The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes
Abstract
:1. Introduction
2. Literature Review Sections
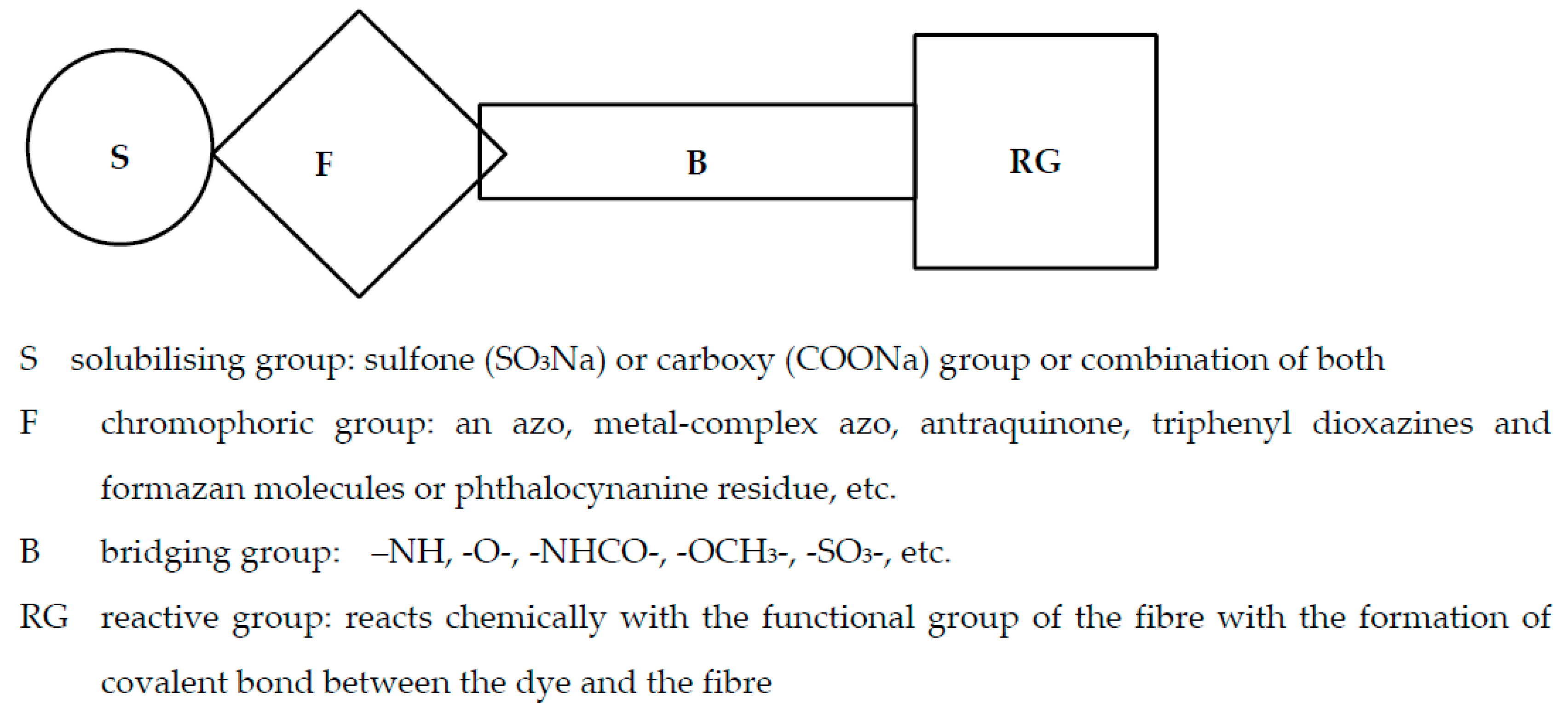
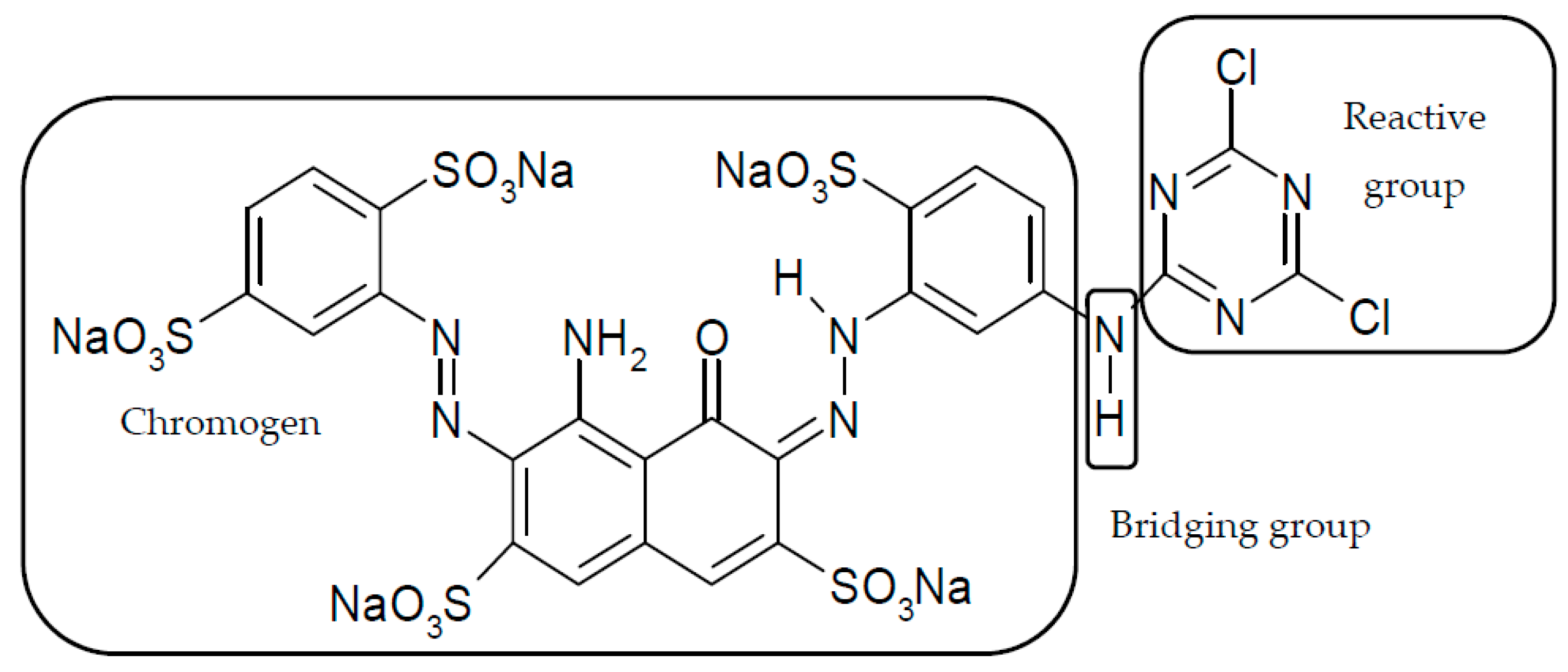
2.1. Reactive Dyes in the Textile Industry—An Overview
- MCT/VS bi-functional dyes—these are reactive dyes containing a monochlorotriazinyl group and a vinyl sulfone group, and they are useful for the dyeing of cellulose fibers.
- VS dyes—these are reactive dyes containing a vinyl sulfone group, and they are useful for the dyeing of cellulose fibers.
- MCT/MCT bi-functional dyes—these are reactive dyes containing two monochlorotriazinyl groups and characterized by high fixation in dyeing polyester/cotton blends.
- MCT dyes—these are reactive dyes containing a monochlorotriazinyl group.
- DCT dyes—these are reactive dyes containing a dichloro triazinyl group.
