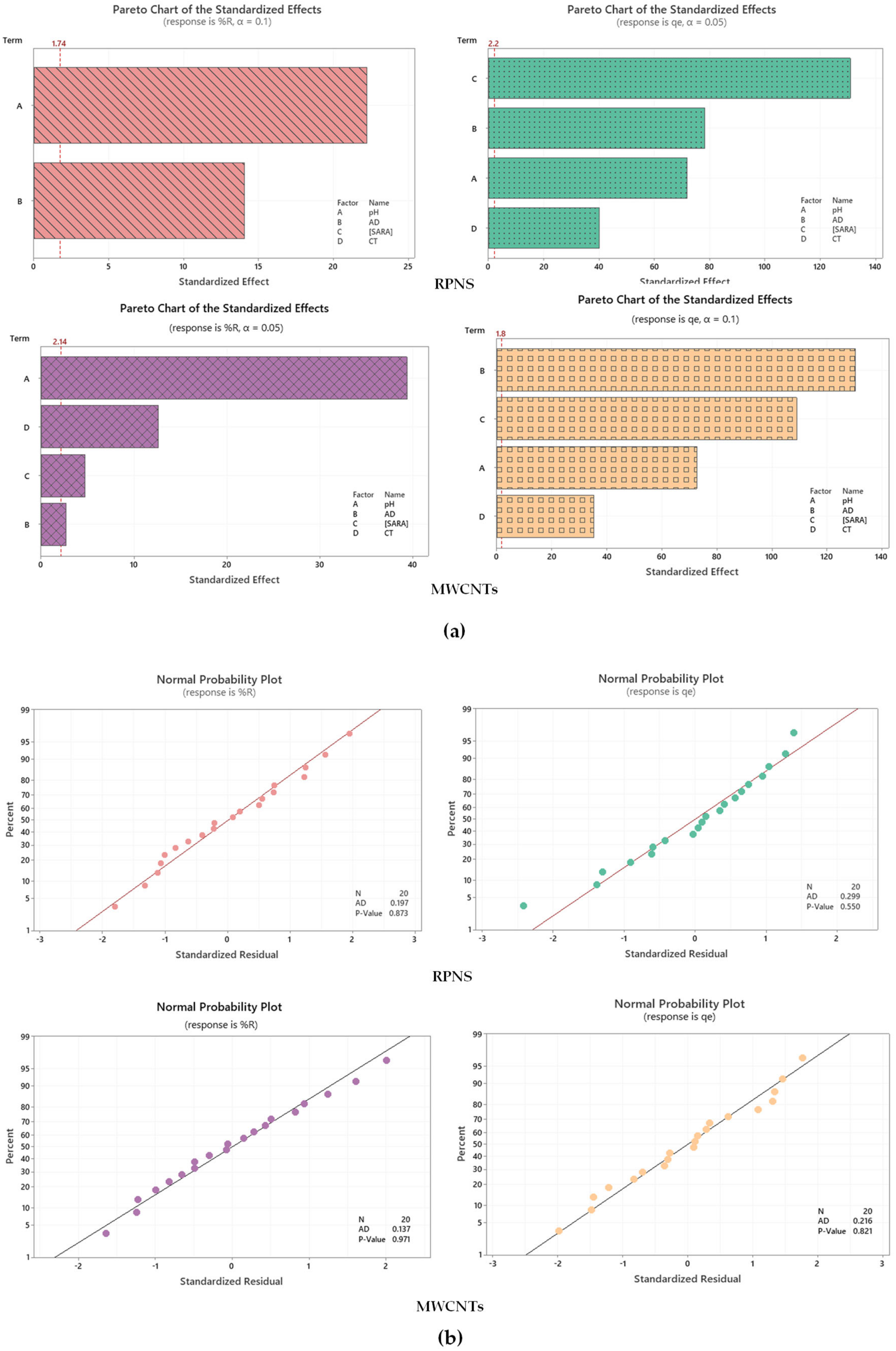
2.3. Assessment of Statistically Significant Independent Variables
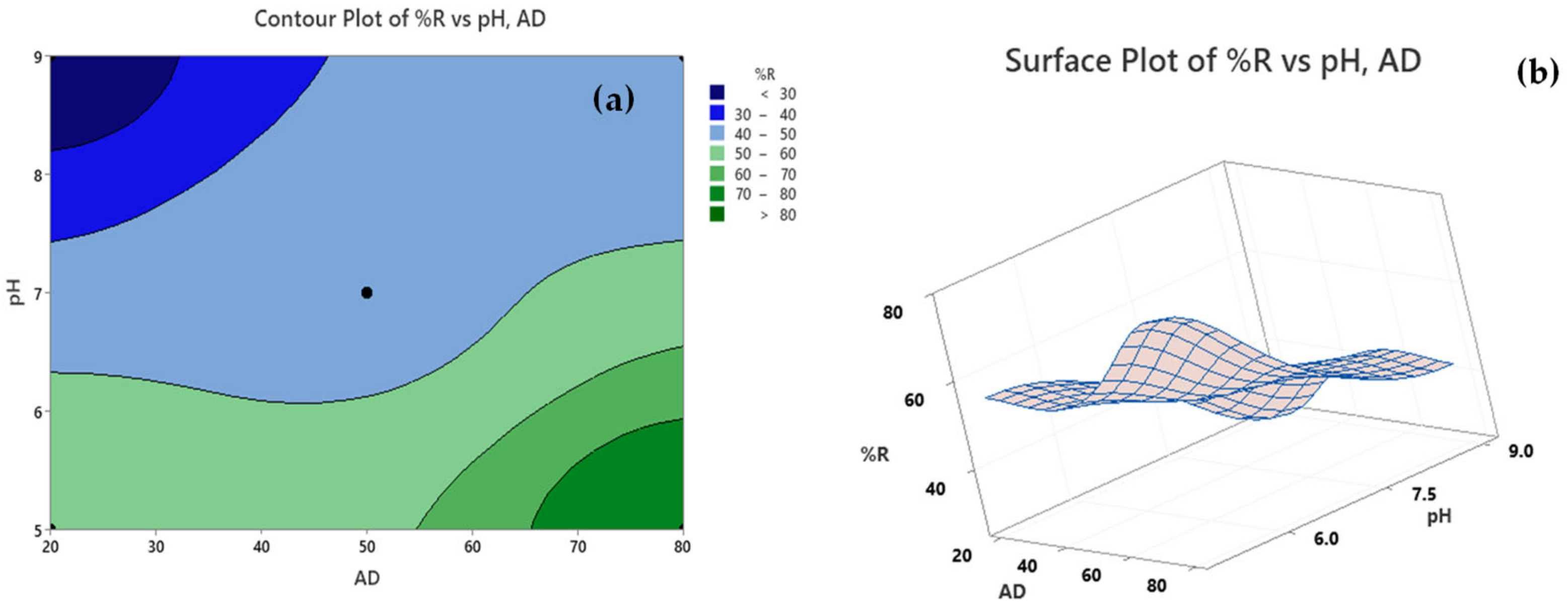
2.4. Contour and Surface Plots
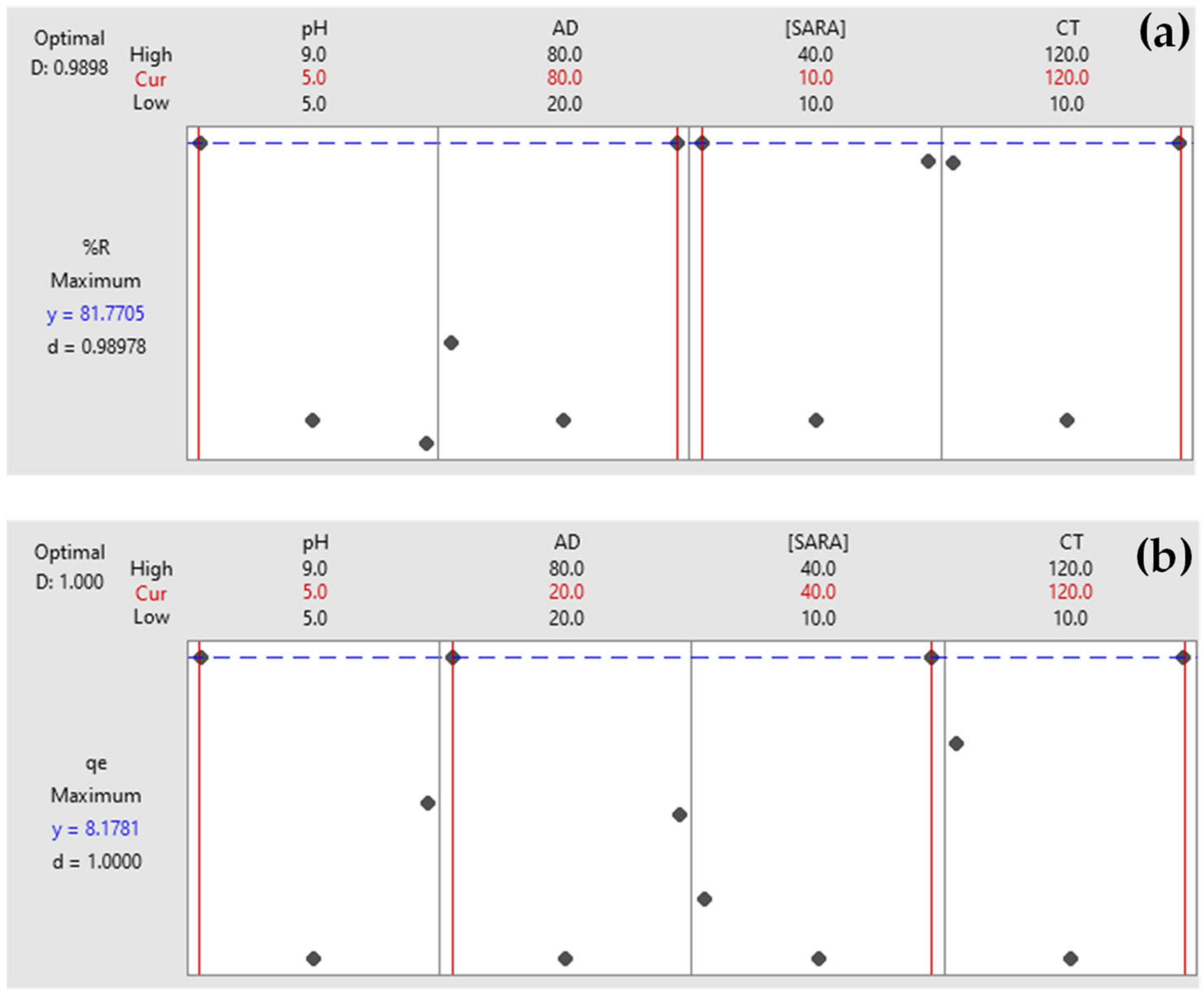
2.5. Response Optimization
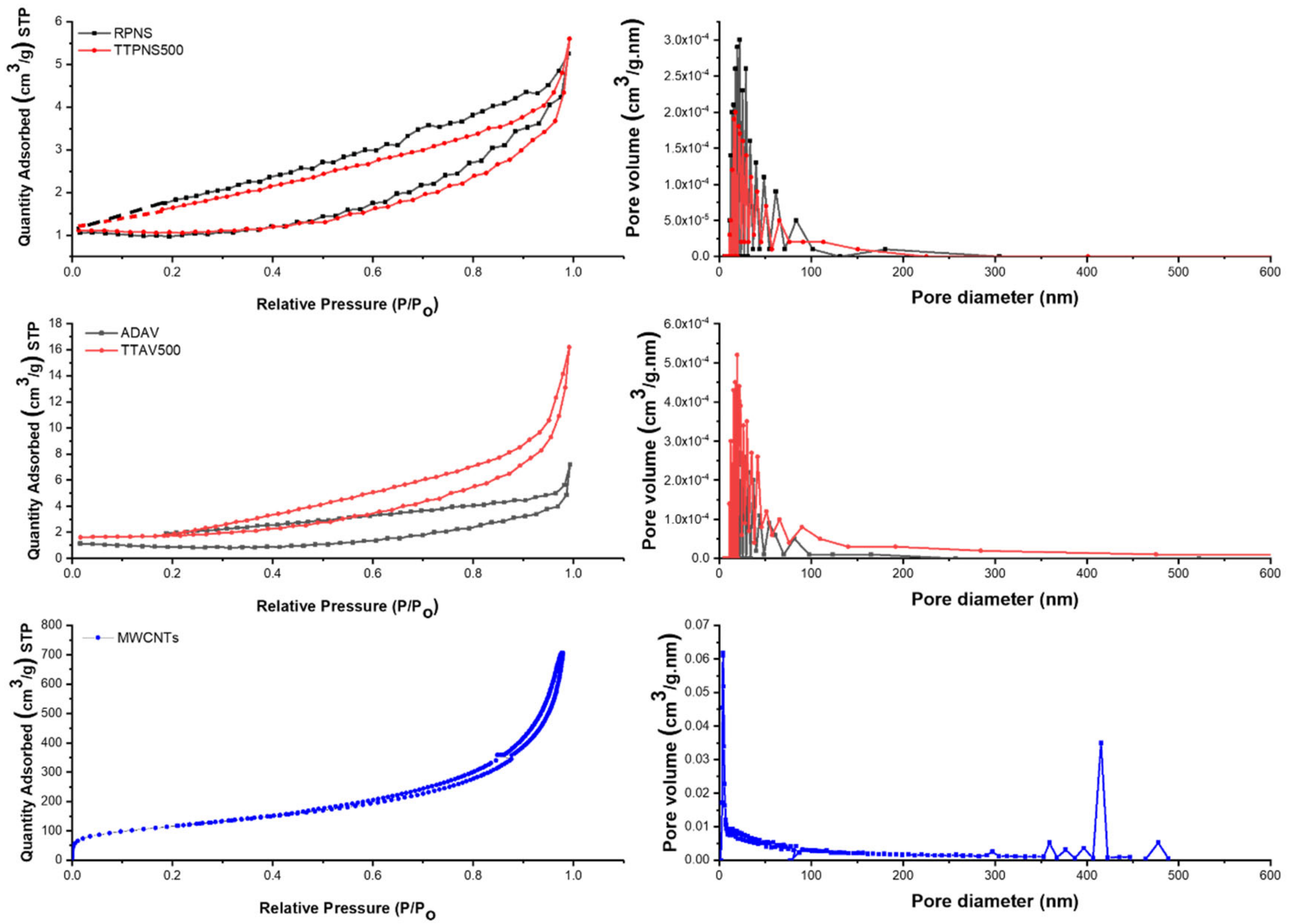
2.6. Characterization of Tested Adsorbents
2.6.1. Thermal Characteristics
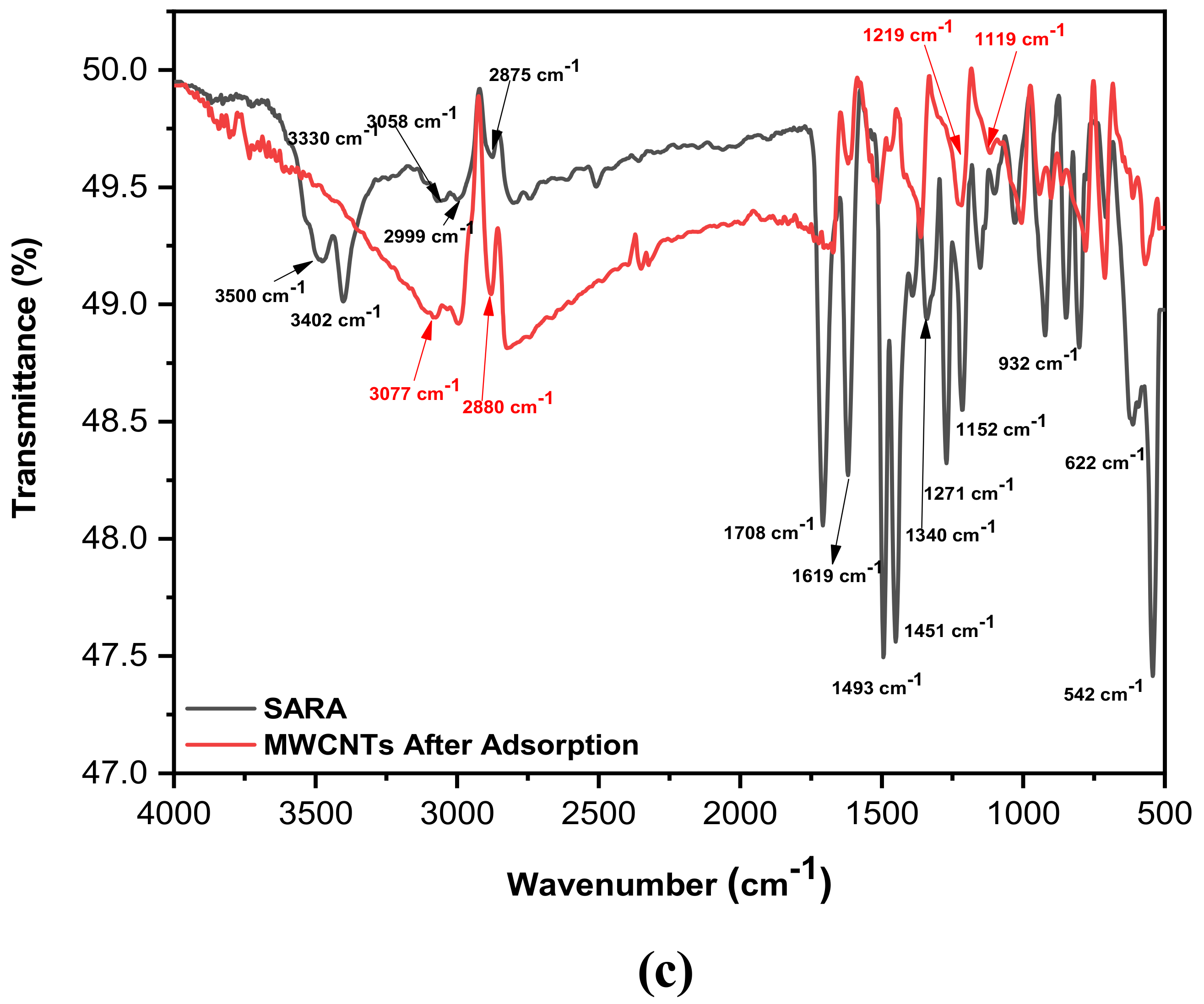
2.6.2. Functional Groups and Adsorption Mechanism
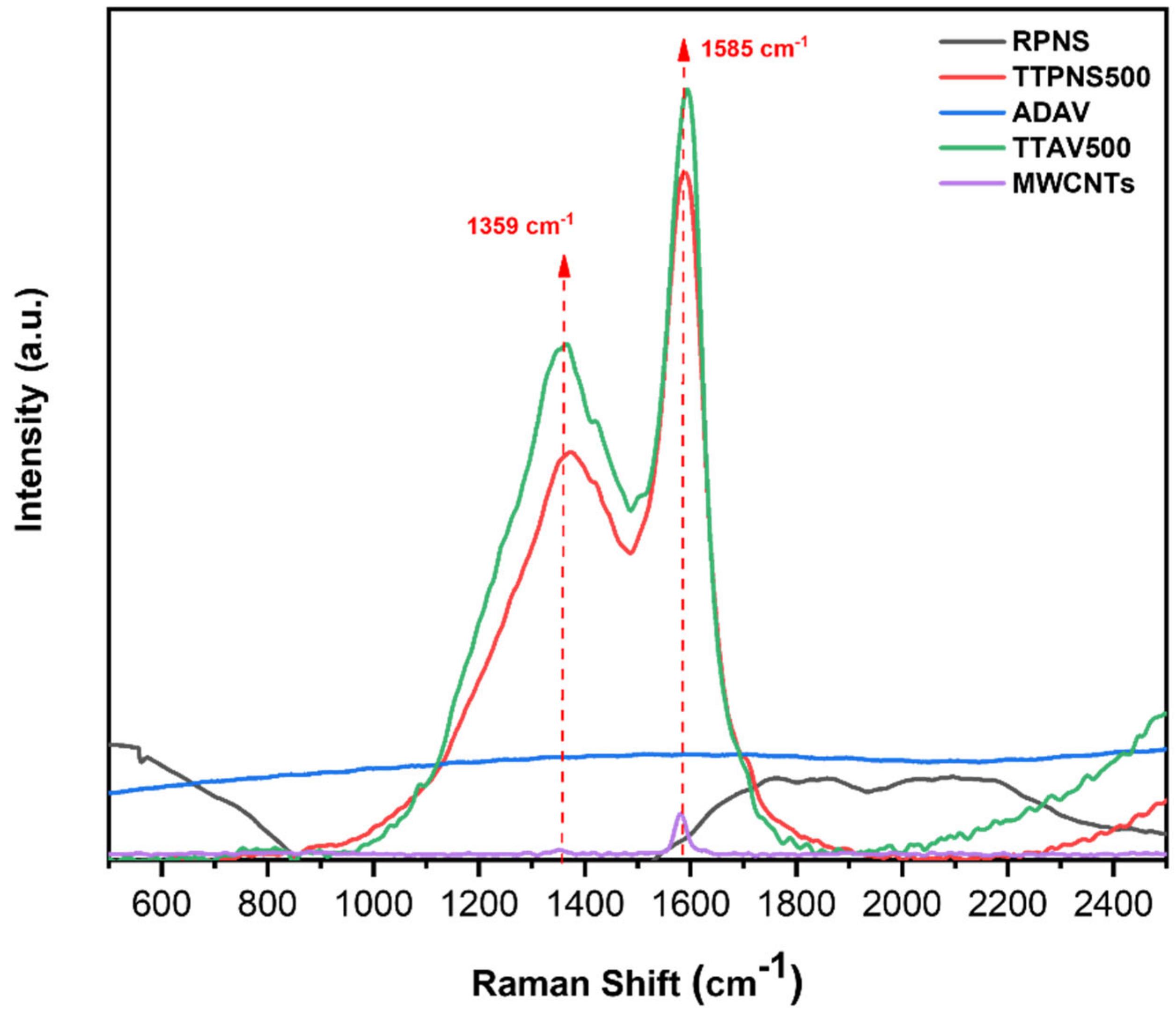
2.6.3. Raman Analysis
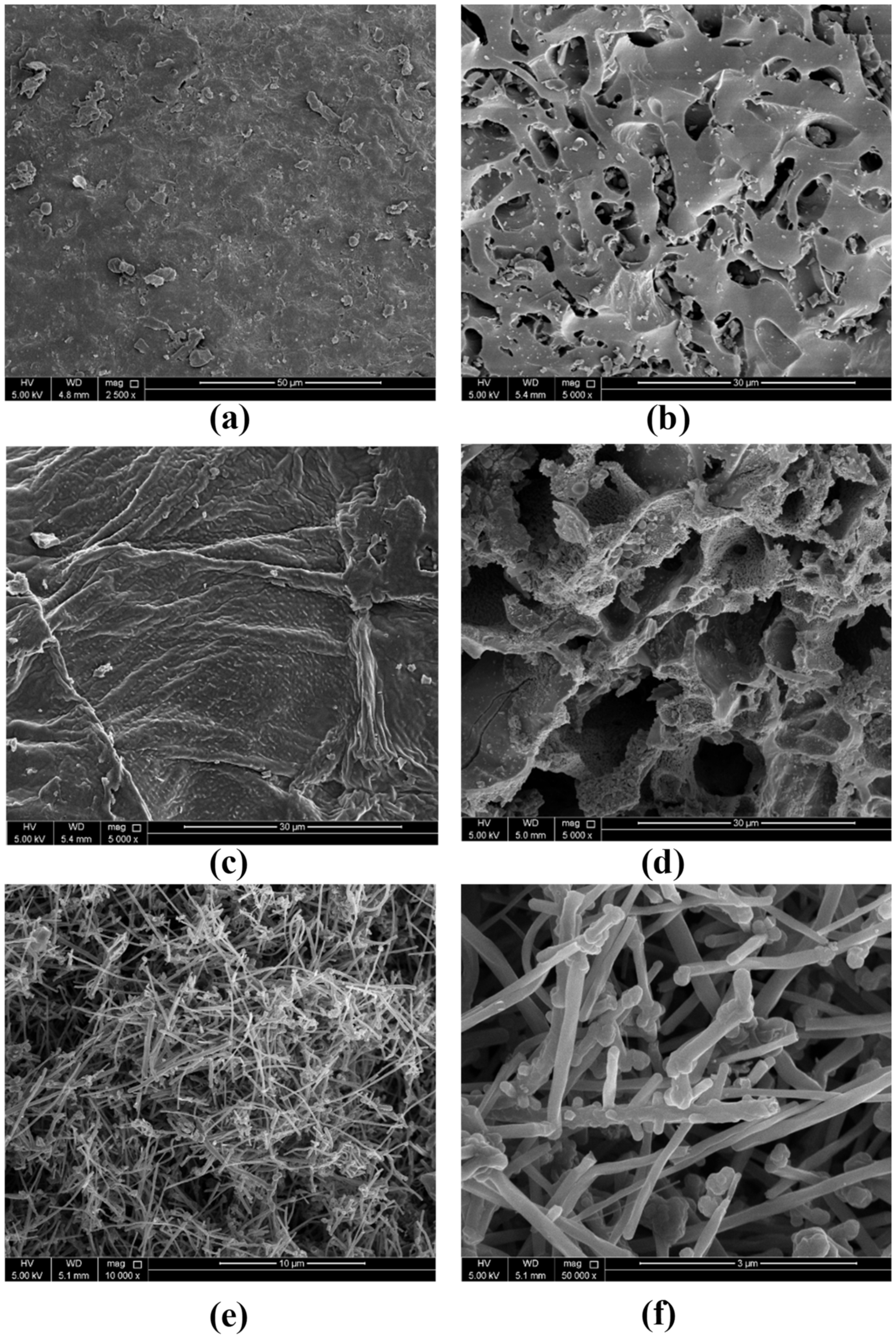
2.6.4. Morphology Characteristics
2.7. Equilibrium and Kinetics Studies
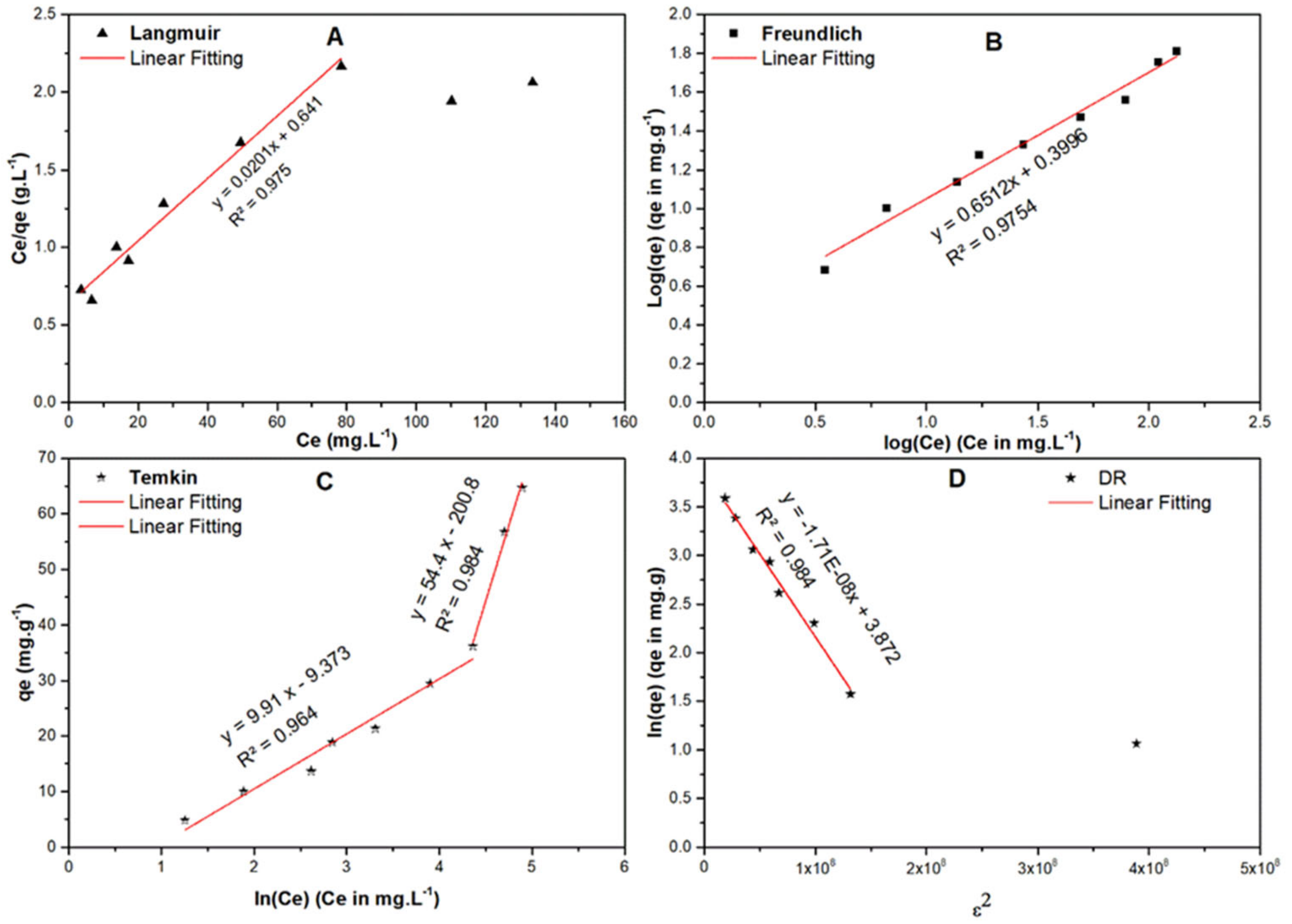
2.7.1. Equilibrium Isotherms
2.7.2. Kinetic Studies
3. Materials and Methods
3.1. Materials and Reagents
3.2. Equilibrium and Kinetic Studies
3.3. Instrumentation and Software
3.4. Preparation of the AWs’ Derived Adsorbents
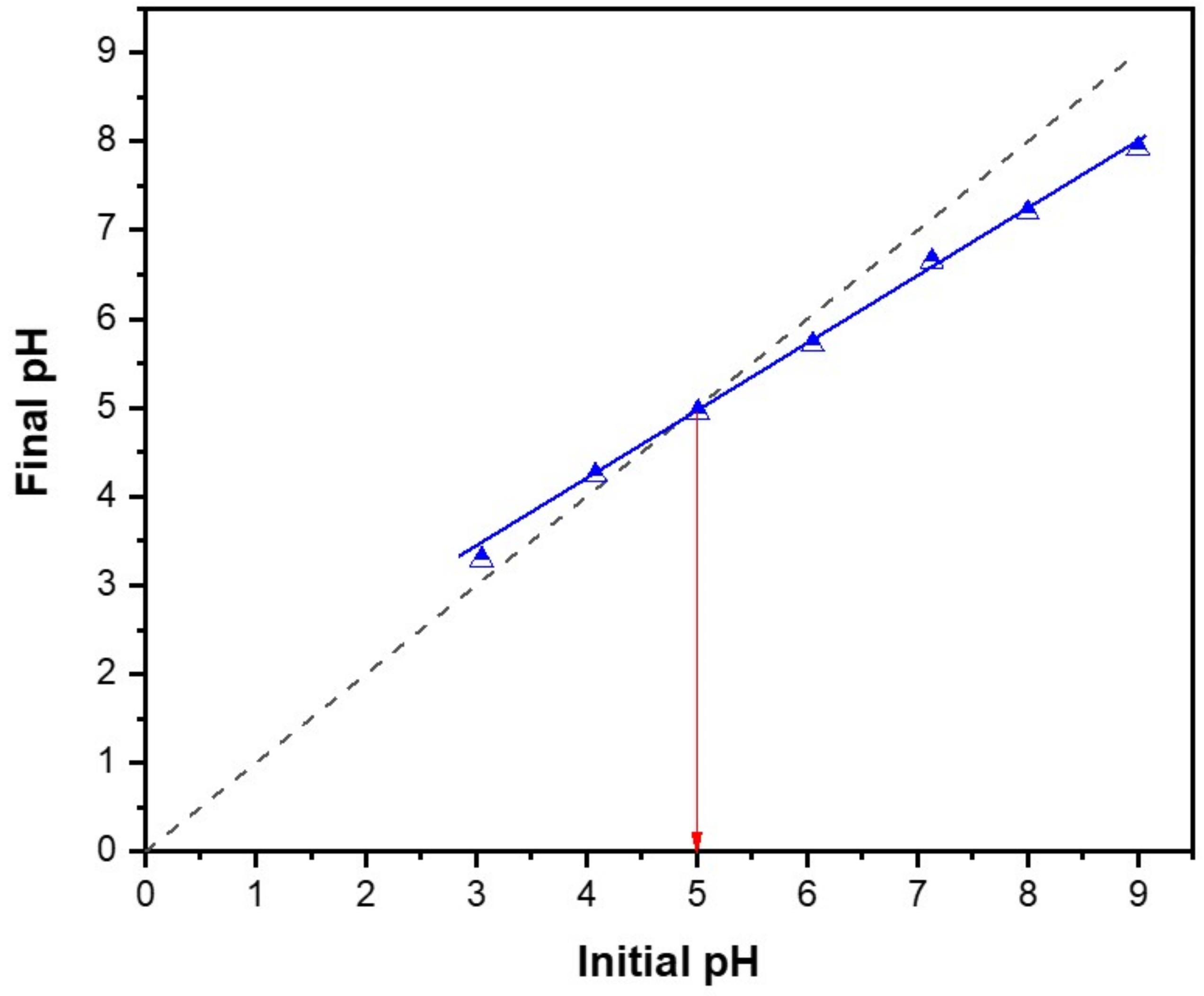
3.5. Determination of the Point of Zero Charge of RPNS
3.6. Plackett-Burman Design (PBD)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pukšec, T.; Leahy, P.; Foley, A.; Markovska, N.; Duić, N. Sustainable development of energy, water and environment systems 2016 (SDEWES2016). Renew. Sust. Energ. Rev. 2018, 82, 1685–1690. [Google Scholar] [CrossRef]
- El-Gendy, A.; El-Shafie, A.S.; Issa, A.; Al-Meer, S.; Al-Saad, K.; El-Azazy, M. Carbon-based materials (CBMS) for determination and remediation of antimicrobials in different substrates: Wastewater and infant foods as examples. In Carbon-Based Material for Environmental Protection and Remediation; Bartoli, M., Frediani, M., Rosi, L., Eds.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/books/carbon-based-material-for-environmental-protection-and-remediation/carbon-based-materials-cbms-for-determination-and-remediation-of-antimicrobials-in-different-substrates (accessed on 22 September 2020). [CrossRef]
- Sarmah, A.K.; Meyer, M.T.; Boxall, A.B.A. A global perspective on the use, sales, exposure pathways, occurrence, fate and effects of veterinary antibiotics (VAs) in the environment. Chemosphere 2006, 65, 725–759. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Watkinson, A.J.; Murby, E.J.; Kolpin, D.W.; Costanzo, S.D. The occurrence of antibiotics in an urban watershed: From wastewater to drinking water. Sci. Total Environ. 2009, 407, 2711–2723. [Google Scholar] [CrossRef] [PubMed]
- Heeb, S.; Fletcher, M.P.; Chhabra, S.R.; Diggle, S.P.; Williams, P.; Cámara, M. Quinolones: From antibiotics to autoinducers. FEMS Microbiol. Rev. 2011, 35, 247–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Renew, J.E.; Smeby, K.L.; Pinkerston, K.; Sedlak, D.L. Assessment of potential antibiotic contaminants in water and preliminary occurrence analysis. Water Resour. 2001, 120, 30–40. [Google Scholar]
- Carabineiro, S.; Thavorn-Amornsri, T.; Pereira, M.; Serp, P.; Figueiredo, J. Comparison between activated carbon, carbon xerogel and carbon nanotubes for the adsorption of the antibiotic ciprofloxacin. Catal. Today 2012, 186, 29–34. [Google Scholar] [CrossRef]
