Sigma-2 Receptor—A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis
Abstract
:1. Introduction
1.1. PGRMC1/Sigma-2 Receptor
1.2. TMEM97/Sigma-2 Receptor
1.3. The Complex of Sigma-2 Receptors
1.4. The Pharmacology of Sigma-2 Receptors
2. Sigma-2 Receptor—A Novel Regulator of Cholesterol Homeostasis
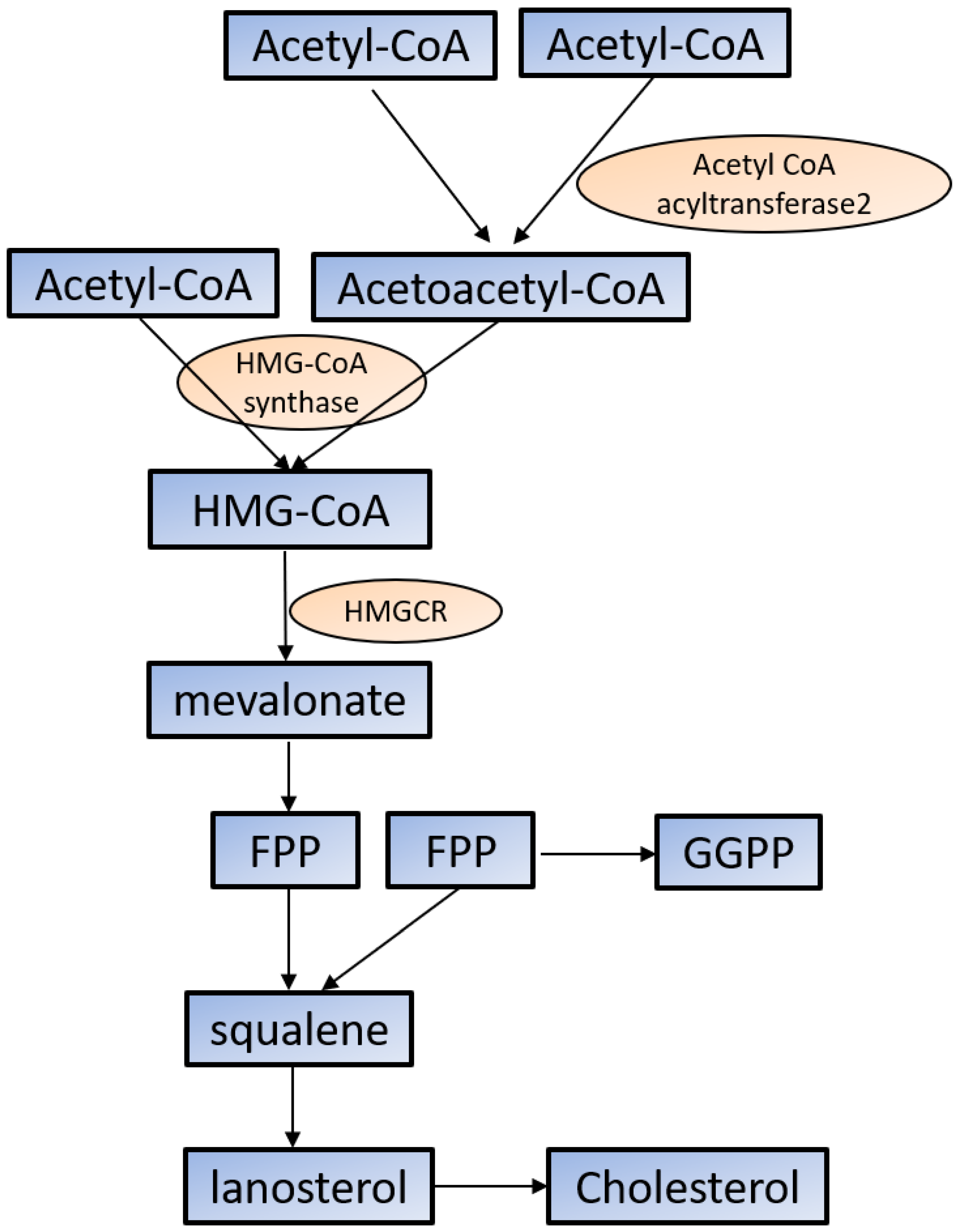
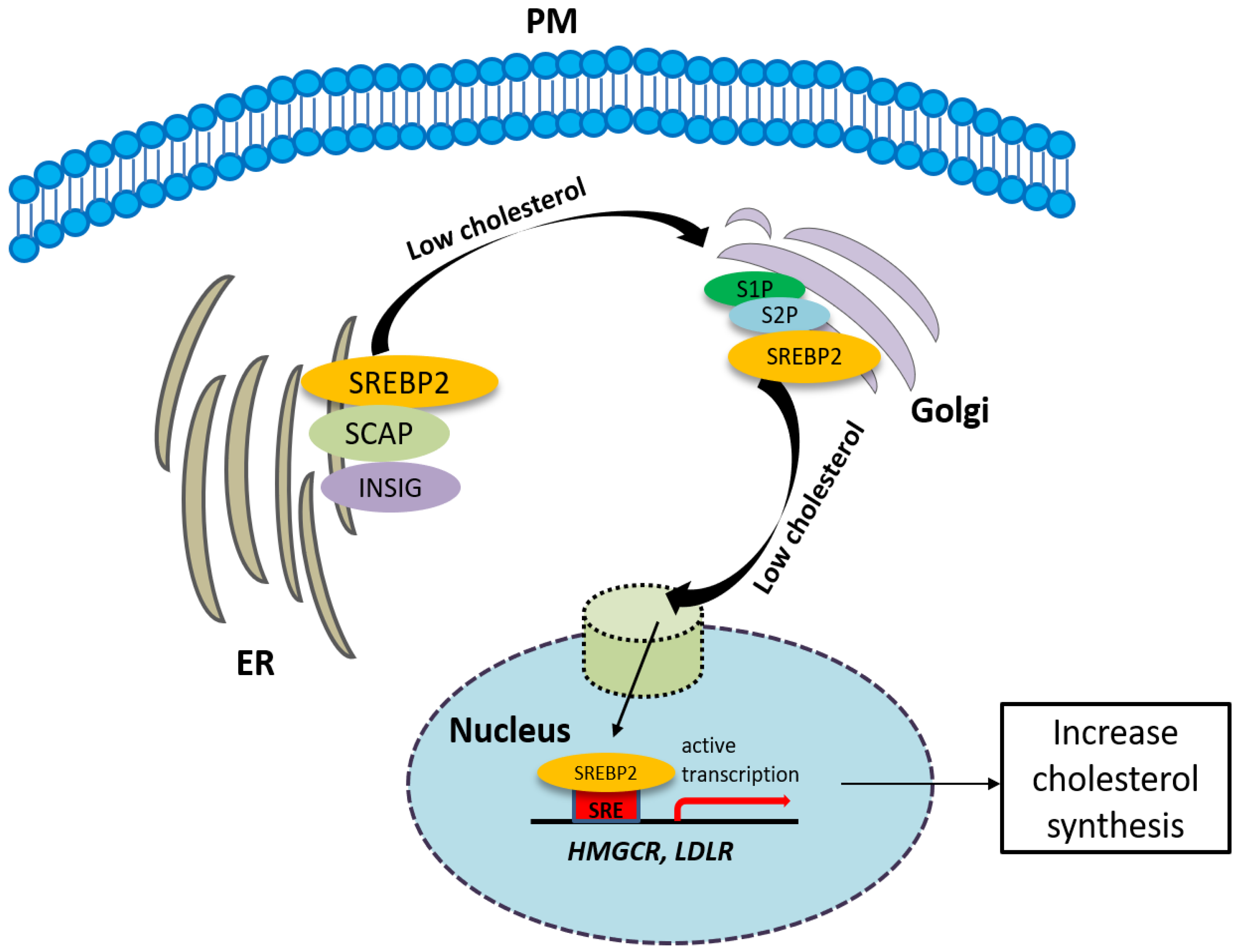
2.1. Cholesterol Synthesis
2.2. Cholesterol Transport
2.3. The Involvement of the Sigma-2 Receptor in Cholesterol Homeostasis
3. Anti-Cancer Effects of Sigma-2 Receptor Ligands
3.1. Increased Cholesterol Synthesis and Uptake
3.2. Enriched Cholesterol-Derived Metabolites: Oxysterols
3.3. The Involvement of Sigma-2 Receptor in Cancer
3.3.1. Sigma-2 Receptor as an Imaging Target for Cancer Diagnosis
3.3.2. Sigma-2 Ligands as Anticancer Agents
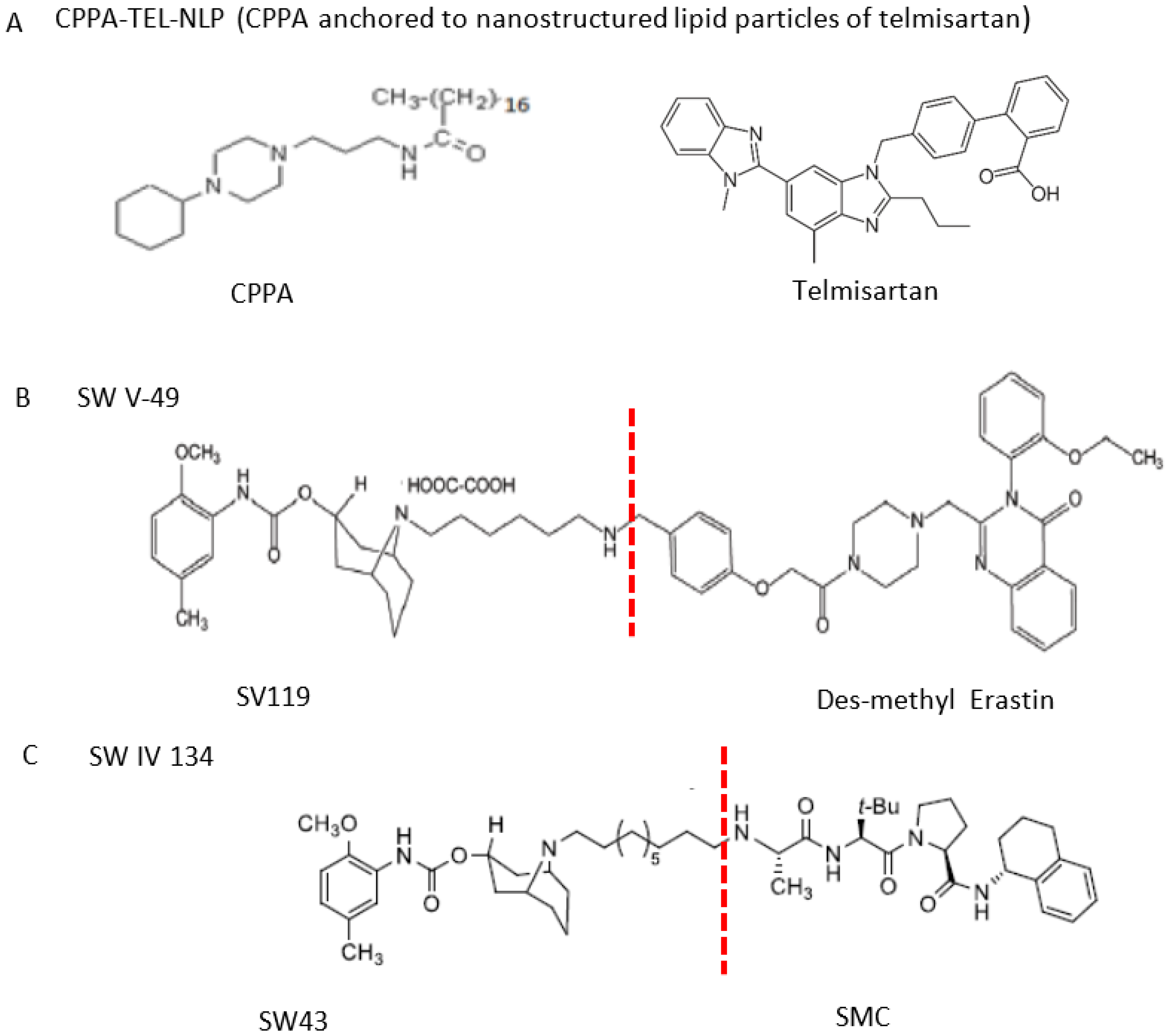
3.3.3. Sigma-2 Receptor Ligands as Anticancer Drug Delivery Vehicles
3.4. Mechanisms of Anti-Cancer by Sigma-2 Ligands Targeting Cholesterol Homeostasis
4. Sigma-2 Receptor Ligands May Target Cholesterol Homeostasis to Treat AD
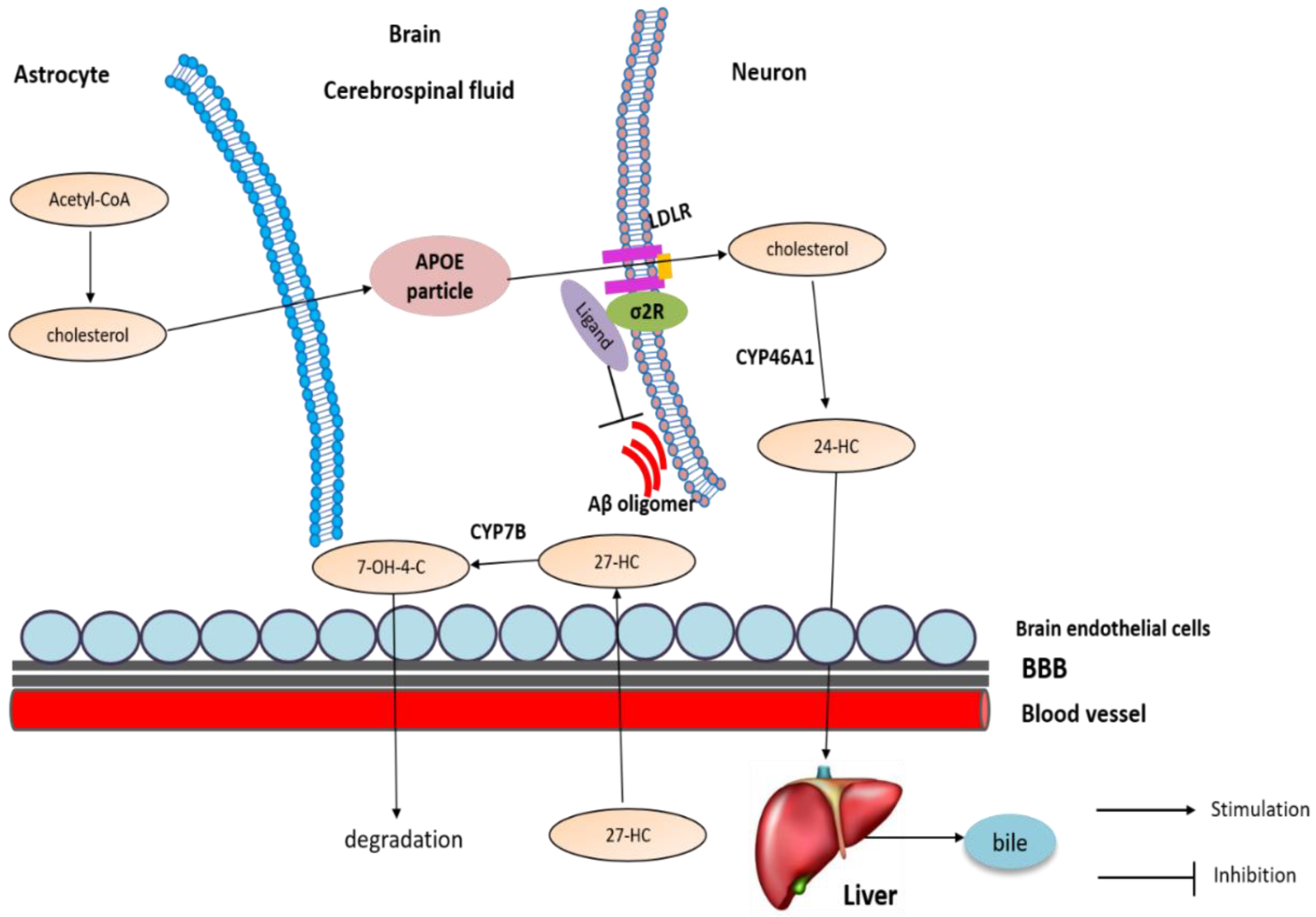
4.1. Cellular Cholesterol Accumulation in AD
4.2. Altered Level of Oxysterols in AD
4.3. The Involvement of Sigma-2 Receptor in AD
4.3.1. Sigma-2 Receptor Ligands as Therapeutic Target of AD
4.3.2. The Potential Mechanism of Sigma-2 Receptor Ligands in AD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Walker, J.M.; Bowen, W.D.; Walker, F.O.; Matsumoto, R.R.; De Costa, B.; Rice, K.C. Sigma receptors: Biology and function. Pharmacol. Rev. 1990, 42, 355–402. [Google Scholar]
- Hellewell, S.B.; Bowen, W.D. A sigma-like binding site in rat pheochromocytoma (PC12) cells: Decreased affinity for (+)-benzomorphans and lower molecular weight suggest a different sigma receptor form from that of guinea pig brain. Brain Res. 1990, 527, 244–253. [Google Scholar] [CrossRef]
- Hayashi, T.; Su, T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 2007, 131, 596–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguinaga, D.; Casanovas, M.; Rivas-Santisteban, R.; Reyes-Resina, I.; Navarro, G.; Franco, R. The sigma-1 receptor as key common factor in cocaine and food-seeking behaviors. J. Mol. Endocrinol. 2019, 63, R81–R92. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Activation of sigma-1 receptor chaperone in the treatment of neuropsychiatric diseases and its clinical implication. J. Pharmacol. Sci. 2015, 127, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Wang, C.; Sun, T. The Roles of Intracellular Chaperone Proteins, Sigma Receptors, in Parkinson’s Disease (PD) and Major Depressive Disorder (MDD). Front. Pharmacol. 2019, 10, 528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, H.R.; Zheng, S.; Gurpinar, E.; Koehl, A.; Manglik, A.; Kruse, A.C. Crystal structure of the human sigma1 receptor. Nature 2016, 532, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Alon, A.; Schmidt, H.R.; Wood, M.D.; Sahn, J.J.; Martin, S.F.; Kruse, A.C. Identification of the gene that codes for the sigma2 receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 7160–7165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colabufo, N.A.; Berardi, F.; Abate, C.; Contino, M.; Niso, M.; Perrone, R. Is the sigma2 receptor a histone binding protein? J. Med. Chem. 2006, 49, 4153–4158. [Google Scholar] [CrossRef]
- Zeng, C.; Vangveravong, S.; Jones, L.A.; Hyrc, K.; Chang, K.C.; Xu, J.; Rothfuss, J.M.; Goldberg, M.P.; Hotchkiss, R.S.; Mach, R.H. Characterization and evaluation of two novel fluorescent sigma-2 receptor ligands as proliferation probes. Mol. Imaging 2011, 10, 420–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Zeng, C.; Chu, W.; Pan, F.; Rothfuss, J.M.; Zhang, F.; Tu, Z.; Zhou, D.; Zeng, D.; Vangveravong, S.; et al. Identification of the PGRMC1 protein complex as the putative sigma-2 receptor binding site. Nat. Commun. 2011, 2, 380. [Google Scholar] [CrossRef] [PubMed]
- Hiranita, T. Identification of the Sigma-2 Receptor: Distinct from the Progesterone Receptor Membrane Component 1 (PGRMC1). J. Alcohol. Drug Depend. 2016, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abate, C.; Niso, M.; Infantino, V.; Menga, A.; Berardi, F. Elements in support of the ‘non-identity’ of the PGRMC1 protein with the sigma2 receptor. Eur. J. Pharmacol. 2015, 758, 16–23. [Google Scholar] [CrossRef]
- Chu, U.B.; Mavlyutov, T.A.; Chu, M.L.; Yang, H.; Schulman, A.; Mesangeau, C.; McCurdy, C.R.; Guo, L.W.; Ruoho, A.E. The Sigma-2 Receptor and Progesterone Receptor Membrane Component 1 are Different Binding Sites Derived From Independent Genes. EBioMedicine 2015, 2, 1806–1813. [Google Scholar] [CrossRef] [Green Version]