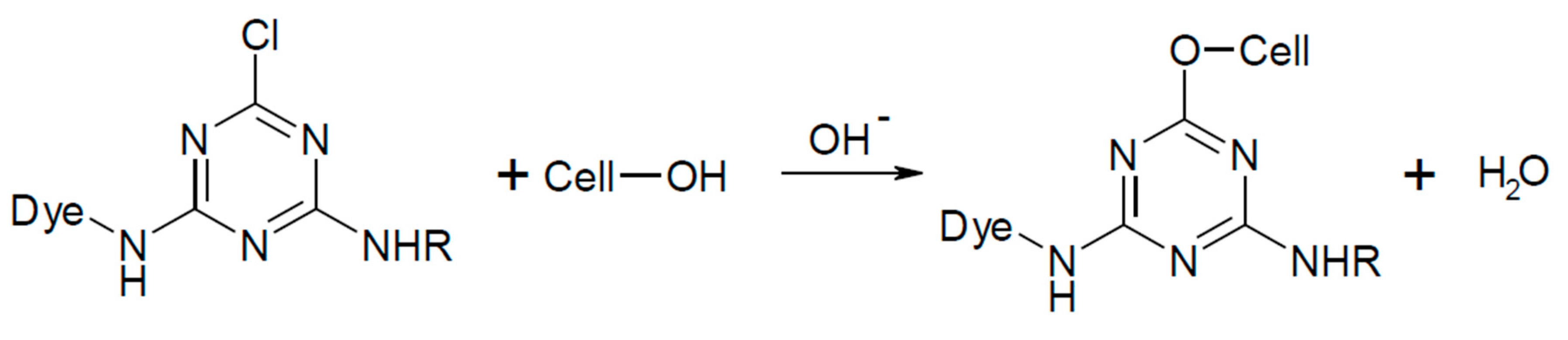
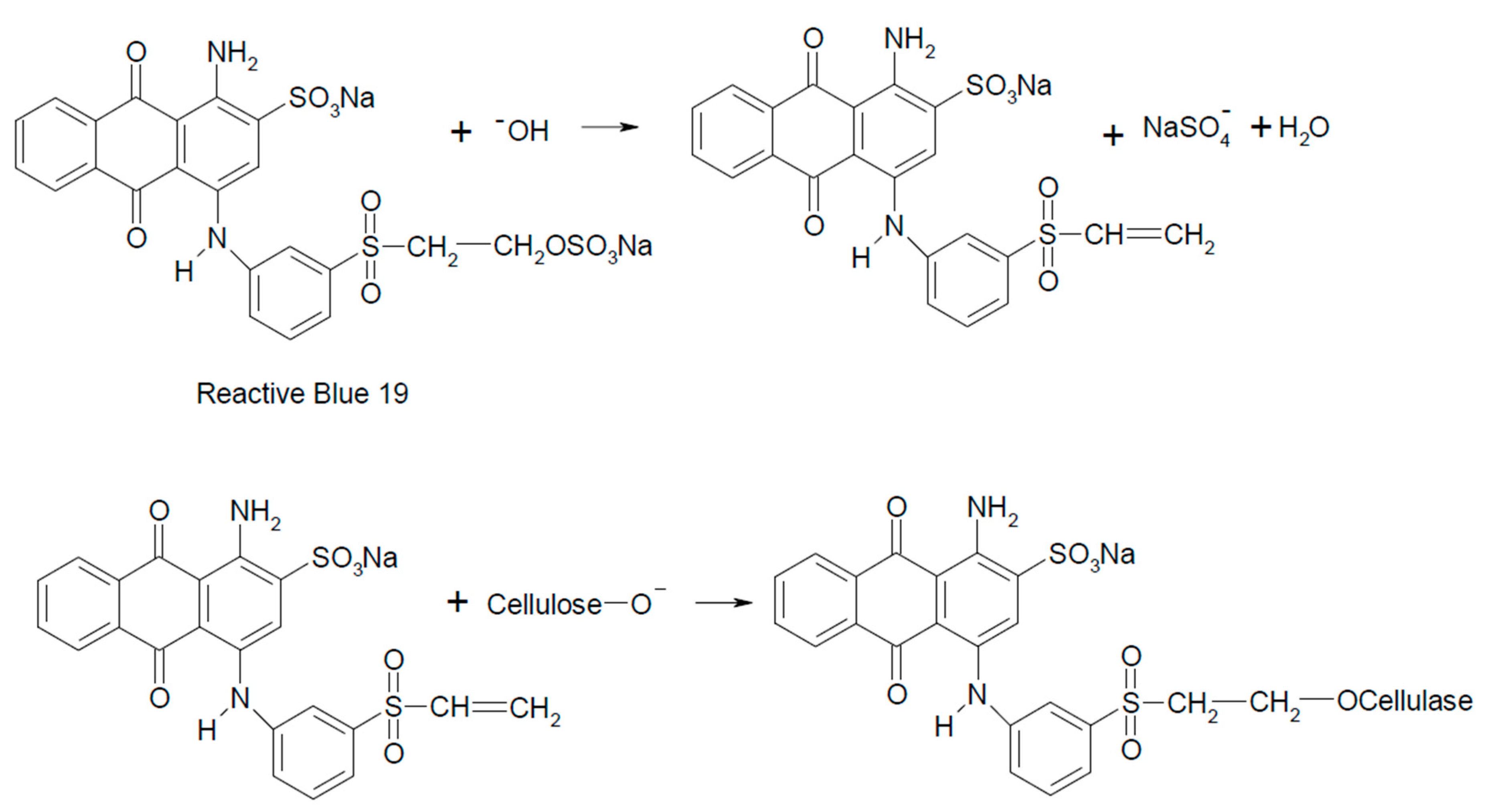
The Formation of a Reactive Dye–Fiber Bond
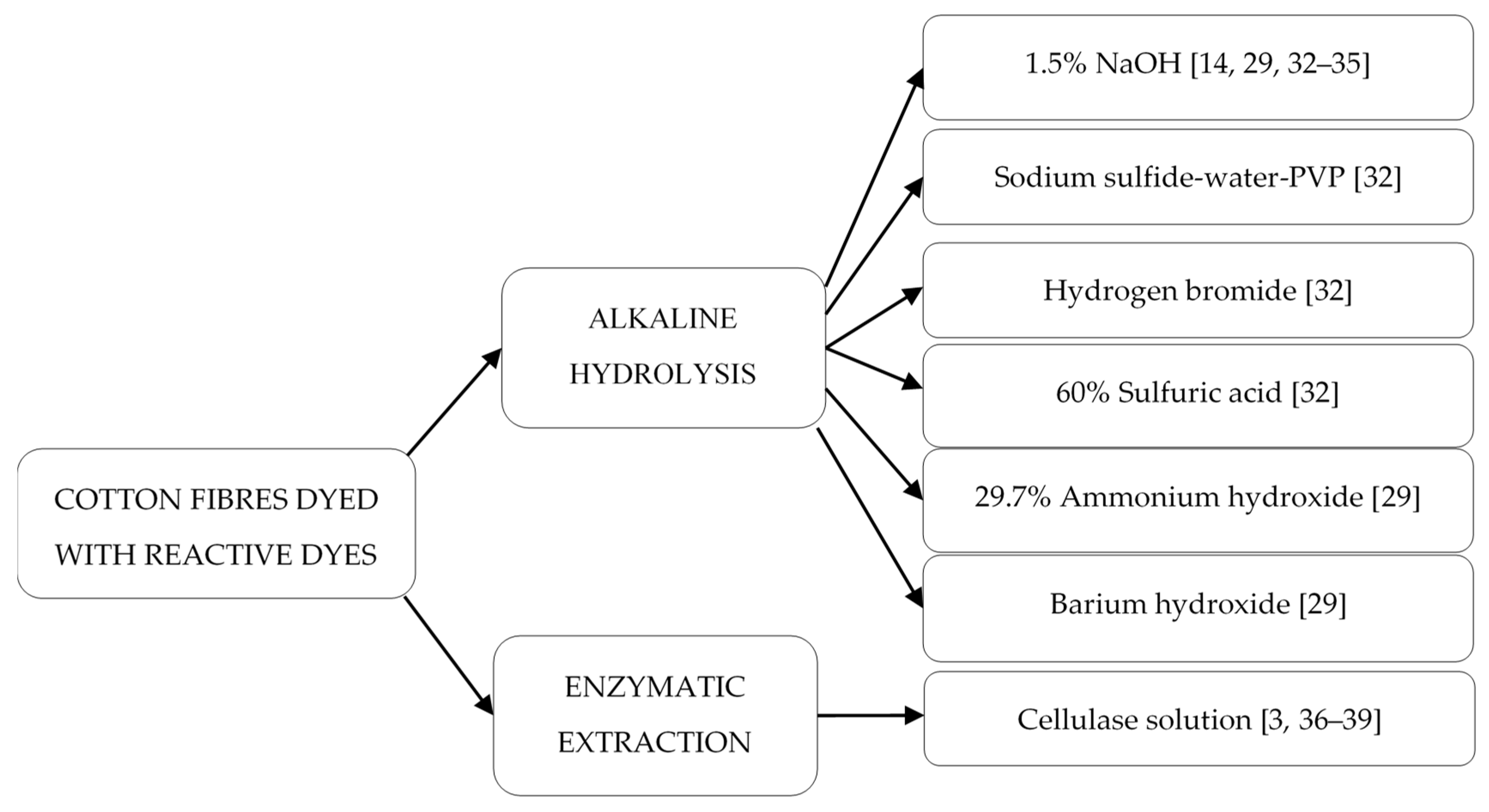
2.2. Extraction of Reactive Dyes from Dyed Textile Fibers for Forensic Purposes
2.2.1. Cleavage of Reactive Dyes from Dyed Cotton Textiles
2.2.2. Extraction of Reactive Dyes from Dyed Cotton Fibers
2.2.3. Extraction of Unknown Reactive Dyes from Dyed Cotton and Viscose
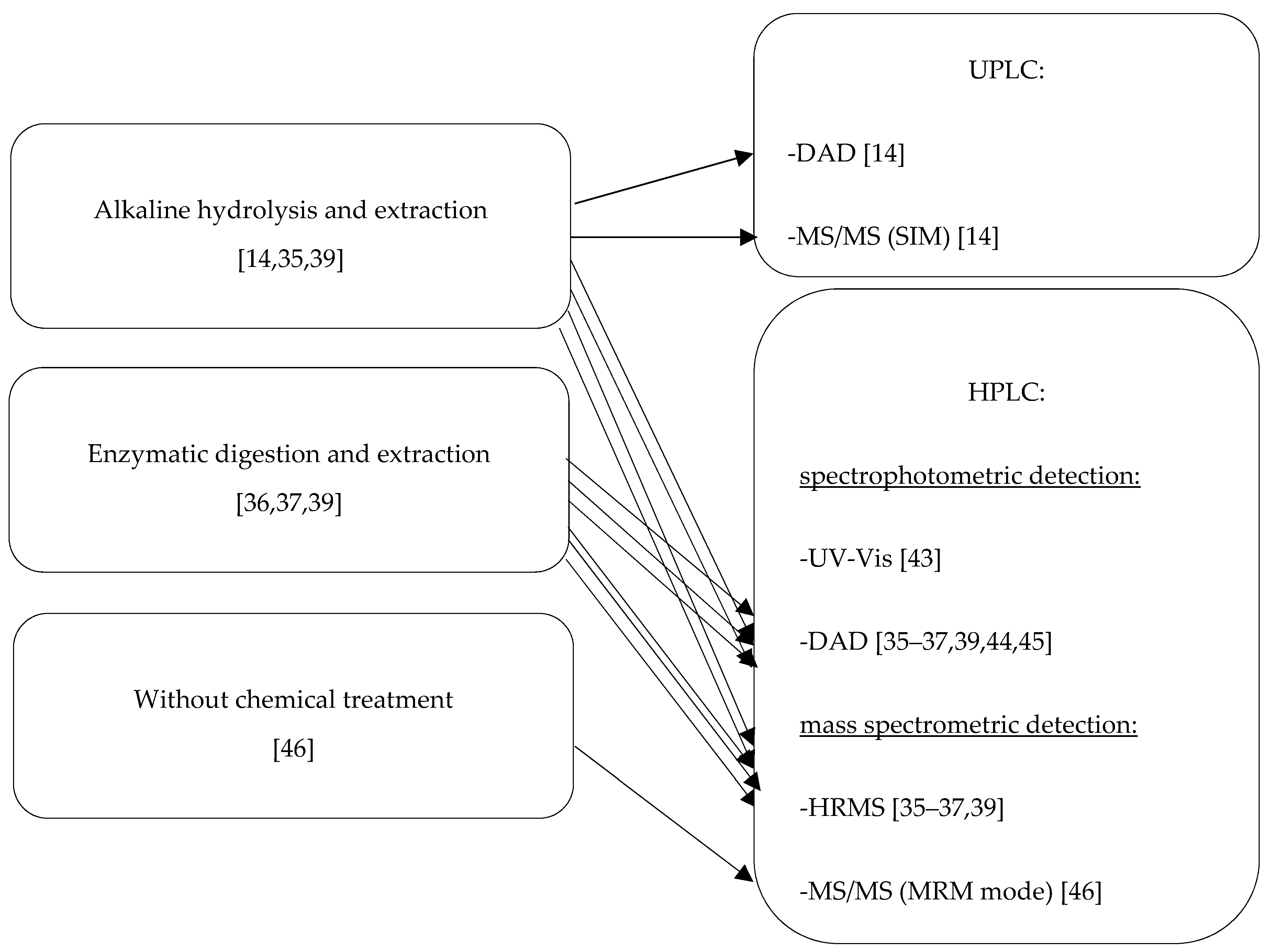
2.3. Identification of Dyed Cotton Fibers for Forensic Purposes Based on Chromatographic Techniques
2.3.1. Chromatographic Conditions
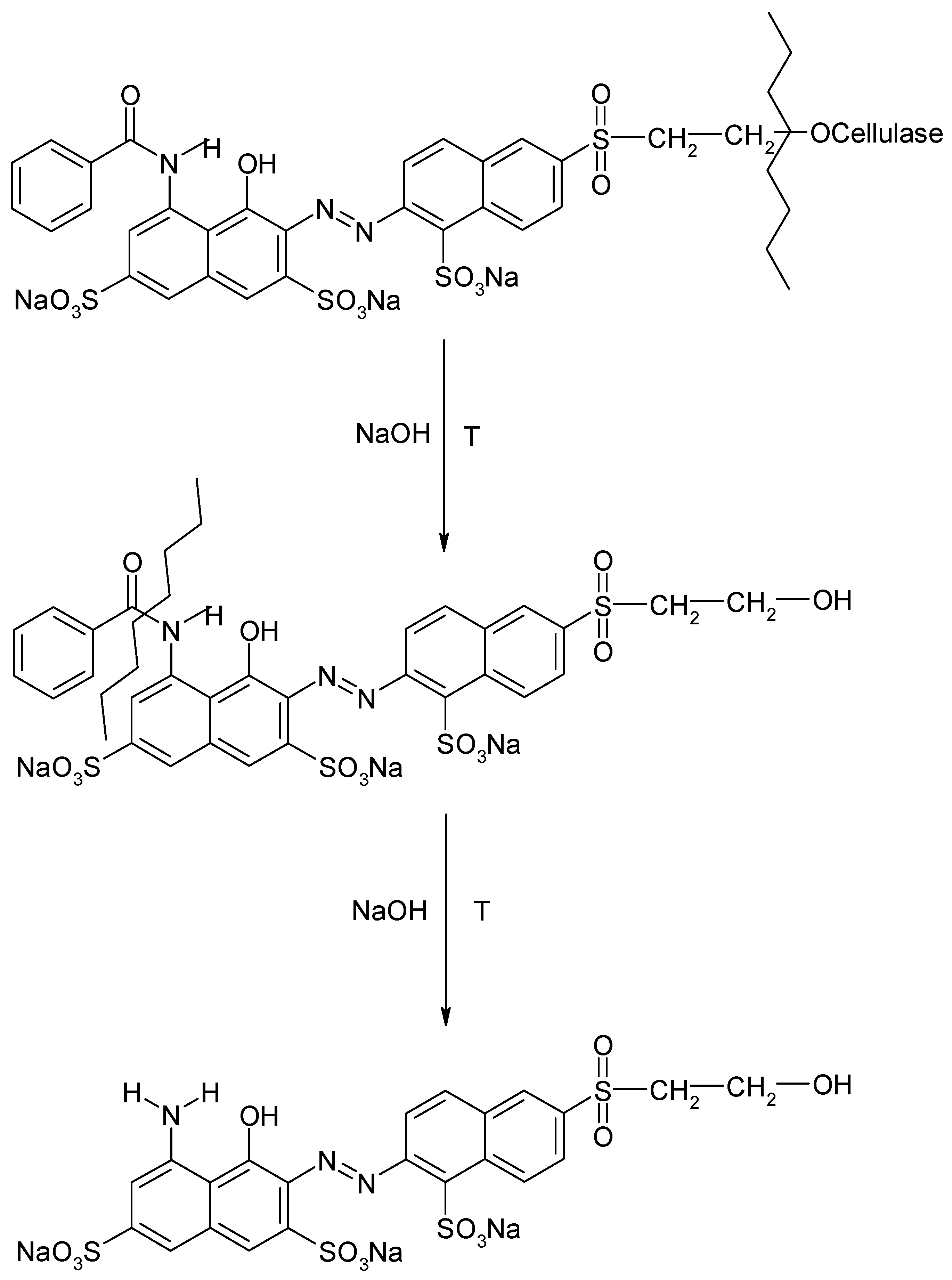
2.3.2. Qualification and Quantification Analysis of Extracted Reactive Dyes for Forensic Purposes
Synthesis of Hydrolyzed and/or Enzymatic Digestion Dye Standards
Identification and Quantification of Extracted Reactive Dyes for Forensic Purposes
2.4. Examination of Dyed Textile Fibers for Forensic Purposes Based on Routinely Used Spectroscopic Techniques
3. Comparison of Chromatographic Methods for the Identification of Dyed Textile Fibers for Forensic Purposes with a Spectroscopic Technique
4. New Challenges in Identifying Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Reichard, E.J.; Bartick, E.G.; Morgan, S.L.; Goodpaster, J.V. Microspectrophotometric analysis of yellow polyester fiber dye loadings with chemometric techniques. Forensic Chem. 2017, 3, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Lepot, L.; De Wael, K.; Gason, F.; Gilbert, B.; Eppe, G.; Malherbe, C. Discrimination of textile dyes in binary mixtures by Raman spectroscopy. J. Raman Spectrosc. 2020, 51, 717–730. [Google Scholar] [CrossRef]
- Góra, P.; Wąs-Gubała, J. Enzymatic extraction of dyes for differentiation of red cotton fibres by TLC coupled with VSC. Sci. Justice 2019, 59, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Grieve, M.; Roux, C.; Wiggins, K.; Champot, C.; Taroni, F. Interpretation of Fibre Evidence Fibres. In Forensic Examination of Fibres, 3rd ed.; Robertson, J., Roux, C., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 345–425. ISBN 9781439828649. [Google Scholar]
- Athey, S.N.; Adams, J.K.; Erdle, L.M.; Jantunen, L.M.; Helm, P.A.; Finkelstein, S.A.; Diamond, M.L. The Widespread Environmental Footprint of Indigo Denim Microfibers from Blue Jeans. Environ. Sci. Technol. Lett. 2020. [Google Scholar] [CrossRef]
- Smigiel-Kaminska, D.; Pospiech, J.; Makowska, J.; Stepnowski, P.; Was-Gubała, J.; Kumirska, J. The identification of polyester fibers dyed with disperse dyes for forensic purposes. Molecules 2019, 24, 613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, N.S.E.; El-Shishtawy, R.M. The use of new technologies in coloration of textile fibers. J. Mater. Sci. 2010, 45, 1143–1153. [Google Scholar] [CrossRef]
- Benghaya, S.; Mrabet, S.; Elharfi, A. A Review on Classifications, Recent Synthesis and Applications of Textile Dyes. Inorg. Chem. Rev. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Ferreira, E.S.B.; Hulme, A.N.; McNab, H.; Quye, A. The natural constituents of historical textile dyes. Chem. Soc. Rev. 2004, 33, 329–336. [Google Scholar] [CrossRef]
- Smith, M.; Thompson, K.; Lennard, F. A literature review of analytical techniques for materials characterisation of painted textiles—Part 2: Spectroscopic and chromatographic analytical instrumentation. J. Inst. Conserv. 2017, 40, 252–266. [Google Scholar] [CrossRef]
- Lewis, D.M. Developments in the chemistry of reactive dyes and their application processes. Coloration Technol. 2014, 130, 382–412. [Google Scholar] [CrossRef]
- Blaus, K. Reactive Dyes for Cellulose Fibers. XXX Semianarium Polskich Kolorystów. 2014, pp. 63–87. Available online: https://pdfslide.net/documents/xxx-seminarium-polskich-kolorystow.html (accessed on 27 October 2020).
- Hoy, S.J. Development and Figures of Merit of Microextraction and Ultra-Performance Liquid Chromatography for Forensic Characterization of Dye Profiles on Trace Acrylic, Nylon, Polyester, and Cotton Textile Fibers. Ph.D. Thesis, University of South Carolina, Portland, OR, USA, 2013. [Google Scholar]
- Mahapatra, N.N. Rective Dyes in Textile Dyes; Woodhead Publishing India Pvt. Ltd.: Delhi, India, 2016; pp. 175–197. ISBN 9789385059049. [Google Scholar]
- Wiggins, K.G. Fibre Dyes Analysis. In Forensic Examination of Fibres, 3rd ed.; Robertson, J., Roux, C., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 227, 239–241. ISBN 9781439828649. [Google Scholar]