- Dong, S.; Sun, Y.; Wu, J.; Wu, B.; Creamer, A.E.; Gao, B. Graphene oxide as filter media to remove levofloxacin and lead from aqueous solution. Chemosphere 2016, 150, 759–764. [Google Scholar] [CrossRef]
- Peng, H.; Pa, B.; Wu, M.; Liu, Y.; Zhang, D.; Xing, B. Adsorption of ofloxacin and norfloxacin on carbon nanotubes: Hydrophobicity-and structure-controlled process. J. Hazard. Mater. 2012, 233, 89–96. [Google Scholar] [CrossRef]
- Dan, A.; Xiaomeng, Z.; Yunv, D.; Chunxing, C.; Yang, Y. Occurrence and removal of quinolone, tetracycline, and macrolide antibiotics from urban wastewater in constructed wetlands. J. Clean. Prod. 2020, 252, 119677. [Google Scholar]
- Ran, Y.; Liang, C.; Rong, S.; Peng, L.; Naibiao, S. Quantification of ultra-trace levels of fluoroquinolones in wastewater by molecularly imprinted solid phase extraction and liquid chromatography triple quadrupole mass. Environ. Technol. Innov. 2020, 19, 100919. [Google Scholar]
- Chansiripornchai, N.; Sasipreeyajan, J. Efficacy of sarafloxacin in broilers after experimental infection with Escherichia coli. Vet. Res. Commun. 2002, 26, 255–262. [Google Scholar] [CrossRef] [PubMed]
- WHO/FAO. FAO Fisheries Technical Paper 469: Responsible Use of Antibiotics in Aquaculture; World Health Organization: Geneva, Switzerland, 2005; Available online: http://www.fao.org/3/a-a0282e.pdf (accessed on 20 September 2020).
- EMEA [European Agency for the Evaluation of Medicinal Products]. Committee for Veterinarian Medicinal Products. Sarafloxacin (Salmonidae); Summary Report (1). 1997. Available online: EMEA/MRL/160/96-FINAL (accessed on 20 September 2020).
- Hektoen, H.; Berge, J.A.; Hormazabal, V.; Yndestad, M. Resistance of antimicrobial agents in marine sediments. Aquaculture 1995, 133, 175–184. [Google Scholar] [CrossRef]
- Jie, Z.; Qiang, H.; Xiaolong, Z.; Xiao, G.; Qiang, S.; Yan, L.; Binghua, Y.; Qian, Z.; Dionysios, D.D. Fabrication of CQDs/Bi5Nb3O15 nanocomposites for photocatalytic degradation of veterinary pharmaceutical sarafloxacin. Catal. Today 2019, in press. [Google Scholar]
- Deivasigamani, P.; Premasis, S.; Marc, L.; Mohan, A.; Maheswari, S.Z.; Michael, S. Photolysis of difloxacin and sarafloxacin in aqueous systems. Chemosphere 2009, 77, 739–746. [Google Scholar]
- Souvik, K.; Deivasigamani, P.; Marc, L.; Michael, S. In vitro residual anti-bacterial activity of difloxacin, sarafloxacin and their photoproducts after photolysis in water. Environ. Pollut. 2009, 157, 2722–2730. [Google Scholar]
- Fu, H.; Li, X.B.; Wang, J.; Lin, P.F.; Chen, C.; Zhang, X.J.; Suffet, I.H. Activated carbon adsorption of quinolone antibiotics in water: Performance, mechanism, and modeling. J. Environ. Sci. 2017, 56, 145–152. [Google Scholar] [CrossRef]
- Hao, W.; Yating, S.; Xiaozhen, G.; Shuangli, Z.; Juanli, D.; Hongping, J.; Lina, H.; Liming, D. Determination and removal of sulfonamides and quinolones from environmental water samples using magnetic adsorbents. J. Sep. Sci. 2016, 39, 4398–4407. [Google Scholar]
- Feng, T.; Daming, S.; Jinsuo, G.; Qian, Z.; Xiaochun, W.; Fei, T.; Xie, Q.; Jingwen, C. Preparation of molecularly imprinted polymer nanoparticles for selective removal of fluoroquinolone antibiotics in aqueous solution. J. Hazard. Mater. 2013, 244, 750–757. [Google Scholar]