- Hellewell, S.B.; Bruce, A.; Feinstein, G.; Orringer, J.; Williams, W.; Bowen, W.D. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: Characterization by ligand binding and photoaffinity labeling. Eur. J. Pharmacol. 1994, 268, 9–18. [Google Scholar] [CrossRef]
- Pati, M.L.; Groza, D.; Riganti, C.; Kopecka, J.; Niso, M.; Berardi, F.; Hager, S.; Heffeter, P.; Hirai, M.; Tsugawa, H.; et al. Sigma-2 receptor and progesterone receptor membrane component 1 (PGRMC1) are two different proteins: Proofs by fluorescent labeling and binding of sigma-2 receptor ligands to PGRMC1. Pharmacol. Res. 2017, 117, 67–74. [Google Scholar] [CrossRef]
- Sanchez-Pulido, L.; Ponting, C.P. TM6SF2 and MAC30, new enzyme homologs in sterol metabolism and common metabolic disease. Front. Genet. 2014, 5, 439. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.C.; Yu, C.F.; Wang, S.C.; Li, H.Y.; Lin, C.M.; Wang, H.H.; Abate, C.; Chiang, C.S. Sigma-2 receptor/TMEM97 agonist PB221 as an alternative drug for brain tumor. BMC Cancer 2019, 19, 473. [Google Scholar] [CrossRef]
- Sahn, J.J.; Mejia, G.L.; Ray, P.R.; Martin, S.F.; Price, T.J. Sigma 2 Receptor/Tmem97 Agonists Produce Long Lasting Antineuropathic Pain Effects in Mice. ACS Chem. Neurosci. 2017, 8, 1801–1811. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.L.; Sahn, J.J.; Ferragud, A.; Yen, R.C.; Satarasinghe, P.N.; Wood, M.D.; Hodges, T.R.; Shi, T.; Prakash, B.A.; Friese, K.M.; et al. Small molecule modulators of sigma2R/Tmem97 reduce alcohol withdrawal-induced behaviors. Neuropsychopharmacology 2018, 43, 1867–1875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vazquez-Rosa, E.; Watson, M.R.; Sahn, J.J.; Hodges, T.R.; Schroeder, R.E.; Cintron-Perez, C.J.; Shin, M.K.; Yin, T.C.; Emery, J.L.; Martin, S.F.; et al. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem. Neurosci. 2019, 10, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Sharma, A.; King, T.I.; Mesangeau, C.; Seminerio, M.; Chin, F.T.; Wilson, L.L.; Matsumoto, R.R.; McLaughlin, J.P.; Avery, B.A.; et al. Discovery of a Highly Selective Sigma-2 Receptor Ligand, 1-(4-(6,7-Dimethoxy-3,4-dihydroisoquinolin-2(1H)-yl)butyl)-3-methyl-1H-benzo[d]im idazol-2(3H)-one (CM398), with Drug-Like Properties and Antinociceptive Effects In Vivo. AAPS J. 2020, 22, 94. [Google Scholar] [CrossRef] [PubMed]
- Franchini, S.; Linciano, P.; Puja, G.; Tait, A.; Borsari, C.; Denora, N.; Iacobazzi, R.M.; Brasili, L.; Sorbi, C. Novel Dithiolane-Based Ligands Combining Sigma and NMDA Receptor Interactions as Potential Neuroprotective Agents. ACS Med. Chem. Lett. 2020, 11, 1028–1034. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Weng, C.C.; Schneider, M.E., Jr.; Puentes, L.; Riad, A.; Xu, K.; Makvandi, M.; Jin, L.; Hawkins, W.G.; Mach, R.H. TMEM97 and PGRMC1 do not mediate sigma-2 ligand-induced cell death. Cell Death Discov. 2019, 5, 58. [Google Scholar] [CrossRef] [Green Version]
- Riad, A.; Zeng, C.; Weng, C.C.; Winters, H.; Xu, K.; Makvandi, M.; Metz, T.; Carlin, S.; Mach, R.H. Sigma-2 Receptor/TMEM97 and PGRMC-1 Increase the Rate of Internalization of LDL by LDL Receptor through the Formation of a Ternary Complex. Sci. Rep. 2018, 8, 16845. [Google Scholar] [CrossRef]
- Riad, A.; Lengyel-Zhand, Z.; Zeng, C.; Weng, C.C.; Lee, V.M.; Trojanowski, J.Q.; Mach, R.H. The Sigma-2 Receptor/TMEM97, PGRMC1, and LDL Receptor Complex Are Responsible for the Cellular Uptake of Abeta42 and Its Protein Aggregates. Mol. Neurobiol. 2020, 57, 3803–3813. [Google Scholar] [CrossRef]
- Yi, B.; Sahn, J.J.; Ardestani, P.M.; Evans, A.K.; Scott, L.L.; Chan, J.Z.; Iyer, S.; Crisp, A.; Zuniga, G.; Pierce, J.T.; et al. Small molecule modulator of sigma 2 receptor is neuroprotective and reduces cognitive deficits and neuroinflammation in experimental models of Alzheimer’s disease. J. Neurochem. 2017, 140, 561–575. [Google Scholar] [CrossRef]
- Zeng, C.; Rothfuss, J.M.; Zhang, J.; Vangveravong, S.; Chu, W.; Li, S.; Tu, Z.; Xu, J.; Mach, R.H. Functional assays to define agonists and antagonists of the sigma-2 receptor. Anal. Biochem. 2014, 448, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Crawford, K.W.; Bowen, W.D. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002, 62, 313–322. [Google Scholar]
- Crawford, K.W.; Coop, A.; Bowen, W.D. Sigma(2) Receptors regulate changes in sphingolipid levels in breast tumor cells. Eur. J. Pharmacol. 2002, 443, 207–209. [Google Scholar] [CrossRef]
