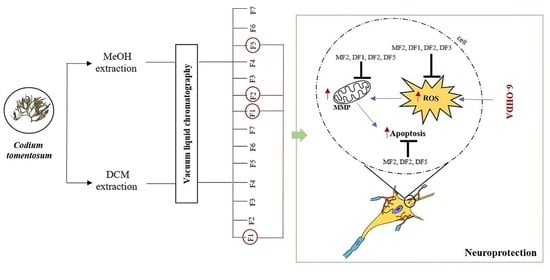
Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium tomentosum
Abstract
:1. Introduction
2. Results
2.1. Yields, Total Phenolic Content and Antioxidant Activity of Codium tomentosum Fractions
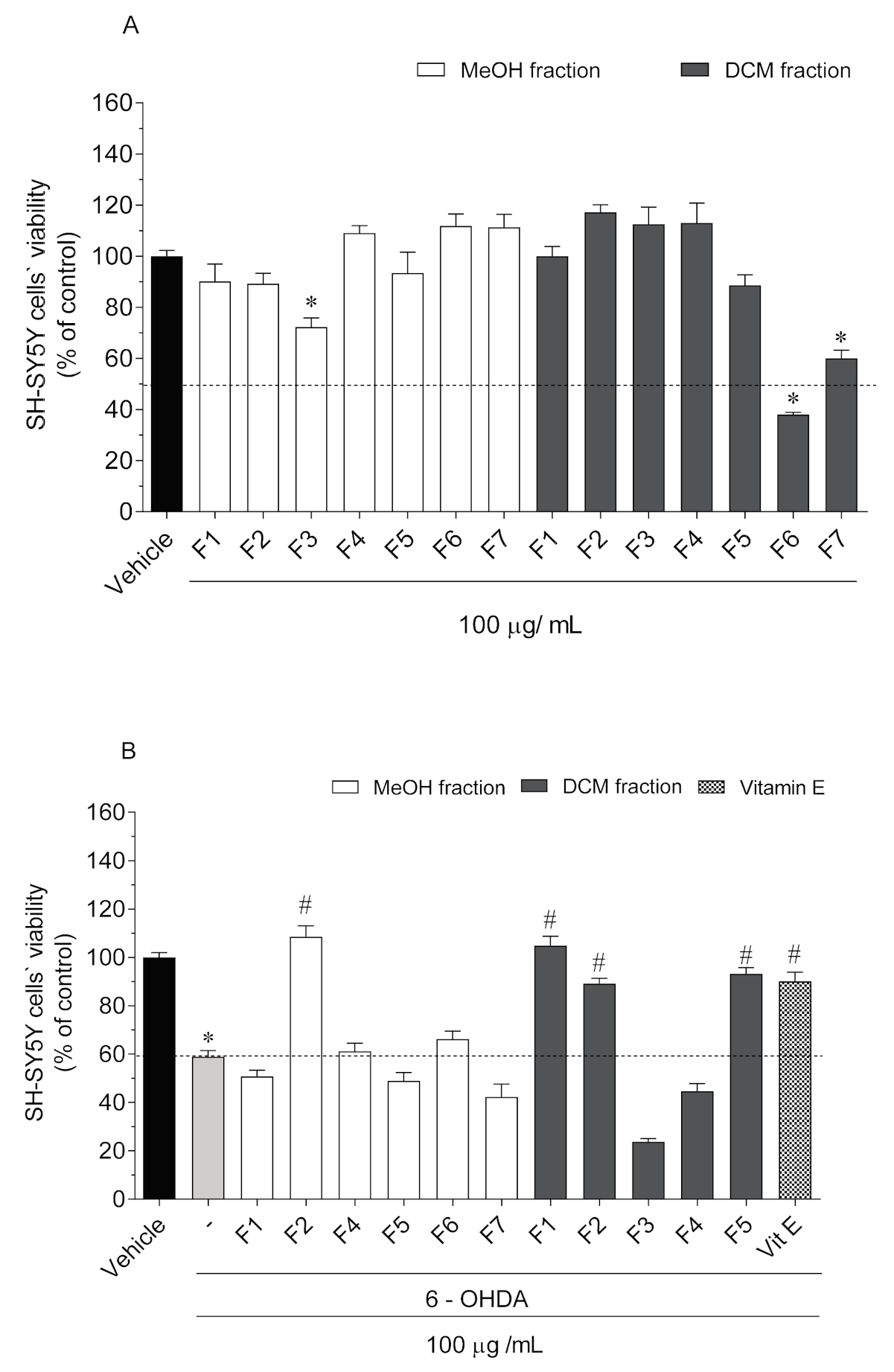
2.2. Neurotoxicity and Neuroprotective Effects of Codium tomentosum Fractions on SH-SY5Y Cells
2.3. Neuroprotective Effects of Codium tomentosum Fractions on PD Hallmarks
2.4. Chemical Characterization of Codium tomentosum Bioactive Fractions
3. Discussion
4. Materials and Methods
4.1. Collection and Preparation of Codium tomentosum Samples
4.2. Seaweed Extraction and Fractionation
4.3. Quantification of Total Phenolic Content (TPC)
4.4. Evaluation of Antioxidant Activity
4.4.1. 2-Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Activity
4.4.2. Oxygen Radical Absorbance Capacity (ORAC-Fluorescein)
4.4.3. Ferric Reducing Antioxidant Power (FRAP)
4.5. Evaluation of the Neuroprotective Potential of Codium tomentosum
4.5.1. Cell Culture Maintenance Conditions
4.5.2. Neurotoxic and Neuroprotective Potential
4.5.3. Neuroprotective Effects on PD Biomarkers
Determination of Intracellular Reactive Oxygen Species (ROS) Levels
Mitochondrial Membrane Potential (MMP)
Caspase-3 Activity
4.6. Chemical Characterization of Codium tomentosum Bioactive Samples
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
4.7. Data and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pangestuti, R.; Kim, S.-K. Neuroprotective effects of marine algae. Mar. Drugs 2011, 9, 803–818. [Google Scholar] [CrossRef]
- El Gamal, A.A. Biological importance of marine algae. Saudi Pharm. J. 2010, 18, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.R. Protein Aggregation and Fibrillogenesis in Cerebral and Systemic Amyloid Disease; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; Volume 65. [Google Scholar]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative diseases: Regenerative mechanisms and novel therapeutic approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radhakrishnan, D.M.; Goyal, V. Parkinson’s disease: A review. Neurol. India 2018, 66, 26. [Google Scholar]
- Del Rey, N.L.-G.; Quiroga-Varela, A.; Garbayo, E.; Carballo-Carbajal, I.; Fernández-Santiago, R.; Monje, M.H.; Trigo-Damas, I.; Blanco-Prieto, M.J.; Blesa, J. Advances in Parkinson’s disease: 200 years later. Front. Neuroanat. 2018, 12, 113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Raymick, J.; Imam, S. Neuroprotective and therapeutic strategies against Parkinson’s disease: Recent perspectives. Int. J. Mol. Sci. 2016, 17, 904. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Alves, C.; Freitas, R.; Martins, A.; Pinteus, S.; Ribeiro, J.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Antioxidant and Neuroprotective Potential of the Brown Seaweed Bifurcaria bifurcata in an in vitro Parkinson’s Disease Model. Mar. Drugs 2019, 17, 85. [Google Scholar] [CrossRef] [Green Version]