- Feng, T.; Min, L.; Suyu, R. Preparation of poly dopamine coated graphene oxide/Fe3O4 imprinted nanoparticles for selective removal of fluoroquinolone antibiotics in water. Sci. Rep. 2017, 7, 5735. [Google Scholar]
- Abdolraouf, S.; Mohsen, N. Removal of sarafloxacin from aqueous solution by a magnetized metal-organic framework; Artificial neural network modeling. Polyhedron 2020, 179, 114342. [Google Scholar]
- Xiangyu, G.; Chufan, K.; Hongliang, H.; Yanjiao, C.; Chongli, Z. Exploration of functional MOFs for efficient removal of fluoroquinolone antibiotics from water. Microporous Mesoporous Mater. 2019, 286, 84–91. [Google Scholar]
- Al-Saad, K.; El-Azazy, M.; Issa, A.A.; Al-Yafie, A.; El-Shafie, A.S.; Al-Sulaiti, M.; Shomar, B. Recycling of date pits into a green adsorbent for removal of heavy metals: A fractional factorial design-based approach. Front. Chem. 2019, 7, 552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Ashraf, A.; Issa, A.A. Eco-structured biosorptive removal of basic fuchsin using pistachio nutshells: A definitive screening design-based approach. Appl. Sci. 2019, 9, 4855. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; Dimassi, S.; El-Shafie, A.S.; Issa, A. Bio-waste Aloe vera leaves as an efficient adsorbent for titan yellow from wastewater: Structuring of a novel adsorbent using Plackett-Burman factorial design. Appl. Sci. 2019, 9, 4856. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; El-Shafie, A.S.; Issa, A.A.; Al-Sulaiti, M.; Al-Yafie, J.; Shomar, B.; Al-Saad, K. Potato peels as an adsorbent for heavy metals from aqueous solutions: Eco-structuring of a green adsorbent operating Plackett–Burman design. J. Chem. 2019, 2019, 4926240. [Google Scholar] [CrossRef] [Green Version]
- El-Azazy, M.; Kalla, R.N.; Issa, A.A.; Al-Sulaiti, M.; El-Shafie, A.S.; Shomar, B.; Al-Saad, K. Pomegranate peels as versatile adsorbents for water purification: Application of Box–Behnken design as a methodological optimization approach. Environ. Prog. Sustain. Energy. 2019, 38, 13223. [Google Scholar] [CrossRef]
- Hassan, S.S.; El-Shafie, A.S.; Zaher, N.; El-Azazy, M. Application of pineapple leaves as adsorbents for removal of rose bengal from wastewater: Process optimization operating face-centered central composite design (FCCCD). Molecules 2020, 25, 3752. [Google Scholar] [CrossRef]
- Jordan, B.N.; Francisco, H.M.L.; Erik, G.P.S.; Fermin, G.V. Evaluation of adsorption processes of metal ions in multi-element aqueous systems by lignocellulosic adsorbents applying different isotherms: A critical review. Chem. Eng. J. 2019, 357, 404–420. [Google Scholar]
- Al-Saad, K.; Issa, A.A.; Idoudi, S.; Shomar, B.; Al-Ghouti, M.A.; Al-Hashimi, N.; El-Azazy, M. Smart synthesis of trimethyl ethoxysilane (TMS) functionalized core–shell magnetic nanosorbents Fe3O4@SiO2: Process optimization and application for extraction of pesticides. Molecules 2020, 25, 4827. [Google Scholar] [CrossRef]
- Huijun, H.; Yongpan, L.; Shaohong, Y.; Jie, L.; He, X.; Zhihong, T. A Review on recent treatment technology for herbicide atrazine in contaminated environment. Int. J. Environ. Res. Public Health. 2019, 16, 5129. [Google Scholar]