- Grundman, M.; Morgan, R.; Lickliter, J.D.; Schneider, L.S.; DeKosky, S.; Izzo, N.J.; Guttendorf, R.; Higgin, M.; Pribyl, J.; Mozzoni, K.; et al. A phase 1 clinical trial of the sigma-2 receptor complex allosteric antagonist CT1812, a novel therapeutic candidate for Alzheimer’s disease. Alzheimers Dement. 2019, 5, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Xu, J.; Zeng, C.; Kirk, M.J.; Mozzoni, K.; Silky, C.; Rehak, C.; Yurko, R.; Look, G.; Rishton, G.; et al. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers II: Sigma-2/PGRMC1 receptors mediate Abeta 42 oligomer binding and synaptotoxicity. PLoS ONE 2014, 9, e111899. [Google Scholar] [CrossRef] [PubMed]
- Brent, P.J.; Pang, G.T. Sigma binding site ligands inhibit cell proliferation in mammary and colon carcinoma cell lines and melanoma cells in culture. Eur. J. Pharmacol. 1995, 278, 151–160. [Google Scholar] [CrossRef]
- Colabufo, N.A.; Berardi, F.; Contino, M.; Niso, M.; Abate, C.; Perrone, R.; Tortorella, V. Antiproliferative and cytotoxic effects of some sigma2 agonists and sigma1 antagonists in tumour cell lines. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 370, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Renaudo, A.; Watry, V.; Chassot, A.A.; Ponzio, G.; Ehrenfeld, J.; Soriani, O. Inhibition of tumor cell proliferation by sigma ligands is associated with K+ Channel inhibition and p27kip1 accumulation. J. Pharmacol. Exp. Ther. 2004, 311, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Azzariti, A.; Colabufo, N.A.; Berardi, F.; Porcelli, L.; Niso, M.; Simone, G.M.; Perrone, R.; Paradiso, A. Cyclohexylpiperazine derivative PB28, a sigma2 agonist and sigma1 antagonist receptor, inhibits cell growth, modulates P-glycoprotein, and synergizes with anthracyclines in breast cancer. Mol. Cancer Ther. 2006, 5, 1807–1816. [Google Scholar] [CrossRef] [Green Version]
- Niso, M.; Abate, C.; Ferorelli, S.; Cassano, G.; Gasparre, G.; Perrone, R.; Berardi, F. Investigation of sigma receptors agonist/antagonist activity through N-(6-methoxytetralin-1-yl)- and N-(6-methoxynaphthalen-1-yl)alkyl derivatives of polymethylpiperidines. Bioorg. Med. Chem. 2013, 21, 1865–1869. [Google Scholar] [CrossRef]
- Ostenfeld, M.S.; Fehrenbacher, N.; Hoyer-Hansen, M.; Thomsen, C.; Farkas, T.; Jaattela, M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005, 65, 8975–8983. [Google Scholar] [CrossRef] [Green Version]
- Ostenfeld, M.S.; Hoyer-Hansen, M.; Bastholm, L.; Fehrenbacher, N.; Olsen, O.D.; Groth-Pedersen, L.; Puustinen, P.; Kirkegaard-Sorensen, T.; Nylandsted, J.; Farkas, T.; et al. Anti-cancer agent siramesine is a lysosomotropic detergent that induces cytoprotective autophagosome accumulation. Autophagy 2008, 4, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Kashiwagi, H.; McDunn, J.E.; Simon, P.O., Jr.; Goedegebuure, P.S.; Vangveravong, S.; Chang, K.; Hotchkiss, R.S.; Mach, R.H.; Hawkins, W.G. Sigma-2 receptor ligands potentiate conventional chemotherapies and improve survival in models of pancreatic adenocarcinoma. J. Transl. Med. 2009, 7, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashiwagi, H.; McDunn, J.E.; Simon, P.O., Jr.; Goedegebuure, P.S.; Xu, J.; Jones, L.; Chang, K.; Johnston, F.; Trinkaus, K.; Hotchkiss, R.S.; et al. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: Applications in diagnostic imaging and therapy. Mol. Cancer 2007, 6, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, E.S.; Mankoff, J.; Makvandi, M.; Chu, W.; Chu, Y.; Mach, R.H.; Zeng, C. Sigma-2 ligands and PARP inhibitors synergistically trigger cell death in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 486, 788–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, W.; Xu, J.; Zhou, D.; Zhang, F.; Jones, L.A.; Wheeler, K.T.; Mach, R.H. New N-substituted 9-azabicyclo[3.3.1]nonan-3alpha-yl phenylcarbamate analogs as sigma2 receptor ligands: Synthesis, in vitro characterization, and evaluation as PET imaging and chemosensitization agents. Bioorg. Med. Chem. 2009, 17, 1222–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and regulation of cholesterol homeostasis. Nat. Rev. Mol. Cell Biol. 2020, 21, 225–245. [Google Scholar] [CrossRef]
- Riscal, R.; Skuli, N.; Simon, M.C. Even Cancer Cells Watch Their Cholesterol! Mol. Cell 2019, 76, 220–231. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Hong, C.; Tontonoz, P. Liver X receptors in lipid metabolism: Opportunities for drug discovery. Nat. Rev. Drug Discov. 2014, 13, 433–444. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef]
- Widenmaier, S.B.; Snyder, N.A.; Nguyen, T.B.; Arduini, A.; Lee, G.Y.; Arruda, A.P.; Saksi, J.; Bartelt, A.; Hotamisligil, G.S. NRF1 Is an ER Membrane Sensor that Is Central to Cholesterol Homeostasis. Cell 2017, 171, 1094–1109.e15. [Google Scholar] [CrossRef]