- Pinteus, S.; Silva, J.; Alves, C.; Horta, A.; Fino, N.; Rodrigues, A.I.; Mendes, S.; Pedrosa, R. Cytoprotective effect of seaweeds with high antioxidant activity from the Peniche coast (Portugal). Food Chem. 2017, 218, 591–599. [Google Scholar] [CrossRef]
- Pinteus, S.; Alves, C.; Monteiro, H.; Araújo, E.; Horta, A.; Pedrosa, R. Asparagopsis armata and Sphaerococcus coronopifolius as a natural source of antimicrobial compounds. World J. Microbiol. Biotechnol. 2015, 31, 445–451. [Google Scholar] [CrossRef]
- Mhadhebi, L.; Mhadhebi, A.; Robert, J.; Bouraoui, A. Antioxidant, Anti-inflammatory and Antiproliferative Effects of Aqueous Extracts of Three Mediterranean Brown Seaweeds of the Genus Cystoseira. Iran J. Pharm. Res. 2014, 13, 207–220. [Google Scholar]
- Alves, C.; Pinteus, S.; Rodrigues, A.; Horta, A.; Pedrosa, R. Algae from Portuguese coast presented high cytotoxicity and antiproliferative effects on an in vitro model of human colorectal cancer. Pharmacogn. Res. 2018, 10, 24. [Google Scholar]
- Silva, J.; Alves, C.; Pinteus, S.; Mendes, S.; Pedrosa, R. Neuroprotective effects of seaweeds against 6-hydroxydopamine-induced cell death on an in vitro human neuroblastoma model. BMC Complement. Altern. Med. 2018, 18, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannan, M.A.; Dash, R.; Haque, M.N.; Mohibbullah, M.; Sohag, A.A.M.; Rahman, M.A.; Uddin, M.J.; Alam, M.; Moon, I.S. Neuroprotective potentials of marine algae and their bioactive metabolites: Pharmacological insights and therapeutic advances. Mar. Drugs 2020, 18, 347. [Google Scholar] [CrossRef]
- Souza, R.B.; Frota, A.F.; Silva, J.; Alves, C.; Neugebauer, A.Z.; Pinteus, S.; Rodrigues, J.A.G.; Cordeiro, E.M.S.; de Almeida, R.R.; Pedrosa, R. In vitro activities of kappa-carrageenan isolated from red marine alga Hypnea musciformis: Antimicrobial, anticancer and neuroprotective potential. Int. J. Biol. Macromol. 2018, 112, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.-H.; Chao, C.-H.; Wu, M.-H.; Sheu, J.-H. A neuroprotective sulfone of marine origin and the in vivo anti-inflammatory activity of an analogue. Eur. J. Med. Chem. 2010, 45, 5998–6004. [Google Scholar] [CrossRef]
- Gao, Y.; Dong, C.; Yin, J.; Shen, J.; Tian, J.; Li, C. Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell. Mol. Neurobiol. 2012, 32, 523–529. [Google Scholar] [CrossRef]
- Valentão, P.; Trindade, P.; Gomes, D.; de Pinho, P.G.; Mouga, T.; Andrade, P.B. Codium tomentosum and Plocamium cartilagineum: Chemistry and antioxidant potential. Food Chem. 2010, 119, 1359–1368. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.-W.; Lee, K.-T.; Kwon, J.; Lee, H.J.; Lee, D.; Mar, W. Neuroprotection against 6-OHDA-induced oxidative stress and apoptosis in SH-SY5Y cells by 5, 7-Dihydroxychromone: Activation of the Nrf2/ARE pathway. Life Sci. 2015, 130, 25–30. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Cirmi, S.; Maugeri, A.; Russo, C.; Lombardo, G.E.; Gangemi, S.; Calapai, G.; Mollace, V.; Navarra, M. Neuroprotective Effect of Bergamot Juice in 6-OHDA-Induced SH-SY5Y Cell Death, an In Vitro Model of Parkinson’s Disease. Pharmaceutics 2020, 12, 326. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Pallares, J.; Parga, J.; Munoz, A.; Rey, P.; Guerra, M.; Labandeira-Garcia, J. Mechanism of 6-hydroxydopamine neurotoxicity: The role of NADPH oxidase and microglial activation in 6-hydroxydopamine-induced degeneration of dopaminergic neurons. J. Neurochem. 2007, 103, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wang, G. Mitochondrial dysfunction in Parkinson’s disease. Transl. Neurodegener. 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abou-Sleiman, P.M.; Muqit, M.M.; Wood, N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006, 7, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Q.; Huang, Y.; Yuan, Y.; Ma, Q.; Du, M.; Cai, T.; Cai, Y. Extraction of polysaccharide from Spirulina and evaluation of its activities. Evid.-Based Complementary Altern. Med. 2018. [Google Scholar] [CrossRef] [Green Version]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Šarkanj, B.; Cikoš, A.-M.; Aladić, K.; Pedisić, S.; Jokić, S. Chemical Diversity of Codium bursa (Olivi) C. Agardh Headspace Compounds, Volatiles, Fatty Acids and Insight into Its Antifungal Activity. Molecules 2019, 24, 842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erekat, N.S. Apoptosis and its Role in Parkinson’s Disease. Exon Publ. 2018. [Google Scholar] [CrossRef] [Green Version]
- Mohd Sairazi, N.S.; Sirajudeen, K. Natural Products and Their Bioactive Compounds: Neuroprotective Potentials against Neurodegenerative Diseases. Evid.-Based Complement. Altern. Med. 2020. [Google Scholar] [CrossRef]
- Bălașa, A.F.; Chircov, C.; Grumezescu, A.M. Marine Biocompounds for Neuroprotection—A Review. Mar. Drugs 2020, 18, 290. [Google Scholar] [CrossRef]
- Huang, C.; Zhang, Z.; Cui, W. Marine-derived natural compounds for the treatment of Parkinson’s disease. Mar. Drugs 2019, 17, 221. [Google Scholar] [CrossRef] [Green Version]
- Carrera, I.; Cacabelos, R. Current drugs and potential future neuroprotective compounds for Parkinson’s disease. Curr. Neuropharmacol. 2019, 17, 295–306. [Google Scholar] [CrossRef]
- Xicoy, H.; Wieringa, B.; Martens, G.J. The role of lipids in Parkinson’s disease. Cells 2019, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, D.; Alves, C.; Horta, A.; Pinteus, S.; Silva, J.; Culioli, G.; Thomas, O.P.; Pedrosa, R. Antitumor and antimicrobial potential of bromoditerpenes isolated from the red alga, Sphaerococcus coronopifolius. Mar. Drugs 2015, 13, 713–726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rey, F.; Cartaxana, P.; Melo, T.; Calado, R.; Pereira, R.; Abreu, H.; Domingues, P.; Cruz, S.; Domingues, M.R. Domesticated populations of Codium tomentosum display lipid extracts with lower seasonal shifts than conspecifics from the wild—relevance for biotechnological applications of this green seaweed. Mar. Drugs 2020, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, E.; Melo, T.; Moreira, A.S.; Alves, E.; Domingues, P.; Calado, R.; Abreu, M.H.; Domingues, M.R. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015, 12, 388–397. [Google Scholar] [CrossRef]