- Yan, C.; Chunping, Y.; Huijun, H.; Guangming, Z.; Kun, Z.; Zhou, Y. Biosorption of Pb(II) ions from aqueous solutions by waste biomass from biotrickling filters: Kinetics, isotherms, and thermodynamics. J. Environ. Eng. 2015, 142, C4015001. [Google Scholar]
- Huijun, H.; Xiang, Z.; Chen, X.; Chen, H.; Huang, H.; Wen, M.; Yang, C. Biosorption of Cd(II) from synthetic wastewater using dry biofilms from biotrickling filters. Int. J. Environ. Sci. Technol. 2017, 15, 1491–1500. [Google Scholar]
- Kim, H.; Hwang, Y.S.; Sharma, V.K. Adsorption of antibiotics and iopromide onto single-walled and multiwalled carbon nanotubes. Chem. Eng. J. 2014, 255, 23–27. [Google Scholar] [CrossRef]
- Yang, W.; Lu, Y.; Zheng, F.; Xue, X.; Li, N.; Liu, D. Adsorption behavior and mechanisms of norfloxacin onto porous resins and carbon nanotube. Chem. Eng. J. 2012, 179, 112–118. [Google Scholar] [CrossRef]
- Seyed, D.A.; Hossein, K.; Jalil, J.; Amir, H.M. Experimental design and response surface modeling for optimization of fluoroquinolone removal from aqueous solution by NaOH-modified rice husk. Desalination Water Treat. 2016, 57, 16456–16465. [Google Scholar]
- Olivia, A.A.; Medhat, A.A.; Marianne, N.; Maissa, Y.S. Adsorptive removal of fluoroquinolones from water by pectin functionalized magnetic nanoparticles: Process optimization using a spectrofluorimetric assay. ACS Sustain. Chem. Eng. 2017, 5, 133–145. [Google Scholar]
- Elazazy, M.S. Factorial design and machine learning strategies: Impacts on pharmaceutical analysis. In Spectroscopic Analyses; Zafar, F., Ed.; IntechOpen: London, UK, 2017; Available online: https://www.intechopen.com/books/spectroscopic-analyses-developments-and-applications/factorial-design-and-machine-learning-strategies-impacts-on-pharmaceutical%20analysis (accessed on 10 September 2020).
- Elazazy, M.S.; El-Hamshary, M.; Sakr, M.; Al-Easa, H.S. Plackett-Burman and Box-Behnken designs as chemometric tools for micro-determination of L-Ornithine. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 193, 397–406. [Google Scholar] [CrossRef]
- Aly, H.; El-Shafie, A.S.; El-Azazy, M. Utilization of 7-chloro-4-nitrobenzo-2-oxa-1,3- diazole (NBD-Cl) for spectrochemical determination of L-ornithine: A multivariate optimization-assisted approach. RSC Adv. 2019, 9, 22106–22115. [Google Scholar] [CrossRef] [Green Version]
- Anderson, T.W.; Darling, D.A. A test of goodness of fit. J. Am. Stat. Assoc. 1954, 49, 765–769. [Google Scholar] [CrossRef]
- Box, G.E.P.; Cox, D.R. An analysis of transformations. J. R. Stat. Soc. B. 1964, 26, 211–252. [Google Scholar] [CrossRef]
- Derringer, G.; Suich, R. Simultaneous optimization of several response variables. J. Qual. Technol. 1980, 12, 214–219. [Google Scholar] [CrossRef]
- Hanbali, G.; Jodeh, S.; Hamed, O.; Bol, R.; Khalaf, B.; Qdemat, A.; Samhan, S. Enhanced ibuprofen adsorption and desorption on synthesized functionalized magnetic multiwall carbon nanotubes from aqueous solution. Materials 2020, 13, 3329. [Google Scholar] [CrossRef] [PubMed]