- Lane-Donovan, C.; Philips, G.T.; Herz, J. More than cholesterol transporters: Lipoprotein receptors in CNS function and neurodegeneration. Neuron 2014, 83, 771–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benjannet, S.; Rhainds, D.; Essalmani, R.; Mayne, J.; Wickham, L.; Jin, W.; Asselin, M.C.; Hamelin, J.; Varret, M.; Allard, D.; et al. NARC-1/PCSK9 and its natural mutants: Zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J. Biol. Chem. 2004, 279, 48865–48875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagace, T.A.; Curtis, D.E.; Garuti, R.; McNutt, M.C.; Park, S.W.; Prather, H.B.; Anderson, N.N.; Ho, Y.K.; Hammer, R.E.; Horton, J.D. Secreted PCSK9 decreases the number of LDL receptors in hepatocytes and in livers of parabiotic mice. J. Clin. Investig. 2006, 116, 2995–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikonen, E. Cellular cholesterol trafficking and compartmentalization. Nat. Rev. Mol. Cell Biol. 2008, 9, 125–138. [Google Scholar] [CrossRef]
- Gu, L.; Saha, S.T.; Thomas, J.; Kaur, M. Targeting cellular cholesterol for anticancer therapy. FEBS J. 2019, 286, 4192–4208. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kasimov, M.R.; Zefirov, A.L. Cholesterol in the Pathogenesis of Alzheimer’s, Parkinson’s Diseases and Autism: Link to Synaptic Dysfunction. Acta Nat. 2017, 9, 26–37. [Google Scholar] [CrossRef]
- Doria, M.; Maugest, L.; Moreau, T.; Lizard, G.; Vejux, A. Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic. Biol. Med. 2016, 101, 393–400. [Google Scholar] [CrossRef]
- Brown, A.J.; Sharpe, L.J.; Rogers, M.J. Oxysterols: From Physiological Tuners to Pharmacological Opportunities. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef] [Green Version]
- Koarai, A.; Yanagisawa, S.; Sugiura, H.; Ichikawa, T.; Kikuchi, T.; Furukawa, K.; Akamatsu, K.; Hirano, T.; Nakanishi, M.; Matsunaga, K.; et al. 25-Hydroxycholesterol enhances cytokine release and Toll-like receptor 3 response in airway epithelial cells. Respir. Res. 2012, 13, 63. [Google Scholar] [CrossRef] [Green Version]
- Dang, E.V.; McDonald, J.G.; Russell, D.W.; Cyster, J.G. Oxysterol Restraint of Cholesterol Synthesis Prevents AIM2 Inflammasome Activation. Cell 2017, 171, 1057–1071.e1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Paz Sanchez-Martinez, M.; Blanco-Favela, F.; Mora-Ruiz, M.D.; Chavez-Rueda, A.K.; Bernabe-Garcia, M.; Chavez-Sanchez, L. IL-17-differentiated macrophages secrete pro-inflammatory cytokines in response to oxidized low-density lipoprotein. Lipids Health Dis. 2017, 16, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fessler, M.B.; Parks, J.S. Intracellular lipid flux and membrane microdomains as organizing principles in inflammatory cell signaling. J. Immunol. 2011, 187, 1529–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, C.R.; Stuart, L.M.; Wilkinson, K.; van Gils, J.M.; Deng, J.; Halle, A.; Rayner, K.J.; Boyer, L.; Zhong, R.; Frazier, W.A.; et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010, 11, 155–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilcox, C.B.; Feddes, G.O.; Willett-Brozick, J.E.; Hsu, L.C.; DeLoia, J.A.; Baysal, B.E. Coordinate up-regulation of TMEM97 and cholesterol biosynthesis genes in normal ovarian surface epithelial cells treated with progesterone: Implications for pathogenesis of ovarian cancer. BMC Cancer 2007, 7, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartz, F.; Kern, L.; Erz, D.; Zhu, M.; Gilbert, D.; Meinhof, T.; Wirkner, U.; Erfle, H.; Muckenthaler, M.; Pepperkok, R.; et al. Identification of cholesterol-regulating genes by targeted RNAi screening. Cell Metab. 2009, 10, 63–75. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi-Fakhari, D.; Wahlster, L.; Bartz, F.; Werenbeck-Ueding, J.; Praggastis, M.; Zhang, J.; Joggerst-Thomalla, B.; Theiss, S.; Grimm, D.; Ory, D.S.; et al. Reduction of TMEM97 increases NPC1 protein levels and restores cholesterol trafficking in Niemann-pick type C1 disease cells. Hum. Mol. Genet. 2016, 25, 3588–3599. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, L.; Jia, R. Role of de novo cholesterol synthesis enzymes in cancer. J. Cancer 2020, 11, 1761–1767. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.H.; Huang, C.H.; Houlihan, S.L.; Regunath, K.; Freed-Pastor, W.A.; Morris, J.P.; Tschaharganeh, D.F.; Kastenhuber, E.R.; Barsotti, A.M.; Culp-Hill, R.; et al. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell 2019, 176, 564–580.e519. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, E.J.; Zelenko, Z.; Neel, B.A.; Antoniou, I.M.; Rajan, L.; Kase, N.; LeRoith, D. Elevated tumor LDLR expression accelerates LDL cholesterol-mediated breast cancer growth in mouse models of hyperlipidemia. Oncogene 2017, 36, 6462–6471. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Ishikawa, T.; Sirianni, R.; Tang, H.; McDonald, J.G.; Yuhanna, I.S.; Thompson, B.; Girard, L.; Mineo, C.; Brekken, R.A.; et al. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep. 2013, 5, 637–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, C.G.; Ramirez, M.E.; Cruz, P.; Epunan, M.J.; Valladares, L.E.; Sierralta, W.D. 27-hydroxycholesterol induces the transition of MCF7 cells into a mesenchymal phenotype. Oncol. Rep. 2011, 26, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Ohm, J.E.; Dhasarathy, A.; Schommer, J.; Roche, C.; Hammer, K.D.; Ghribi, O. The cholesterol metabolite 27-hydroxycholesterol regulates p53 activity and increases cell proliferation via MDM2 in breast cancer cells. Mol. Cell. Biochem. 2015, 410, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Shen, Z.; Liu, J.; Chen, J.; Liu, Y.; Hu, C.; Li, Z.; Li, Y. The ROS-mediated activation of STAT-3/VEGF signaling is involved in the 27-hydroxycholesterol-induced angiogenesis in human breast cancer cells. Toxicol. Lett. 2016, 264, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Nury, T.; Zarrouk, A.; Yammine, A.; Mackrill, J.J.; Vejux, A.; Lizard, G. Oxiapoptophagy: A type of cell death induced by some oxysterols. Br. J. Pharmacol. 2020. [Google Scholar] [CrossRef]
- Guo, F.; Hong, W.; Yang, M.; Xu, D.; Bai, Q.; Li, X.; Chen, Z. Upregulation of 24(R/S),25-epoxycholesterol and 27-hydroxycholesterol suppresses the proliferation and migration of gastric cancer cells. Biochem. Biophys. Res. Commun. 2018, 504, 892–898. [Google Scholar] [CrossRef]
- Warns, J.; Marwarha, G.; Freking, N.; Ghribi, O. 27-hydroxycholesterol decreases cell proliferation in colon cancer cell lines. Biochimie 2018, 153, 171–180. [Google Scholar] [CrossRef]
- Al-Nabulsi, I.; Mach, R.H.; Wang, L.M.; Wallen, C.A.; Keng, P.C.; Sten, K.; Childers, S.R.; Wheeler, K.T. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br. J. Cancer 1999, 81, 925–933. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, K.T.; Wang, L.M.; Wallen, C.A.; Childers, S.R.; Cline, J.M.; Keng, P.C.; Mach, R.H. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br. J. Cancer 2000, 82, 1223–1232. [Google Scholar] [CrossRef]
- Dehdashti, F.; Laforest, R.; Gao, F.; Shoghi, K.I.; Aft, R.L.; Nussenbaum, B.; Kreisel, F.H.; Bartlett, N.L.; Cashen, A.; Wagner-Johnston, N.; et al. Assessment of cellular proliferation in tumors by PET using 18F-ISO-1. J. Nucl. Med. 2013, 54, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Dence, C.S.; Ponde, D.E.; Jones, L.; Wheeler, K.T.; Welch, M.J.; Mach, R.H. Carbon-11 labeled sigma2 receptor ligands for imaging breast cancer. Nucl. Med. Biol. 2005, 32, 423–430. [Google Scholar] [CrossRef] [PubMed]
- McDonald, E.S.; Doot, R.K.; Young, A.J.; Schubert, E.K.; Tchou, J.; Pryma, D.A.; Farwell, M.D.; Nayak, A.; Ziober, A.; Feldman, M.D.; et al. Breast Cancer (18)F-ISO-1 Uptake as a Marker of Proliferation Status. J. Nucl. Med. 2020, 61, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Riad, A.; Mach, R.H. The Biological Function of Sigma-2 Receptor/TMEM97 and Its Utility in PET Imaging Studies in Cancer. Cancers 2020, 12, 1877. [Google Scholar] [CrossRef]
- Cantonero, C.; Camello, P.J.; Abate, C.; Berardi, F.; Salido, G.M.; Rosado, J.A.; Redondo, P.C. NO1, a New Sigma 2 Receptor/TMEM97 Fluorescent Ligand, Downregulates SOCE and Promotes Apoptosis in the Triple Negative Breast Cancer Cell Lines. Cancers 2020, 12, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, M.; Tilahun, E.D.; Lieberman, B.P.; Anderson, R.C.; Zeng, C.; Xu, K.; Hou, C.; McDonald, E.S.; Pryma, D.A.; Mach, R.H. The sigma-2 receptor as a therapeutic target for drug delivery in triple negative breast cancer. Biochem. Biophys. Res. Commun. 2015, 467, 1070–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohman, K.A.; Hashim, Y.M.; Vangveravong, S.; Nywening, T.M.; Cullinan, D.R.; Goedegebuure, S.P.; Liu, J.; Van Tine, B.A.; Tiriac, H.; Tuveson, D.A.; et al. Conjugation to the sigma-2 ligand SV119 overcomes uptake blockade and converts dm-Erastin into a potent pancreatic cancer therapeutic. Oncotarget 2016, 7, 33529–33541. [Google Scholar] [CrossRef] [Green Version]
- Puri, R.; Kaur Bhatia, R.; Shankar Pandey, R.; Kumar Jain, U.; Katare, O.P.; Madan, J. Sigma-2 receptor ligand anchored telmisartan loaded nanostructured lipid particles augmented drug delivery, cytotoxicity, apoptosis and cellular uptake in prostate cancer cells. Drug Dev. Ind. Pharm. 2016, 42, 2020–2030. [Google Scholar] [CrossRef]
- Takahashi, S.; Uemura, H.; Seeni, A.; Tang, M.; Komiya, M.; Long, N.; Ishiguro, H.; Kubota, Y.; Shirai, T. Therapeutic targeting of angiotensin II receptor type 1 to regulate androgen receptor in prostate cancer. Prostate 2012, 72, 1559–1572. [Google Scholar] [CrossRef]