- Chakraborty, J.N. Dyeing with reactive dye. In Fundamentals and Practices in Colouration of Textiles; Woodhead Publishing India Pvt. Ltd.: Delhi, India, 2010; pp. 57–75. ISBN 9789380308463. [Google Scholar]
- Lewis, D.M. The chemistry of reactive dyes and their application process. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 303–364. ISBN 9780081016510. [Google Scholar]
- Shang, S.M. Process Control in dyeing od textiles. In Process Control in Textile Manufacturing; Majumdar, A., Das, A., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 300–338. ISBN 9780857090270. [Google Scholar]
- Chattopadhyay, D.P. Chemistry of dyeing. In Handbook of Textile and Industrial Dyeing; Clark, M., Ed.; Woodhead Publishing: Cambridge, UK, 2011; pp. 150–183. ISBN 9781845696955. [Google Scholar]
- Zollinger, H. Reactive Azo Dyes. In Color Chemistry: Synthesis, Properties and Application of Organic Dyes and Pigments, 3rd ed.; Verlag Helvetica Acta, Willej-VCH: Weinheim, Germany, 2003; pp. 225–240. ISBN 9783906390239. [Google Scholar]
- Pal, P. Treatment Technology for Textile Water Treatment: Case Studies. In Industrial Water Treatment Process Technology; Butterworth—Heinemann: Cambridge, UK, 2017; pp. 243–511. ISBN 9780128103920. [Google Scholar]
- Khatri, A.; Peerzada, M.H.; Mohsin, M.; White, M. A review on developments in dyeing cotton fabrics with reactive dyes for reducing effluent pollution. J. Clean. Prod. 2015, 87, 50–57. [Google Scholar] [CrossRef]
- El Harfi, S.; El Harfi, A. Classifications, properties and applications of textile dye: A review. Appl. J. Environ. Eng. Sci. 2017, 3, 311–320. [Google Scholar] [CrossRef] [Green Version]
- Soleimani-Gorgani, A.; Karami, Z. The effect of biodegradable organic acids on the improvement of cotton ink-jet printing and antibacterial activity. Fibers Polym. 2016, 17, 512–520. [Google Scholar] [CrossRef]
- Cantrell, S.; Roux, C.; Maynard, P.; Robertson, J. A textile fibre survey as an aid to the interpretation of fibre evidence in the Sydney region. Forensic Sci. Int. 2001, 123, 48–53. [Google Scholar] [CrossRef]
- Watt, R.; Roux, J.; Robertson, J. The population of coloured textile fibers in domestic washing machine. Sci. Justice 2005, 45, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Grieve, M.C.; Biermann, T.W. The population of coloured textile fibres on outdoor surfaces. Sci. Justice 1997, 37, 231–239. [Google Scholar] [CrossRef]
- Dockery, C.R.; Stefan, A.R.; Nieuwland, A.A.; Roberson, S.N.; Baguley, B.M.; Hendrix, J.E.; Morgan, S.L. Automated extraction of direct, reactive, and vat dyes from cellulosic fibers for forensic analysis by capillary electrophoresis. Anal. Bioanal. Chem. 2009, 394, 2095–2103. [Google Scholar] [CrossRef]
- Morgan, S.L. Validation of Forensic Characterization and Chemical Identification of Dyes Extracted from Milimeter-length Fibers. In Final Report; University of South Carolina: Columbia, SC, USA, 2015. [Google Scholar]
- Božič, M.; Kokol, V. Ecological alternatives to the reduction and oxidation processes in dyeing with vat and sulphur dyes. Dyes Pigm. 2008, 76, 299–309. [Google Scholar] [CrossRef]
- Home, J.M.; Dudley, R.J. Thin-layer chromatography of dyes extracted from cellulosic fibres. Forensic Sci. Int. 1981, 17, 71–78. [Google Scholar] [CrossRef]
- Sirén, H.; Sulkava, R. Determination of black dyes from cotton and wool fibres by capillary zone electrophoresis with UV detection: Application of marker technique. J. Chromatogr. A 1995, 717, 149–155. [Google Scholar] [CrossRef]
- Xu, X.; Leijenhorst, H.A.L.; Van Den Hoven, P.; De Koeijer, J.A.; Logtenberg, H. Analysis of single textile fibres by sample-induced isotachophoresis—Micellar electrokinetic capillary chromatography. Sci. Justice 2001, 41, 93–105. [Google Scholar] [CrossRef]
- Sultana, N.; Williams, K.; Ankeny, M.; Vinueza, N.R. Degradation studies of CI Reactive Blue 19 on biodegraded cellulosic fabrics via liquid chromatography-photodiode array detection coupled to high resolution mass spectrometry. Coloration Technol. 2019, 135, 475–483. [Google Scholar] [CrossRef]
- Carey, A.; Rodewijk, N.; Xu, X.; Van Der Weerd, J. Identification of dyes on single textile fibers by HPLC-DAD-MS. Anal. Chem. 2013, 85, 11335–11343. [Google Scholar] [CrossRef] [PubMed]
- Schotman, T.G.; Xu, X.; Rodewijk, N.; van der Weerd, J. Application of dye analysis in forensic fibre and textile examination: Case examples. Forensic Sci. Int. 2017, 278, 338–350. [Google Scholar] [CrossRef]
- Bundeskriminalamt. Collective Work, European Fibres Group, Best Practice Guidelines—Thin Layer Chromatography; Bundeskriminalamt: Wiesbaden, Germany, 2001.