- Pathak, P.D.; Mandavgane, S.A.; Kulkarni, B.D. Characterizing fruit and vegetable peels as bioadsorbents. Curr. Sci. 2016, 110, 2114–2123. [Google Scholar] [CrossRef]
- Horn, M.M.; Martins, V.C.A.; Plepis, A.M.D. Interaction of anionic collagen with chitosan: Effect on thermal and morphological characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Ralph, J.; Lapierre, C.; Boerjan, W. Lignin structure and its engineering. Curr. Opin. Biotech. 2019, 56, 240–249. [Google Scholar] [CrossRef]
- Kacurakova, M.; Capek, P.; Sasinkova, V.; Wellner, N.; Ebringerova, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Huang, A.; Zhou, Q.; Liu, J.; Fei, B.; Sun, S. Distinction of three wood species by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2008, 883, 160–166. [Google Scholar] [CrossRef]
- Evans, P.A. Differentiating “hard” from “soft” woods using Fourier transform infrared and Fourier transform Raman spectroscopy. Spectrochim. Acta A Mol. Spectrosc. 1991, 47, 1441–1447. [Google Scholar] [CrossRef]
- Sills, D.L.; Gossett, J.M. Using FTIR to predict saccharification from enzymatic hydrolysis of alkali pretreated biomasses. Biotechnol. Bioeng. 2012, 109, 353–362. [Google Scholar] [CrossRef]
- Kurrey, R.; Mahilang, M.; Deb, M.K.; Nirmalkar, J.; Shrivas, K.; Pervez, S.; Rai, M.K.; Rai, J. A direct DRS-FTIR probe for rapid detection and quantification of fluoroquinolone antibiotics in poultry egg-yolk. Food Chem. 2019, 270, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, R.M.; Webster, F.X. Spectrometric Identification of Organic Compounds, 6th ed.; John Wiley and Sons: New York, NY, USA, 2002. [Google Scholar]
- Şentürk, I.; Alzein, M. Adsorptive removal of basic blue 41 using pistachio shell adsorbent—Performance in batch and column system. Sustain. Chem. Pharm. 2020, 16, 100254. [Google Scholar] [CrossRef]
- Ferro-García, M.A.; Rivera-Utrilla, J.; Bautista-Toledo, I.; Moreno-Castilla, C. Adsorption of humic substances on activated carbon from aqueous solutions and their effect on the removal of Cr (III) ions. Langmuir 1998, 14, 1880–1886. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Świątkowski, A. Removal of chlorophenols from aqueous solutions by sorption onto walnut, pistachio and hazelnut shells. Pol. J. Chem. Technol. 2015, 17, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Thommes, M.; Kaneko, K.; Neimark, A.V. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1052–1069. [Google Scholar] [CrossRef] [Green Version]
- Langmuir, I. Adsorption of gases on glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Wang, J. Comparison of linearization methods for modeling the Langmuir adsorption isotherm. J. Mol. Liq. 2019, 296, 111850. [Google Scholar] [CrossRef]
- Araújo, C.S.T.; Almeida, I.L.S.; Rezende, H.C.; Marcionilio, S.M.L.O.; Léon, J.J.L.; de Matos, T.N. Elucidation of mechanism involved in adsorption of Pb (II) onto Lobeira fruit (Solanum lycocarpum) using Langmuir, Freundlich and Temkin isotherms. Microchem. J. 2018, 137, 348–354. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium (VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
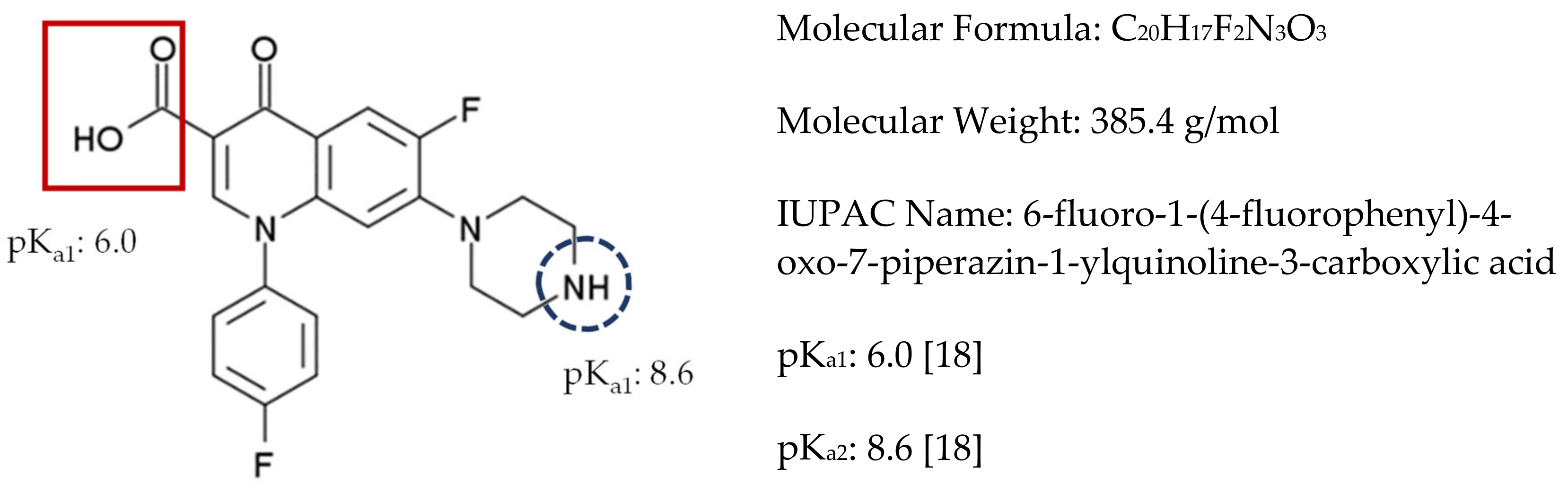