- Kim, H.K.; Youm, J.B.; Lee, S.R.; Lim, S.E.; Lee, S.Y.; Ko, T.H.; Long le, T.; Nilius, B.; Won du, N.; Noh, J.H.; et al. The angiotensin receptor blocker and PPAR-gamma agonist, telmisartan, delays inactivation of voltage-gated sodium channel in rat heart: Novel mechanism of drug action. Pflugers Arch. 2012, 464, 631–643. [Google Scholar] [CrossRef]
- Friedmann Angeli, J.P.; Krysko, D.V.; Conrad, M. Ferroptosis at the crossroads of cancer-acquired drug resistance and immune evasion. Nat. Rev. Cancer 2019, 19, 405–414. [Google Scholar] [CrossRef]
- Jensen, S.; Seidelin, J.B.; LaCasse, E.C.; Nielsen, O.H. SMAC mimetics and RIPK inhibitors as therapeutics for chronic inflammatory diseases. Sci. Signal. 2020, 13, eaax8295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornick, J.R.; Vangveravong, S.; Spitzer, D.; Abate, C.; Berardi, F.; Goedegebuure, P.; Mach, R.H.; Hawkins, W.G. Lysosomal membrane permeabilization is an early event in Sigma-2 receptor ligand mediated cell death in pancreatic cancer. J. Exp. Clin. Cancer Res. 2012, 31, 41. [Google Scholar] [CrossRef] [Green Version]
- Mir, S.U.; Schwarze, S.R.; Jin, L.; Zhang, J.; Friend, W.; Miriyala, S.; St Clair, D.; Craven, R.J. Progesterone receptor membrane component 1/Sigma-2 receptor associates with MAP1LC3B and promotes autophagy. Autophagy 2013, 9, 1566–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pati, M.L.; Hornick, J.R.; Niso, M.; Berardi, F.; Spitzer, D.; Abate, C.; Hawkins, W. Sigma-2 receptor agonist derivatives of 1-Cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine (PB28) induce cell death via mitochondrial superoxide production and caspase activation in pancreatic cancer. BMC Cancer 2017, 17, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, C.; Rothfuss, J.; Zhang, J.; Chu, W.; Vangveravong, S.; Tu, Z.; Pan, F.; Chang, K.C.; Hotchkiss, R.; Mach, R.H. Sigma-2 ligands induce tumour cell death by multiple signalling pathways. Br. J. Cancer 2012, 106, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Ryu, C.S.; Klein, K.; Zanger, U.M. Membrane Associated Progesterone Receptors: Promiscuous Proteins with Pleiotropic Functions—Focus on Interactions with Cytochromes P450. Front. Pharmacol. 2017, 8, 159. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Resh, M.D. Cholesterol depletion from the plasma membrane triggers ligand-independent activation of the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 49631–49637. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, I.; Liang, J.; Hedeen, D.; Roberts, K.J.; Zhang, Y.; Ha, B.; Latorraca, N.R.; Faust, B.; Dror, R.O.; Beachy, P.A.; et al. Smoothened stimulation by membrane sterols drives Hedgehog pathway activity. Nature 2019, 571, 284–288. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef] [Green Version]
- Badana, A.K.; Chintala, M.; Gavara, M.M.; Naik, S.; Kumari, S.; Kappala, V.R.; Iska, B.R.; Malla, R.R. Lipid rafts disruption induces apoptosis by attenuating expression of LRP6 and survivin in triple negative breast cancer. Biomed. Pharmacother. 2018, 97, 359–368. [Google Scholar] [CrossRef]
- Ma, X.; Bi, E.; Huang, C.; Lu, Y.; Xue, G.; Guo, X.; Wang, A.; Yang, M.; Qian, J.; Dong, C.; et al. Cholesterol negatively regulates IL-9-producing CD8(+) T cell differentiation and antitumor activity. J. Exp. Med. 2018, 215, 1555–1569. [Google Scholar] [CrossRef] [PubMed]
- Eibinger, G.; Fauler, G.; Bernhart, E.; Frank, S.; Hammer, A.; Wintersperger, A.; Eder, H.; Heinemann, A.; Mischel, P.S.; Malle, E.; et al. On the role of 25-hydroxycholesterol synthesis by glioblastoma cell lines. Implications for chemotactic monocyte recruitment. Exp. Cell Res. 2013, 319, 1828–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baek, A.E.; Yu, Y.A.; He, S.; Wardell, S.E.; Chang, C.Y.; Kwon, S.; Pillai, R.V.; McDowell, H.B.; Thompson, J.W.; Dubois, L.G.; et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat. Commun. 2017, 8, 864. [Google Scholar] [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Primers 2015, 1, 15056. [Google Scholar] [CrossRef]
- Karran, E.; Mercken, M.; De Strooper, B. The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011, 10, 698–712. [Google Scholar] [CrossRef]
- Di Paolo, G.; Kim, T.W. Linking lipids to Alzheimer’s disease: Cholesterol and beyond. Nat. Rev. Neurosci. 2011, 12, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Sims, R.; Hill, M.; Williams, J. The multiplex model of the genetics of Alzheimer’s disease. Nat. Neurosci. 2020, 23, 311–322. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.C.; Bu, G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef]
- Karasinska, J.M.; Hayden, M.R. Cholesterol metabolism in Huntington disease. Nat. Rev. Neurol. 2011, 7, 561–572. [Google Scholar] [CrossRef]