- Feng, C.; Sultana, N.; Sui, X.; Chen, Y.; Brooks, E.; Ankeny, M.A.; Vinueza, N.R. High-resolution mass spectrometry analysis of reactive dye derivatives removed from biodegraded dyed cotton by chemical and enzymatic methods. AATCC J. Res. 2020, 7, 9–18. [Google Scholar] [CrossRef]
- Laing, D.K.; Dudley, R.J.; Hartshorne, A.W.; Home, J.M.; Rickard, R.A.; Bennett, D.C. The extraction and classification of dyes from cotton and viscose fibres. Forensic Sci. 1991, 50, 23–35. [Google Scholar] [CrossRef]
- Lewis, S.W. Chapter 11: Analysis of dyes using chromatography. In Identification of Textile Fibres; Houck, M., Ed.; Woodhead Publishing: Cambridge, UK, 2009; pp. 203–223. ISBN 9781845692667. [Google Scholar]
- Dorrien, D.M. Discrimination of Automobile Carpet Fibers Using Various Analytical Techniques and the Subsequent Creation of a Comprehensive Database. Ph.D. Thesis, University of Central Florida, Orlando, FL, USA, 2006. [Google Scholar]
- Zotou, A.; Eleftheriadis, I.; Heli, M.; Pegiadou, S. Ion Ion-pair high performance liquid chromatographic study of the hydrolysis behaviour of reactive fluorotriazinic dyes. Dyes Pigm. 2002, 53, 267–275. [Google Scholar] [CrossRef]
- Chemchame, Y.; Popikov, I.V.; Soufiaoui, M. Study of analytical methods for quantifying unfixed form of bifunctional reactive dyes used in dyeing cellulosic fibers (cotton). Fibers Polym. 2010, 11, 565–571. [Google Scholar] [CrossRef]
- Chemchame, Y.; Popikov, I.V.; Soufiaoui, M. Study on analytical methods for quantifying the non-adsorbed reactive dye forms in an exhausted dyebath. Coloration Technol. 2012, 128, 169–175. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, J.; Mei, H.; Shi, H.; Guo, H.; Zhang, G.; Wang, P.; Lu, L.; Zheng, X. A sensitive HPLC-MS/MS method for the analysis of fiber dyes. Forensic Chem. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Nayar, S.B.; Freeman, H.S. Hydrolyzed reactive dyes. Part 1: Analyses via fast atom bombardment and electrospray mass spectrometry. Dyes Pigm. 2008, 79, 89–100. [Google Scholar] [CrossRef]
- Chalmers, J.; Edwards, H.; Hargreaves, M. Infrared and Raman Spectroscopy in Forensic Science; John Wiley and Sons: Hoboken, NJ, USA, 2012; ISBN 9780470749067. [Google Scholar]
- Goodpaster, J.V.; Liszewski, E.A. Forensic analysis of dyed textile fibers. Anal. Bioanal. Chem. 2009, 394, 2009–2018. [Google Scholar] [CrossRef] [PubMed]
- Was-Gubala, J.; Starczak, R. UV-Vis microspectrophotometry as a method of differentiation between cotton fibre evidence coloured with reactive dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 142, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Was-Gubala, J.; Starczak, R. Nondestructive identification of dye mixtures in polyester and cotton fibers using Raman spectroscopy and ultraviolet-visible (UV-Vis) microspectrophotometry. Appl. Spectrosc. 2015, 69, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Buzzini, P.; Massonnet, G. Discrimination of Colored Acrylic, Cotton, and Wool Textile Fibers Using Micro-Raman Spectroscopy. Part 1: In situ Detection and Characterization of Dyes. J. Forensic Sci. 2013, 58, 1593–1600. [Google Scholar] [CrossRef]
- Buzzini, P.; Massonnet, G. The analysis of colored acrylic, cotton, and wool textile fibers using micro-Raman spectroscopy. Part 2: Comparison with the traditional methods of fiber examination. J. Forensic Sci. 2015, 60, 712–720. [Google Scholar] [CrossRef]
- Was-Gubala, J.; Machnowski, W. Application of Raman spectroscopy for differentiation among cotton and viscose fibers dyed with several dye classes. Spectrosc. Lett. 2014, 47, 527–535. [Google Scholar] [CrossRef] [Green Version]
- Puchowicz, D.; Giesz, P.; Kozanecki, M.; Cieślak, M. Surface-enhanced Raman spectroscopy (SERS) in cotton fabrics analysis. Talanta 2019, 195, 516–524. [Google Scholar] [CrossRef]
- Zaffiono, C.; Bruni, S.; Guglielmi, V.; De Luca, E. Fourier-transform surface-enhanced Raman spectroscopy (FT-SERS) applied to the identification of natural dyes in textile fibers: An extractionless approach to the analysis. J. Raman Spectrosc. 2014, 45, 211–218. [Google Scholar] [CrossRef]
- Degano, I.; Ribechini, E.; Modugno, F.; Colombini, M.P. Analytical Methods for the Characterization of Organic Dyes in Artworks and in Historical Textiles. Appl. Spectrosc. Rev. 2009, 44, 363–410. [Google Scholar] [CrossRef]