| Adsorbent | Analytical Approach Used | Kinetics Model | Isotherm Model | Surface Area (m2/g) | qe (mg/g) | %R | References |
|---|---|---|---|---|---|---|---|
| Raw pistachio nutshells (RPNS) [A] Thermally treated PNS at 500 °C (TTPNS500) [B] Air-dried Aloe vera leaves (ADAV) [C] Thermally treated AV leaves at 500 °C (TTAV500) [D] Multi-walled carbon nanotubes (MWCNTs) [E] | Plackett-Burman design (PBD) | PSO * | Freundlich | 4.24 [A] 76.94 [B] 3.94 [C] 7.56 [D] 482 [E] | 49.75 [A] | 82.39% [A] 96.20% [E] | Current approach |
| Powdered activated carbon (PAC) | Univariate analysis | PSO * | Freundlich | ND ** | 120 | 80–96% | [20] |
| Molecularly imprinted polymer nanoparticles (nanoMCN@MIPs) [A] Nonimprinted polymer nanoparticles NanoMCN@NIPs [B] Mesoporous carbon nanoparticles MCNs [C] | Univariate analysis | ND ** | ND ** | 126 [A] 102 [B] 622 [C] | ND ** | Deionized water “>90% [A] 30-40% [B and C]” Spiked seawater “>90% [A]” | [22] |
| Polydopamine-coated graphene oxide/Fe3O4 (PDA@ GO/Fe3O4) imprinted nanoparticles coupled with magnetic separation | Univariate analysis | PSO * | Langmuir | 50.34 | 70.9 | >95% | [23] |
| Magnetized metal-organic framework (MOF) (Fe3O4/MIL-101(Fe)) | Response surface methodology (RSM) | ND ** | Langmuir | 1624.91 | 81.31 | >93% | [24] |
| MIL-101(Cr)–SO3H a stable mesoporous MOF with polar –SO3H groups in the structure | Univariate analysis | ND ** | ND ** | ND ** | 898.2 | ND ** | [25] |
| Candidate Adsorbent | %R | qe (mg/g) |
|---|---|---|
| Upcycled AWs | ||
| RPNS | 63.45 | 7.614 |
| TTPNS500 | 7.871 | 0.944 |
| ADAV | 29.15 | 3.498 |
| TTAV500 | 2.101 | 0.252 |
| Factors | −1 | 0 | +1 |
|---|---|---|---|
| pH (A, pH unit) | 5.0 | 7.0 | 9.0 |
| Adsorbent Dose (AD, B, mg/15 mL) | 20.0 | 50.0 | 80.0 |
| Initial Drug Concentration ([SARA], C, ppm) | 10.0 | 25.0 | 40.0 |
| Contact Time (CT, D, min.) | 10.0 | 65.5 | 120.0 |
| Variables | RPNS | MWCNTs | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial No | pH | AD | [SARA] | CT | %R Obs. * | %R Pred. ** | RE *** | qe Obs. * | qe Pred. ** | RE *** | %R Obs. * | %R Pred. ** | RE *** | qe Obs. * | qe Pred. ** | RE *** |
| 01 | 9(+) | 20(-) | 10(-) | 10(-) | 26.70 | 24.89 | 0.07 | 1.15 | 1.09 | 0.05 | 38.73 | 39.66 | 0.02 | 2.05 | 2.05 | 0 |
| 02 | 9(+) | 20(-) | 40(+) | 10(-) | 21.75 | 23.66 | 0.08 | 4.22 | 4.02 | 0.05 | 33.55 | 32.48 | 0.03 | 6.47 | 6.53 | 0.01 |
| 03 | 5(-) | 20(-) | 10(-) | 120(+) | 58.04 | 56.67 | 0.02 | 3.60 | 3.47 | 0.04 | 96.20 | 97.19 | 0.01 | 6.59 | 6.46 | 0.02 |
| 04 | 7(0) | 50(0) | 25(0) | 65(0) | 47.29 | 47.09 | 0.004 | 2.36 | 2.29 | 0.03 | 73.97 | 70.5 | 0.05 | 3.60 | 3.59 | 0.00 |
| 05 | 9(+) | 80(+) | 40(+) | 10(-) | 41.48 | 40.69 | 0.02 | 2.10 | 2.03 | 0.03 | 37.16 | 36.61 | 0.01 | 1.65 | 1.64 | 0.01 |
| 06 | 5(-) | 80(+) | 10(-) | 10(-) | 75.51 | 79.25 | 0.05 | 1.08 | 1.00 | 0.08 | 87.69 | 87.79 | 0.00 | 1.10 | 1.12 | 0.02 |
| 07 | 9(+) | 80(+) | 10(-) | 120(+) | 46.22 | 44.15 | 0.05 | 0.67 | 0.60 | 0.12 | 61.36 | 59.43 | 0.03 | 0.77 | 0.75 | 0.03 |
| 08 | 5(-) | 80(+) | 40(+) | 10(-) | 79.00 | 77.04 | 0.03 | 3.99 | 3.83 | 0.04 | 82.26 | 83.13 | 0.01 | 3.64 | 3.55 | 0.03 |