- Lopes, G.; Sousa, C.; Bernardo, J.; Andrade, P.B.; Valentão, P.; Ferreres, F.; Mouga, T. Sterol profiles in 18 macroalgae of the portuguese coast. J. Phycol. 2011, 47, 1210–1218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xiao, J.; Zhou, N.; Zheng, M.; Luo, X.; Jiang, H.; Chen, K. A genetic algorithm based support vector machine model for blood-brain barrier penetration prediction. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akhtar, A.; Andleeb, A.; Waris, T.S.; Bazzar, M.; Moradi, A.-R.; Awan, N.R.; Yar, M. Neurodegenerative diseases and effective drug delivery: A review of challenges and novel therapeutics. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC− fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Ouazia, D.; Levros, L.-C., Jr.; Rassart, E.; Desrosiers, R. Dopamine down-regulation of protein L-isoaspartyl methyltransferase is dependent on reactive oxygen species in SH-SY5Y cells. Neuroscience 2014, 267, 263–276. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |


| Crude Extract | Fraction | Yield (%) | TPC a | DPPH b | FRAP c | ORAC d |
|---|---|---|---|---|---|---|
| MeOH | MF1 | 0.09 | 2.24 ± 0.15 | >100 | 282.87 ± 14.27 | 99.91 ± 0.57 |
| MF2 | 3.29 | 3.96 ± 0.26 | >100 | 288.40 ± 11.42 | 28.92 ± 0.51 | |
| MF3 | 0.88 | 2.58 ± 0.13 | >100 | 282.99 ± 2.51 | 52.60 ± 1.28 | |
| MF4 | 0.57 | 7.30 ± 0.47 | >100 | 271.33 ± 7.34 | 74.76 ± 0.48 | |
| MF5 | 0.65 | 4.32 ± 0.30 | >100 | 273.01 ± 1.81 | 66.32 ± 0.95 | |
| MF6 | 6.73 | 4.38 ± 0.12 | >100 | 292.73 ± 3.73 | 72.73 ± 1.36 | |
| MF7 | 16.89 | 2.47 ± 0.05 | >100 | 23.79 ± 8.45 | 98.55 ± 2.28 | |
| CH2Cl2 | DF1 | 0.58 | 4.18 ± 0.13 | >100 | 24.40 ± 1.22 | 38.88 ± 0.73 |
| DF2 | 1.52 | 10.43 ± 0.40 | >100 | 67.67 ± 8.05 | 62.06 ± 1.67 | |
| DF3 | 1.53 | 5.51 ± 0.75 | >100 | 825.73 ± 32.59 | 98.84 ± 6.00 | |
| DF4 | 1.00 | 3.51 ± 0.20 | >100 | 1008.27 ± 18.18 | 144.15 ± 2.02 | |
| DF5 | 6.35 | 4.30 ± 0.23 | >100 | 636.21 ± 22.21 | 94.56 ± 1.02 | |
| DF6 | 13.88 | 4.57 ± 0.17 | >100 | 356.95 ± 3.73 | 165.34 ± 6.42 | |
| DF7 | 8.58 | 5.69 ± 0.16 | >100 | 582.11 ± 20.81 | 193.85 ± 14.30 | |
| BHT | - | - | >100 | 2821.50 ± 51.27 | 142.87 ± 8.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, J.; Martins, A.; Alves, C.; Pinteus, S.; Gaspar, H.; Alfonso, A.; Pedrosa, R. Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium tomentosum. Molecules 2020, 25, 5478. https://doi.org/10.3390/molecules25225478
Silva J, Martins A, Alves C, Pinteus S, Gaspar H, Alfonso A, Pedrosa R. Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium tomentosum. Molecules. 2020; 25(22):5478. https://doi.org/10.3390/molecules25225478
Chicago/Turabian StyleSilva, Joana, Alice Martins, Celso Alves, Susete Pinteus, Helena Gaspar, Amparo Alfonso, and Rui Pedrosa. 2020. "Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium tomentosum" Molecules 25, no. 22: 5478. https://doi.org/10.3390/molecules25225478
APA StyleSilva, J., Martins, A., Alves, C., Pinteus, S., Gaspar, H., Alfonso, A., & Pedrosa, R. (2020). Natural Approaches for Neurological Disorders—The Neuroprotective Potential of Codium tomentosum. Molecules, 25(22), 5478. https://doi.org/10.3390/molecules25225478