- Casadio, F.; Leona, M.; Lombardi, J.R.; Van Duyne, R. Identification of Organic Colorants in Fibers, Paints, and Glazes by Surface Enhanced Raman Spectroscopy. Acc. Chem. Res. 2010, 43, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, F.; Porcinai, S.; Lombardi, J.R.; Leona, M. Statistical methods and library search approaches for fast and reliable identification of dyes using surface-enhanced Raman spectroscopy (SERS). Anal. Meth. 2013, 5, 4201–4212. [Google Scholar] [CrossRef]
- Sciutto, G.; Prati, S.; Bonacini, I.; Litti, L.; Meneghetti, M.; Mazzeo, R. A new integrated TLC/MU-ATR/SERS advanced approach for the identification of trace amounts of dyes in mixtures. Anal. Chim. Acta 2017, 991, 104–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirkbride, K.P. Infrared microspectroscopy of fibres. In Forensic Examination of Fibres, 3rd ed.; Robertson, J., Roux, C., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 245–289. ISBN 9781439828649. [Google Scholar]
- Grieve, M.C.; Griffin, R.M.E.; Malone, R. An assessment of the value of blue, red, and black cotton fibers as target fibers in forensic science investigations. Sci. Justice 1998, 38, 27–37. [Google Scholar] [CrossRef]
- Kokot, S.; Crawford, K.; Rintoul, L.; Meyer, U. A DRIFTS study of reactive dye states on cotton fabric. Vib. Spectrosc. 1997, 15, 103–111. [Google Scholar] [CrossRef]
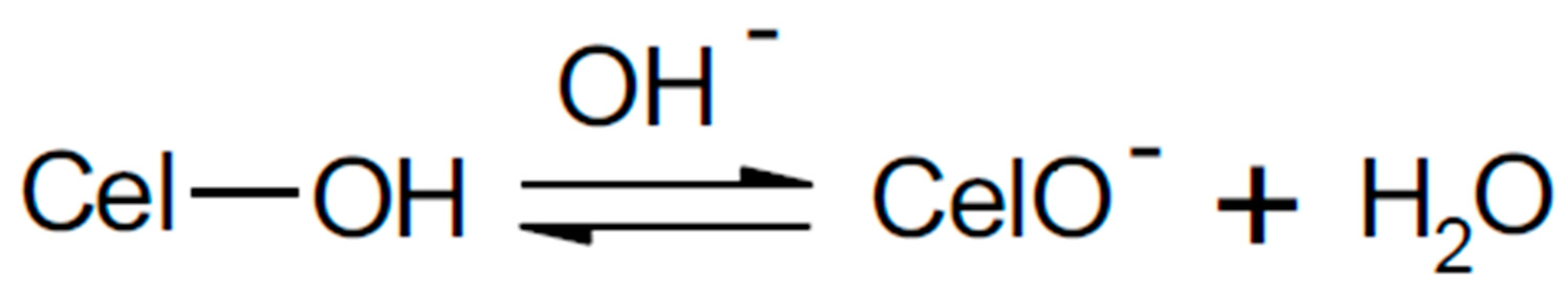


| Reactive Group | Chemical Structure |
|---|---|
| Monochlorotriazine |  |
| Dichlorotriazine | 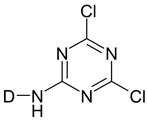 |
| Dichloropyrimidine |  |
| Dichloroquinoxaline | 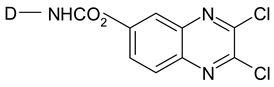 |
| Aminochlorotriazine |  |
| Monofluorotriazine | 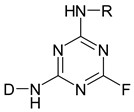 |
| Fluorochloropirymidine |  |
| Aminofluorotriazine | 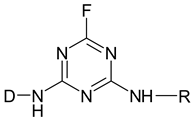 |
| Vinyl Sulphone | 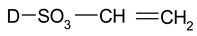 |
| Sulphatoethylsulphone | 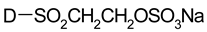 |
| Extraction Procedure | ||||||||
|---|---|---|---|---|---|---|---|---|
| No. Method | Fiber/Dyes | Cleavage of Covalent Bonds of Reactive Dyes With Functional Groups on the Cotton Fibers | Solvents | Fiber Length | Volume | Temperature | Time/Additional Information | Lit. |
| 1 | Dyes extracted from manufacturers’ pattern cards and casework materials (50 different reactive dyes) | Hydrolysis |
| n.d. | n.d. | Sulfuric acid at room temp.; other solvents at 100 °C | n.d. | [32] |
| 2 | Black cotton/Names of reactive dyes not presented | Alkaline hydrolysis | 1.5% NaOH; 10% MeOH (v/v) | 0.25–5.5 cm 2 of black cloth (cotton) | n.d. | 100 °C | 25 min Most of the solvent was evaporated and the precipitate was dissolved into 300 µL of water–methanol (1:1, v/v). Next, the samples were diluted with water (1:10) and filtered through 0.45-/zm PTFE filters and analyzed using capillary zone electrophoresis with UV detection | [33] |
| 3 | Cotton 35% and Polyester 65%/black/Cibacron (reactive dyes) Dianix (disperse dyes) Cotton/brown/ Brown A-96138, reactive dyes: 1.66% Procion Yellow H-EXL 0.62% Procion Crimson H-EXL 1.72% Procion Blue H-ERD Cotton/marine/ Marine A-9514, reactive dyes: 0.96% Procion Yellow H-E4R 1.6% Procion Red H-EXL 5.16% Procion Navy H-ER | Alkaline hydrolysis | 0.27 M (1.5%) NaOH; 50% MeOH | A single fiber of 2–15 mm | 5 µL | 100 °C | The extraction time varies depending on the extraction efficiency of the dye(s). Resin H(+) form was used to remove NaOH. Next, the samples were analyzed using micellar electrokinetic capillary chromatography | [34] |
| 4 | Cotton dyed fabrics and standard dyes: Reactive Blue 21, Reactive Yellow 160, Reactive Orange 72, Reactive Blue 19, Reactive Yellow 176, Reactive Violet 5, Reactive Black 5, Reactive Blue 250, Reactive Red 198, Reactive Blue 220, Reactive Red 180, Reactive Red 239/241 | Alkaline hydrolysis in automated extraction system | Solvent systems:
| 10-cm threads of fiber (yarns consisting of a bundle of twisted fibers) | 500 µL | n.p. | n.d. Three methods to remove alkaline and alkaline earth cations from the extract: (1) Cation exchange resin using Dowex® HCR-W2, H+ form, spherical beads, 16–40 mesh; (2) Solid-phase extraction (SPE) using Waters Oasis HLB 6-cm3 cartridges. (3) Precipitation reactions using ammonium bicarbonate to precipitate barium carbonate. Next, the samples were analyzed using capillary electrophoresis | [29] |
| 5 | Reactive Yellow 160, Reactive Blue 220, Reactive Orange 72 | Alkaline hydrolysis | 1.5% NaOH | Fibers of lengths 10 mm, 5 mm, and 1 mm | 50 µL | 100 °C | 1 h The resulting solution was then treated with 25 µL of 0.375 M hydrochloric acid (equimolar with the sodium hydroxide), 25 μL of 10 mM ammonium acetate adjusted to pH 9.3 to neutralize any excess sodium hydroxide remaining prior to UPLC-DAD-MS/MS | [14] |