| 09 | 7(0) | 50(0) | 25(0) | 65(0) | 49.12 | 47.09 | 0.04 | 2.45 | 2.29 | 0.07 | 71.94 | 70.5 | 0.02 | 3.60 | 3.59 | 0.00 |
| 10 | 9(+) | 80(+) | 10(-) | 120(+) | 40.60 | 44.15 | 0.08 | 0.65 | 0.60 | 0.08 | 58.97 | 59.43 | 0.01 | 0.74 | 0.75 | 0.01 |
| 11 | 5(-) | 20(-) | 40(+) | 120(+) | 51.74 | 54.81 | 0.06 | 7.92 | 8.18 | 0.03 | 94.77 | 92.78 | 0.02 | 19.95 | 20.52 | 0.03 |
| 12 | 7(0) | 50(0) | 25(0) | 65(0) | 48.35 | 47.09 | 0.03 | 2.20 | 2.29 | 0.04 | 68.75 | 70.5 | 0.02 | 3.61 | 3.59 | 0.01 |
| 13 | 7(0) | 50(0) | 25(0) | 65(0) | 45.19 | 47.09 | 0.04 | 2.10 | 2.29 | 0.08 | 70.40 | 70.5 | 0.00 | 3.57 | 3.59 | 0.01 |
| 14 | 5(-) | 20(-) | 10(-) | 10(-) | 55.50 | 54.57 | 0.02 | 2.33 | 2.45 | 0.05 | 85.75 | 85.18 | 0.01 | 4.49 | 4.45 | 0.01 |
| 15 | 7(0) | 50(0) | 25(0) | 65(0) | 46.84 | 47.09 | 0.01 | 2.19 | 2.29 | 0.04 | 68.34 | 70.5 | 0.03 | 3.51 | 3.59 | 0.02 |
| 16 | 7(0) | 50(0) | 25(0) | 65(0) | 49.72 | 47.09 | 0.05 | 2.21 | 2.29 | 0.03 | 70.76 | 70.5 | 0.00 | 3.54 | 3.59 | 0.01 |
| 17 | 7(0) | 50(0) | 25(0) | 65(0) | 45.78 | 47.09 | 0.03 | 2.18 | 2.29 | 0.05 | 72.13 | 70.5 | 0.02 | 3.61 | 3.59 | 0.06 |
| 18 | 7(0) | 50(0) | 25(0) | 65(0) | 44.59 | 47.09 | 0.05 | 2.19 | 2.29 | 0.04 | 67.59 | 70.5 | 0.04 | 3.68 | 3.59 | 0.03 |
| 19 | 5(-) | 80(+) | 40(+) | 120(+) | 82.39 | 79.53 | 0.03 | 4.93 | 5.11 | 0.03 | 94.67 | 95.27 | 0.01 | 5.14 | 5.16 | 0.00 |
| 20 | 9(+) | 20(-) | 40(+) | 120(+) | 25.85 | 25.05 | 0.03 | 5.17 | 5.32 | 0.03 | 48.21 | 50.34 | 0.04 | 9.64 | 9.50 | 0.01 |
| RPNS | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Response | %R | qe | ||||||||
| Source | DF * | Adj SS * | Adj MS * | F-Value | P-Value | DF * | Adj SS * | Adj MS * | F-Value | P-Value |
| Model | 2 | 24.1383 | 12.0692 | 345.40 | 0.000 | 6 | 3.65834 | 0.60972 | 5342.19 | 0.000 |
| Blocks | 1 | 0.01266 | 0.01266 | 110.94 | 0.000 | |||||
| Linear | 2 | 24.1383 | 12.0692 | 345.40 | 0.000 | 4 | 2.77354 | 0.69339 | 6075.22 | 0.000 |
| pH | 1 | 17.2545 | 17.2545 | 493.80 | 0.000 | 1 | 0.58908 | 0.58908 | 5161.32 | 0.000 |
| AD | 1 | 6.8838 | 6.8838 | 197.01 | 0.000 | 1 | 0.69691 | 0.69691 | 6106.10 | 0.000 |
| [SARA] | 1 | 1.95224 | 1.95224 | 17104.89 | 0.000 | |||||
| CT | 1 | 0.18325 | 0.18325 | 1605.59 | 0.000 | |||||
| Error | 17 | 0.0398 | 0.0398 | 1.15 | 0.300 | 13 | 0.04748 | 0.04748 | 416.02 | 0.000 |
| Curvature | 1 | 0.5940 | 0.0349 | 1 | 0.00148 | 0.00011 | ||||
| Lack-of-Fit | 8 | 0.3342 | 0.0418 | 1.52 | 0.284 | 6 | 0.00037 | 0.00006 | 0.38 | 0.868 |
| Pure Error | 8 | 0.2200 | 0.0275 | 7 | 0.00112 | 0.00016 | ||||
| Total | 19 | 24.7323 | 19 | 3.65982 | ||||||
| MWCNTs | ||||||||||
| Response | %R | qe | ||||||||
| Source | DF * | Adj SS * | Adj MS * | F-Value | P-Value | DF * | Adj SS * | Adj MS * | F-Value | P-Value |
| Model | 5 | 968610 | 193722 | 347.94 | 0.000 | 5 | 11.9604 | 2.39209 | 7091.84 | 0.000 |
| Linear | 4 | 968590 | 242147 | 434.91 | 0.000 | 4 | 11.9160 | 2.97899 | 8831.84 | 0.000 |
| pH | 1 | 863285 | 863285 | 1550.52 | 0.000 | 1 | 1.7798 | 1.77984 | 5276.69 | 0.000 |
| AD | 1 | 3976 | 3976 | 7.14 | 0.018 | 1 | 5.7161 | 5.71612 | 16946.62 | 0.000 |
| [SARA] | 1 | 12530 | 12530 | 22.51 | 0.000 | 1 | 4.0004 | 4.00044 | 11860.15 | 0.000 |