- Gong, J.S.; Sawamura, N.; Zou, K.; Sakai, J.; Yanagisawa, K.; Michikawa, M. Amyloid beta-protein affects cholesterol metabolism in cultured neurons: Implications for pivotal role of cholesterol in the amyloid cascade. J. Neurosci. Res. 2002, 70, 438–446. [Google Scholar] [CrossRef]
- Mohamed, A.; Saavedra, L.; Di Pardo, A.; Sipione, S.; Posse de Chaves, E. Beta-amyloid inhibits protein prenylation and induces cholesterol sequestration by impairing SREBP-2 cleavage. J. Neurosci. 2012, 32, 6490–6500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.Y.; Kwon, O.H.; Park, M.K.; Kim, T.W.; Chung, S. Elevated cellular cholesterol in Familial Alzheimer’s presenilin 1 mutation is associated with lipid raft localization of beta-amyloid precursor protein. PLoS ONE 2019, 14, e0210535. [Google Scholar] [CrossRef]
- Pappolla, M.A.; Bryant-Thomas, T.K.; Herbert, D.; Pacheco, J.; Fabra Garcia, M.; Manjon, M.; Girones, X.; Henry, T.L.; Matsubara, E.; Zambon, D.; et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 2003, 61, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.S.; Tseng, P.T.; Stubbs, B.; Chen, T.Y.; Tang, C.H.; Li, D.J.; Yang, W.C.; Chen, Y.W.; Wu, C.K.; Veronese, N.; et al. Use of statins and the risk of dementia and mild cognitive impairment: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 5804. [Google Scholar] [CrossRef]
- Poly, T.N.; Islam, M.M.; Walther, B.A.; Yang, H.C.; Wu, C.C.; Lin, M.C.; Li, Y.C. Association between Use of Statin and Risk of Dementia: A Meta-Analysis of Observational Studies. Neuroepidemiology 2020, 54, 214–226. [Google Scholar] [CrossRef]
- Wagstaff, L.R.; Mitton, M.W.; Arvik, B.M.; Doraiswamy, P.M. Statin-associated memory loss: Analysis of 60 case reports and review of the literature. Pharmacotherapy 2003, 23, 871–880. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, Y.Y.; Liu, X.G.; Kuo, S.H.; Liu, N.; Song, Q.Y.; Wang, M.W. Cholesterol, 24-Hydroxycholesterol, and 27-Hydroxycholesterol as Surrogate Biomarkers in Cerebrospinal Fluid in Mild Cognitive Impairment and Alzheimer’s Disease: A Meta-Analysis. J. Alzheimers Dis. 2016, 51, 45–55. [Google Scholar] [CrossRef]
- Kolsch, H.; Heun, R.; Kerksiek, A.; Bergmann, K.V.; Maier, W.; Lutjohann, D. Altered levels of plasma 24S- and 27-hydroxycholesterol in demented patients. Neurosci. Lett. 2004, 368, 303–308. [Google Scholar] [CrossRef]
- Heverin, M.; Bogdanovic, N.; Lutjohann, D.; Bayer, T.; Pikuleva, I.; Bretillon, L.; Diczfalusy, U.; Winblad, B.; Bjorkhem, I. Changes in the levels of cerebral and extracerebral sterols in the brain of patients with Alzheimer’s disease. J. Lipid Res. 2004, 45, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Brown, J., 3rd; Theisler, C.; Silberman, S.; Magnuson, D.; Gottardi-Littell, N.; Lee, J.M.; Yager, D.; Crowley, J.; Sambamurti, K.; Rahman, M.M.; et al. Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J. Biol. Chem. 2004, 279, 34674–34681. [Google Scholar] [CrossRef] [Green Version]
- Yau, J.L.; Rasmuson, S.; Andrew, R.; Graham, M.; Noble, J.; Olsson, T.; Fuchs, E.; Lathe, R.; Seckl, J.R. Dehydroepiandrosterone 7-hydroxylase CYP7B: Predominant expression in primate hippocampus and reduced expression in Alzheimer’s disease. Neuroscience 2003, 121, 307–314. [Google Scholar] [CrossRef]
- Bouchard, P.; Quirion, R. [3H]1,3-di(2-tolyl)guanidine and [3H](+)pentazocine binding sites in the rat brain: Autoradiographic visualization of the putative sigma1 and sigma2 receptor subtypes. Neuroscience 1997, 76, 467–477. [Google Scholar] [CrossRef]
- Sahlholm, K.; Liao, F.; Holtzman, D.M.; Xu, J.; Mach, R.H. Sigma-2 receptor binding is decreased in female, but not male, APP/PS1 mice. Biochem. Biophys. Res. Commun. 2015, 460, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Izzo, N.J.; Staniszewski, A.; To, L.; Fa, M.; Teich, A.F.; Saeed, F.; Wostein, H.; Walko, T., 3rd; Vaswani, A.; Wardius, M.; et al. Alzheimer’s therapeutics targeting amyloid beta 1-42 oligomers I: Abeta 42 oligomer binding to specific neuronal receptors is displaced by drug candidates that improve cognitive deficits. PLoS ONE 2014, 9, e111898. [Google Scholar] [CrossRef]
- Hartley, D.M.; Walsh, D.M.; Ye, C.P.; Diehl, T.; Vasquez, S.; Vassilev, P.M.; Teplow, D.B.; Selkoe, D.J. Protofibrillar intermediates of amyloid beta-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J. Neurosci. 1999, 19, 8876–8884. [Google Scholar] [CrossRef] [Green Version]
- Xiong, H.; Callaghan, D.; Jones, A.; Walker, D.G.; Lue, L.F.; Beach, T.G.; Sue, L.I.; Woulfe, J.; Xu, H.; Stanimirovic, D.B.; et al. Cholesterol retention in Alzheimer’s brain is responsible for high beta- and gamma-secretase activities and Abeta production. Neurobiol. Dis. 2008, 29, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Simons, M.; Keller, P.; De Strooper, B.; Beyreuther, K.; Dotti, C.G.; Simons, K. Cholesterol depletion inhibits the generation of beta-amyloid in hippocampal neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 6460–6464. [Google Scholar] [CrossRef] [Green Version]