| 6 | Cotton/Reactive Blue 19 | Alkaline hydrolysis | 0.15% sodium hydroxide solution | 3 mg fabric strip was cut from 2 × 2 cm fabric samples | 1 mL | 80 °C | 1 h After 1 h, the vail was cooled to room temperature, neutralized with 30 μL of 1 N hydrochloric acid solution, filtered with a 0.2 μm polytetrafluoroethylene filter and analyzed using HPLC-DAD-HRMS | [35] |
| 7 | Cotton/Reactive Yellow 145 Cotton/Reactive Red 120 Cotton/Reactive Orange 16 | Enzymatic extraction | 10 μL of NaOH solution; 4 °C, 4 h. Next, the NaOH solution was removed and the fiber was rinsed in acetic acid solution and twice in cellulase solution. Then, the fiber was submerged in 10 μL of cellulase solution and mixed in a thermo mixer (Eppendorf Comfort, 50 °C, 550 rpm). | 10 mm piece of fiber | 10 μL | 50 °C | 20 h Afterward, the samples were centrifuged (5000 rpm, 5 min), and 10 μL of methanol was added. Next, the samples were analyzed using HPLC-DAD-MS | [36] |
| 8 | Cotton/Reactive Orange 122 Cotton/Reactive Red 195 | Enzymatic extraction | 10 μL of NaOH solution (3 M, 4 °C, 4 h. Next, the NaOH solution was removed and the fiber was rinsed twice in acetic acid solution (0.5 M) and in cellulase solution 0.01 g in 10 mL acetic acid solution at pH 5). Then, the fiber was submerged in 10 μL of cellulase solution and mixed in a thermo mixer (Eppendorf Comfort, 50 °C, 550 rpm) | 10 mm of fiber | 10 μL | 50 °C | 20 h Afterward, the samples were centrifuged (5000 rpm, 5 min), and 10 μL of methanol was added. Next, the samples were analyzed using HPLC-DAD-MS | [37] |
| 9 | 21 pieces of red clothing, of a similar shade of color, made solely of cotton or with addition of other type of fibers polyesters, modal, elastane/Names of reactive dyes not presented | Enzymatic extraction Cellulase from
| Basic procedure: 50 μL NaOH solution for 4 h at 4 °C. Next, the fibers were washed first in 50 μL of 0.5 M acetic acid solution and then twice in 150 μL of a 1.6 g/dm3 cellulase solution in acetate buffer (pH = 5). Next, the fibers were again covered with 150 μL of a cellulose solution
| 4 (1) and 8 (2) threads of a length of 1 cm | 150 μL | 50 °C; 55 °C, 60 °C, respectively | 20 h After that centrifugation 5 min, 7000 rpm. Next, the samples were analyzed using TLC coupled with VSC | [3,38] |
| 10 | Degraded Cotton/Reactive Red 198 Degraded Cotton/Reactive Black 5 Degraded Cotton/Reactive Blue 49 Degraded Cotton/Reactive Orange 35 | Two methods: Chemical Treatment Method Before treatment, the degraded fabrics were washed three times with different solvents (2-mL water, 2-mL methanol, and 2-mL acetonitrile) in turn to remove impurities that may interfere with treatment. | 1 mL of 1.5% NaOH solution with constant stirring. | 3 mg of fabric samples | 1 mL | 80 °C | 1 h After 1 h, the sample was cool down, neutralized by adding 300 µL of 1 M HCl solution, and filtered with a polytetrafluoroethylene (PTFE) syringe filter. Next, the samples were analyzed using HPLC-HRMS | [39] |
| Enzymatic Digestion Method Before treatment, the degraded fabrics were washed by 1 mL of water, 1 mL of methanol, and 1 mL of acetonitrile. Finally, the washed fabric samples were dried at RT and were ready for enzymatic treatment. | 100 μL of 3 M NaOH solution was added to the vial, and the vial was placed in a container containing ice for 4 h. Then, the NaOH solution was removed, and 500 μL of 0.5 M acetic acid was added and incubated for 1 min; then, acetic acid was removed and 1.5 mL of buffer solution (0.1 M sodium acetate, pH 5 with acetic acid) was added and kept for 1 min; the buffer solution was removed and 1 mL of enzyme solution (90-g cellulase in 50-mL buffer) was added. The vials were sealed and placed in a shaking bath | 3 mg of fabric samples | 1 mL | 50 °C | 24 h After 24 h, the vials were removed from the shaker and sonicated for 30 s, the solutions were filtered with a polytetrafluoroethylene (PTFE) syringe filter. Next, the samples were analyzed using HPLC-HRMS | |||
| No. Method | Analytes | Technique | Column | The Mobile Phase | Gradient Program | The Mobile Phase Flow Rate | Injection | Lit. |
|---|---|---|---|---|---|---|---|---|
| 1 | Table 2 No. 5 | UPLC | BEH C18 column (1.7 μm, 2.1 × 50 mm, Waters Acquity UPLC®) heated to 40 °C | A—10 mM ammonium acetate (pH 9.3) B—acetonitrile | UPLC-DAD-MS/MS 0 min 95% A; 5% B 0–2 min 50% A; 50% B 2–3 min 95% A; 5% B 3–5 min 95% A; 5% B | 0.4 mL/min | 10 μL | [14] |