| CT | 1 | 88799 | 88799 | 159.49 | 0.000 | 1 | 0.4196 | 0.41957 | 1243.89 | 0.000 |
| Curvature | 1 | 20 | 20 | 0.04 | 0.852 | 1 | 0.0445 | 0.04448 | 131.86 | 0.000 |
| Error | 14 | 7795 | 557 | 14 | 0.0047 | 0.00034 | ||||
| Lack-of-Fit | 6 | 2173 | 362 | 0.52 | 0.783 | 6 | 0.0026 | 0.00043 | 1.60 | 0.262 |
| Pure Error | 8 | 5622 | 703 | 8 | 0.0021 | 0.00027 | ||||
| Total | 19 | 976405 | 19 | 11.9652 | ||||||
| Parameters | RPNS | TTPNS500 | ADAV | TTAV500 | MWCNTs |
|---|---|---|---|---|---|
| Raman ID: IG Ratio | 0.61 | 0.65 | 0.24 | 0.67 | 0.11 |
| Langmuir surface area (SA) (m2/g) | 4.24 | 76.94 | 3.94 | 7.56 | 482.01 |
| Total pore volume (cm3/g) | 0.0082 | 0.0072 | 0.0112 | 0.0261 | 1.0778 |
| Average pore radius (°A) | 61.7 | 89.8 | 98.6 | 80.8 | 52.8 |
| Isotherm | Equations (Generalized/ Linearized Forms) | Parameters | Value | |
|---|---|---|---|---|
| Langmuir | (mg/g) | 49.75 | ||
| (L·mole−1) | 0.0314 | |||
| R2 | 0.975 | |||
| Freundlich | 0.651 | |||
| (mole/g) (L/mole)1/n | 2.509 | |||
| R2 | 0.9785 | |||
| Temkin | (J/mole) | 250.0 | 45.54 | |
| (L/mole) | 0.389 | 0.025 | ||
| R2 | 0.964 | 0.984 | ||
| DR | 1.7 × 10−8 | 1.98 × 10−9 | ||
| (kJ/mole) | 5.423 | 15.891 | ||
| (mg/g) | 48.04 | 6.23 | ||
| R2 | 0.984 | |||
| Models | Parameter | Value | |
|---|---|---|---|
| Pseudo-first order (PFO) | K1 (min−1) | 0.021 | |
| qe (mg/g) | 8.868 | ||
| R2 | 0.889 | ||
| Pseudo-second order (PSO) Where K2 is rate constant (g·mg−1·min−1) | K2 (g·mg−1·min−1) | 0.162 | |
| qe(mg/g) | 13.94 | ||
| R2 | 0.999 | ||
| Elovich equation is is used to predict the sorption mechanism, where qt is adsorbed quantity at time t; while α and β are initial sorption concentration rate (mg·g−1·min−1), and desorption constant (g/mg), respectively. | Α | 7.1 × 103 | |
| Β | 0.392 | ||
| R2 | 0.978 | ||
| Weber-Morris intraparticle diffusion model is used to study the formed layers around the adsorbent and rate-controlling step, which is expressed as , where KI is intraparticle diffusion rate constant (mg·g−1·min−0.5), and C is the boundary thickness effect. | KI | 2.19 | 0.55 |
| C | 19.02 | 26.16 | |
| R2 | 0.934 | 0.898 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Azazy, M.; El-Shafie, A.S.; Elgendy, A.; Issa, A.A.; Al-Meer, S.; Al-Saad, K.A. A Comparison between Different Agro-wastes and Carbon Nanotubes for Removal of Sarafloxacin from Wastewater: Kinetics and Equilibrium Studies. Molecules 2020, 25, 5429. https://doi.org/10.3390/molecules25225429
El-Azazy M, El-Shafie AS, Elgendy A, Issa AA, Al-Meer S, Al-Saad KA. A Comparison between Different Agro-wastes and Carbon Nanotubes for Removal of Sarafloxacin from Wastewater: Kinetics and Equilibrium Studies. Molecules. 2020; 25(22):5429. https://doi.org/10.3390/molecules25225429
Chicago/Turabian StyleEl-Azazy, Marwa, Ahmed S. El-Shafie, Ahmed Elgendy, Ahmed A. Issa, Saeed Al-Meer, and Khalid A. Al-Saad. 2020. "A Comparison between Different Agro-wastes and Carbon Nanotubes for Removal of Sarafloxacin from Wastewater: Kinetics and Equilibrium Studies" Molecules 25, no. 22: 5429. https://doi.org/10.3390/molecules25225429