- Osenkowski, P.; Ye, W.; Wang, R.; Wolfe, M.S.; Selkoe, D.J. Direct and potent regulation of gamma-secretase by its lipid microenvironment. J. Biol. Chem. 2008, 283, 22529–22540. [Google Scholar] [CrossRef] [Green Version]
- Vetrivel, K.S.; Cheng, H.; Kim, S.H.; Chen, Y.; Barnes, N.Y.; Parent, A.T.; Sisodia, S.S.; Thinakaran, G. Spatial segregation of gamma-secretase and substrates in distinct membrane domains. J. Biol. Chem. 2005, 280, 25892–25900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetrivel, K.S.; Meckler, X.; Chen, Y.; Nguyen, P.D.; Seidah, N.G.; Vassar, R.; Wong, P.C.; Fukata, M.; Kounnas, M.Z.; Thinakaran, G. Alzheimer disease Abeta production in the absence of S-palmitoylation-dependent targeting of BACE1 to lipid rafts. J. Biol. Chem. 2009, 284, 3793–3803. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, A.M.; Ferreira, A. Increased membrane cholesterol might render mature hippocampal neurons more susceptible to beta-amyloid-induced calpain activation and tau toxicity. J. Neurosci. 2009, 29, 4640–4651. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, N.; Gong, J.S.; Chang, T.Y.; Yanagisawa, K.; Michikawa, M. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J. Neurochem. 2003, 84, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Steffensen, K.R.; Jakobsson, T.; Gustafsson, J.A. Targeting liver X receptors in inflammation. Expert Opin. Ther. Targets 2013, 17, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, C.; Wei, Z.; Wang, Y.; Zhang, X.; Fu, Y. Activation of liver X receptors inhibit LPS-induced inflammatory response in primary bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2018, 197, 87–92. [Google Scholar] [CrossRef]
- Testa, G.; Gamba, P.; Badilli, U.; Gargiulo, S.; Maina, M.; Guina, T.; Calfapietra, S.; Biasi, F.; Cavalli, R.; Poli, G.; et al. Loading into nanoparticles improves quercetin’s efficacy in preventing neuroinflammation induced by oxysterols. PLoS ONE 2014, 9, e96795. [Google Scholar] [CrossRef] [Green Version]

| Compound | Putative Action | Assays Used | Reference |
|---|---|---|---|
CB-184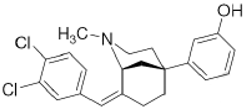 | Agonist (sigma-2/TMEM97) | Apoptosis assay, Lactate dehydrogenase (LDH) release | [30,31] |
CM398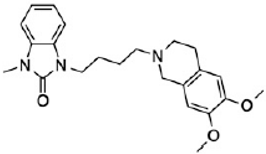 | ? (sigma-2/TMEM97) | [23] | |
CT1812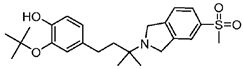 | Antagonist (sigma-2/PGRMC1) | Trafficking assay | [32,33] |
DKR-1677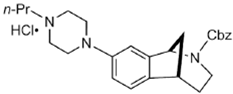 | ? (sigma-2/TMEM97) | [22] | |
DTG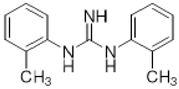 | Agonist (sigma-1 and sigma-2/TMEM97) | Apoptosis assay, LDH release | [34,35,36] |
JVW-1034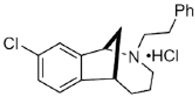 | ? (sigma-2/TMEM97) | [21] | |
PB221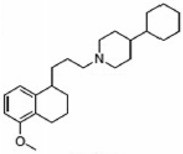 | Agonist (sigma-2/TMEM97) | (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay | [19] |
PB28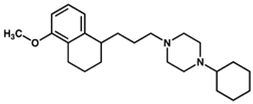 | Agonist (sigma-2/TMEM97) | Apoptosis assay, in vivo tumor xenografts | [35,37] |
RHM-4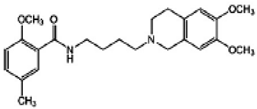 | Antagonist (sigma-2) | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) cell viability assay, Caspase-3 activation assay | [29] |
SAS-0132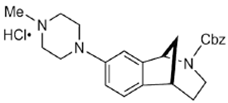 | Antagonist (sigma-2/PGRMC1) | Ca2+ assay | [28] |
Siramesine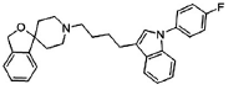 | Agonist (sigma-2/TMEM97) | LDH release, apoptosis assay, in vivo tumor xenografts | [38,39,40] |
SV119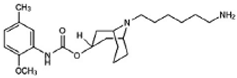 | Agonist (sigma-2/TMEM97) | LDH release, apoptosis assay, in vivo tumor xenografts | [41,42,43] |
UKH-1114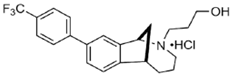 | Agonist (sigma-2/TMEM97) | Pain relief | [20] |
WC-26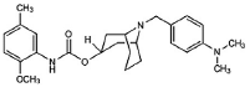 | Agonist (sigma-2/TMEM97) | Apoptosis assay, LDH release | [42,43,44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Zeng, C.; Wang, C.; Sun, M.; Yin, D.; Sun, T. Sigma-2 Receptor—A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis. Molecules 2020, 25, 5439. https://doi.org/10.3390/molecules25225439
Yang K, Zeng C, Wang C, Sun M, Yin D, Sun T. Sigma-2 Receptor—A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis. Molecules. 2020; 25(22):5439. https://doi.org/10.3390/molecules25225439
Chicago/Turabian StyleYang, Kai, Cheng Zeng, Changcai Wang, Meng Sun, Dan Yin, and Taolei Sun. 2020. "Sigma-2 Receptor—A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis" Molecules 25, no. 22: 5439. https://doi.org/10.3390/molecules25225439