| 2 | Table 2 No. 6 | HPLC-DAD-HRMS | a Zorbax Eclipse Plus C18 (2.1 × 50 mm, 3.5 μm) column Pre-column a Zorbax Eclipse Plus C18 narrow bore guard column (2.1 × 12.5 mm, 5 μm) temp. 40 °C | A—20 mmol/L ammonium formate with formic acid in water (pH = 4) B—70/30 MeOH/ACN | 3% B from 0 to 1 min, 3%–60% B from 1 to 1.5 min, 60%–90% B from 1.5 to 7 min, holding 90% B from 7 to 9 min then returning to 3% B at 9 to 9.5 min. A 4 min post-run of 3% B was used to re-equilibrate the column | 0.5 mL/min | 10 μL | [35] |
| 3 | Table 2 No. 7 | HPLC | Grom-sil 120 ODS-5 ST (3 μm, 2 × 150 mm) Grace Davison Discovery Sciences, Deerfield, USA Precolumn AJO-4286 Temp. 22 °C | A—10 mM ammonium acetate in water: MeOH (95:5, v/v) B—25 mM ammonium acetate in ACN: MeOH (50: 50, v/v) | 50% B (0–53 min) 100% B (53–67 min) | n. d. | 10 μL | [36] |
| 4 | Table 2 No. 8 | HPLC | Grom-sil 120 ODS-5 ST (150 × 2.0 mm i.d., 3 μm) Grace Davison Discovery Sciences, Deerfield, USA Precolumn AJO-4286 Temp. 22 °C | A—10 mM ammonium acetate in water: MeOH (95:5, v/v) B—25 mM ammonium acetate in ACN: MeOH (50:50, v/v) | Linear gradient Total time 78 min | n. d. | 20 μL | [37] |
| 5 | Table 2 No. 10 | HPLC | Zorbax Eclipse Plus C18 (2.1 × 50 mm, 3.5 μm) column with a Zorbax Eclipse Plus C18 narrow bore guard column (2.1 × 12.5 mm, 5 μm); Temp. 40 °C | A—water with 20-mM ammonium formate and formic acid (pH = 4) B—70:30, v/v) methanol/acetonitrile | 3% B from 0 to 1 min, 3%–60% B from 1 to 1.5 min, 60%–90% B from 1.5 to 7 min, holding at 90% B from 7 to 9 min, and 3% B at 9 to 9.5 min. A 4-min post run of 3% B before the next run was performed. | 0.5 mL/min | 10 μL | [39] |
| 6 | Cibacron Yellow F-4G Cibacron Blue F-R | Ion-pair HPLC | Nucleosil 100–5, C18, 150 × 4.6 mm I.D. analytical column Macherey-Nagel GmbH & Co.KG | A 47:53 v/v mixture of acetonitile and 0.05 M ammonium acetate buffer containing 1 mM trimethylammonium bromide (CTAB) ion-pairing agent | isocratic mode | 0.8 mL/min each dye eluted separately 0.6 mL/min a mixture of dyes | n.d. | [43] |
| 7 | The C.I. Reactive Red 195, C.I. Reactive Yellow 145, C.I. Reactive Blue 221 | HPLC | BDS Hypersil C18 column (150 mm × 4.6 mm, 5 µm) | A—90% deionized water and 10% acetonitrile containing 0.1% ammonium acetate (of buffer of pH 6). B—90% acetonitrile and 10% deionized water containing 0.1% ammonium acetate of buffer of pH 6 | 0.00 min 100% A; 0% B 10.00 min 0% A; 100% B 10.10 min10 0% A; 0% B 20.00 min 0% A; 0% B | 0.75 mL/min | 20 μL | [44] |
| 8 | The C.I. Reactive Red 195, C.I. Reactive Yellow 145, C.I. Reactive Blue 221 | HPLC | BDS Hypersil C18 column (150 mm × 4.6 mm, 5 µm) | A—90% deionized water and 10% acetonitrile containing 0.1% ammonium acetate (of buffer of pH 6). B—90% acetonitrile and 10% deionized water containing 0.1% ammonium acetate of buffer of pH 6 | 0.00 min 100% A; 0% B 10.00 min 0% A; 100% B 10.10 min10 0% A; 0% B 20.00 min 0% A; 0% B | 0.75 mL/min | 20 μL | [45] |
| 9 | Reactive orange 16 (RO16) | HPLC-MS/MS | Symmetry C18 (50 mm × 1.0 mm I.D., 3.5 μm, Ireland) | (A) 0.1% Acetic acid in water (B) 0.1% Acetic acid in acetonitrile | From 95:5 A:B (v/v) to 24:76 A:B (v/v)A (0–15 min), 100% B (for 0.1 min and retention for 2 min), 95:5 A:B (v/v) (for 0.1 min and retention for 2 min) | 0.3 mL/min | 10 μL | [46] |
| No. | Analytes | Technique | Detector | Scanned Wavelength Range | Retention Time | Value [m/z] | LOD | Lit. |
|---|---|---|---|---|---|---|---|---|
| 1 | Table 2 No. 5 | UPLC | DAD MS(ESI) tandem quadrupole mass spectrometer MS/MS—Selected Reaction Monitoring (SRM) mode | 300–700 nm for Reactive Yellow 160, Reactive Blue 220, and Reactive Orange 72 at 405 nm, 610 nm, and 478 nm, respectively | Several peaks between 0.75 and 2.75 min within which multiple additional components are present | m/z 652, 572, 554, and 614 from the analysis the Reactive Yellow 160; Reactive Blue 220 did not did not appear in UPLC-MS; m/z 572, 474, 492, and 417 from the analysis of Reactive Orange 72 | DAD—0.33–1.42 ppb MS/MS—3 pg to 83 pg 1 mm extract of Reactive Yellow 160 was detected and arguably quantifiable | [14] |
| 2 | Table 2 No. 6 | HPLC | DAD ESI(-)-(Q-TOF)MS | 200–800 nm. The main wavelengths for absorbance analysis 254 nm, 620 nm, and 660 nm; the quantitative analysis at 620 nm | 4.6 min RB19-OH Degraded products were also monitored | Q-TOF MS RB19-OH a theoretical m/z 501.0432; monitored m/z 501.0432 | 0.12 ± 0.07 µg/mL (DAD) n.d. (HRMS) | [35] |
| 3 | Table 2 No. 7 | HPLC | Diode array detection (DAD) MS(ESI)-LTQ Orbitrap (HRMS) | 200–800 nm λmax 494 nm for Reactive Orange 16 λmax 417 nm for Reactive Yellow 145 λmax 540 nm for Reactive Red 120 150–2000 m/z | Between 14.5 and 24.8 min | Calculated mass [m/z] e.g., Reactive Orange 16 pure m/z 572.00980 m/z 474.04242 Reactive Orange 16 fiber m/z 774.14806 m/z 816.15863 (the dye molecule connected to two cellulose units) | e.g., Reactive Orange 16 DAD—37.3 µg/L powder/0.06 mm fiber HRMS—4.2 µg/L powder/0.011 mm fiber | [36] |
| 4 | Table 2 No. 8 | HPLC | DAD MS(ESI)-LTQ Orbitrap (HRMS) | 200–800 nm 150–2000 m/z | Reactive Orange 122 16.4 min 17.5 min 19.9 min Reactive Orange 195 16 min 14.2 min 17.5 min | Reactive Orange 122 584.5629 [M+2glu-2H]2- 746.616 [M+4glu-2H]2- -(red dye) 624.5407 [M+2glu-2H]2- -(yellow dye) -(blue dye) | n.d. | [37] |
| 5 | Table 2 No. 10 | HPLC | Diode array detection (DAD) MS(negative ESI)-QTOF (HRMS) | 200 to 800 nm. The main wavelengths for absorbance analyses were set to 254, 515, 610, 620, and 660 nm 200–800 nm (λmax 515 nm for RR198 λmax 620 nm for RBlk5 λmax 600 nm for RB49 λmax 430 nm and 254 nm for RO35 100–1600 m/z | RR198 45.6 min 51.0 min | The hydrolyzed form of dye RR198 at m/z 397.5020 (doubly charged deprotonated ion) The hydrolyzed form of dye RBlk5 at m/z 370.5093 (doubly charged deprotonated ion) The hydrolyzed form of dye RB49 at m/z 397.5354 (doubly charged deprotonated ion) The hydrolyzed form of dye RO35 at m/z 363.5312 (doubly charged deprotonated ion) | n.d. | [39] |
| 6 | Cibacron Yellow F-4G Cibacron Blue F-R | Ion-pair HPLC | UV absorbance | 275 nm | Blue 4.70 min Hydrolyzed Blue 3.44 min Yellow 3.32 min Hydrolyzed Yellow 2.38 min Blue in mixture (m) 7.54 min Hydrolyzed Blue (m) 5.07 min Yellow in mixture (m) 4.57 min Hydrolyzed Yellow (m) 3.16 min | n.d. | [43] | |
| 7 | The C.I. Reactive Red 195, C.I. Reactive Yellow 145, C.I. Reactive Blue 221 | HPLC | DAD | 200–800 nm λmax 540 nm Reactive Red 195 λmax 420 nm Reactive Yellow 145 λmax 610 nm Reactive Blue 221 | Total runtime of 20 min e.g., several peaks with different RT belonging to different dye forms for Disperse Red 195 tR = 1.77 min; tR = 2.75 min; major peak at tR = 5.54 min attributed to the active form of Reactive Red 195. In the opposite, the Reactive Yellow 145 and Reactive Blue 221 show only one big peak respectively at RT 6.71 min and RT 6.64 min | n.d. | [44] | |
| 8 | The C.I. Reactive Red 195, C.I. Reactive Yellow 145, C.I. Reactive Blue 221 | HPLC | DAD | 200–800 nm λmax 540 nm Reactive Red 195 λmax 420 nm Reactive Yellow 145 λmax 610 nm Reactive Blue 221 | Total runtime of 20 min e.g., several peaks with different RT belonging to different dye forms for Disperse Red 195 tR = 1.79 min, 2.2 min, 2.34 min, 2.92 min, 3.55 min and 4.11 min—the inactive forms; two major peaks at tR = 4.73 min and tR = 5.96 min attributed to the partially hydrolyzed forms of Reactive Red 195. Peak at tR = 5.33 min attributed to the completely hydrolyzed form. In the opposite, the Reactive Yellow 145 and Reactive Blue 221 showed only one big peak respectively at RT 2.74 min and RT 2.71 min of the inactivated forms and at 5.15 min (RY145), 5.24 min, 5.91 min (RB221) of the partially hydrolyzed forms and at tR = 6.30 min attributed to the completely hydrolyzed form of RB221 | n.d. | [45] | |
| 9 | Reactive orange 16 (RO16) | HPLC-MS/MS | MS(ESI) quadrupled type tandem MS MS/MS–MRM mode | n.d. | 5.09 min | Molecular weight 617.54 285.4 [M-2H]2- 285.4→236.85 285.4→264 | MS/MS—2.10 ng/mL | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śmigiel-Kamińska, D.; Wąs-Gubała, J.; Stepnowski, P.; Kumirska, J. The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes. Molecules 2020, 25, 5435. https://doi.org/10.3390/molecules25225435
Śmigiel-Kamińska D, Wąs-Gubała J, Stepnowski P, Kumirska J. The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes. Molecules. 2020; 25(22):5435. https://doi.org/10.3390/molecules25225435
Chicago/Turabian StyleŚmigiel-Kamińska, Daria, Jolanta Wąs-Gubała, Piotr Stepnowski, and Jolanta Kumirska. 2020. "The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes" Molecules 25, no. 22: 5435. https://doi.org/10.3390/molecules25225435
APA StyleŚmigiel-Kamińska, D., Wąs-Gubała, J., Stepnowski, P., & Kumirska, J. (2020). The Identification of Cotton Fibers Dyed with Reactive Dyes for Forensic Purposes. Molecules, 25(22), 5435. https://doi.org/10.3390/molecules25225435








