Synthesis of 5?-Thiamine-Capped RNA
Abstract
:1. Introduction
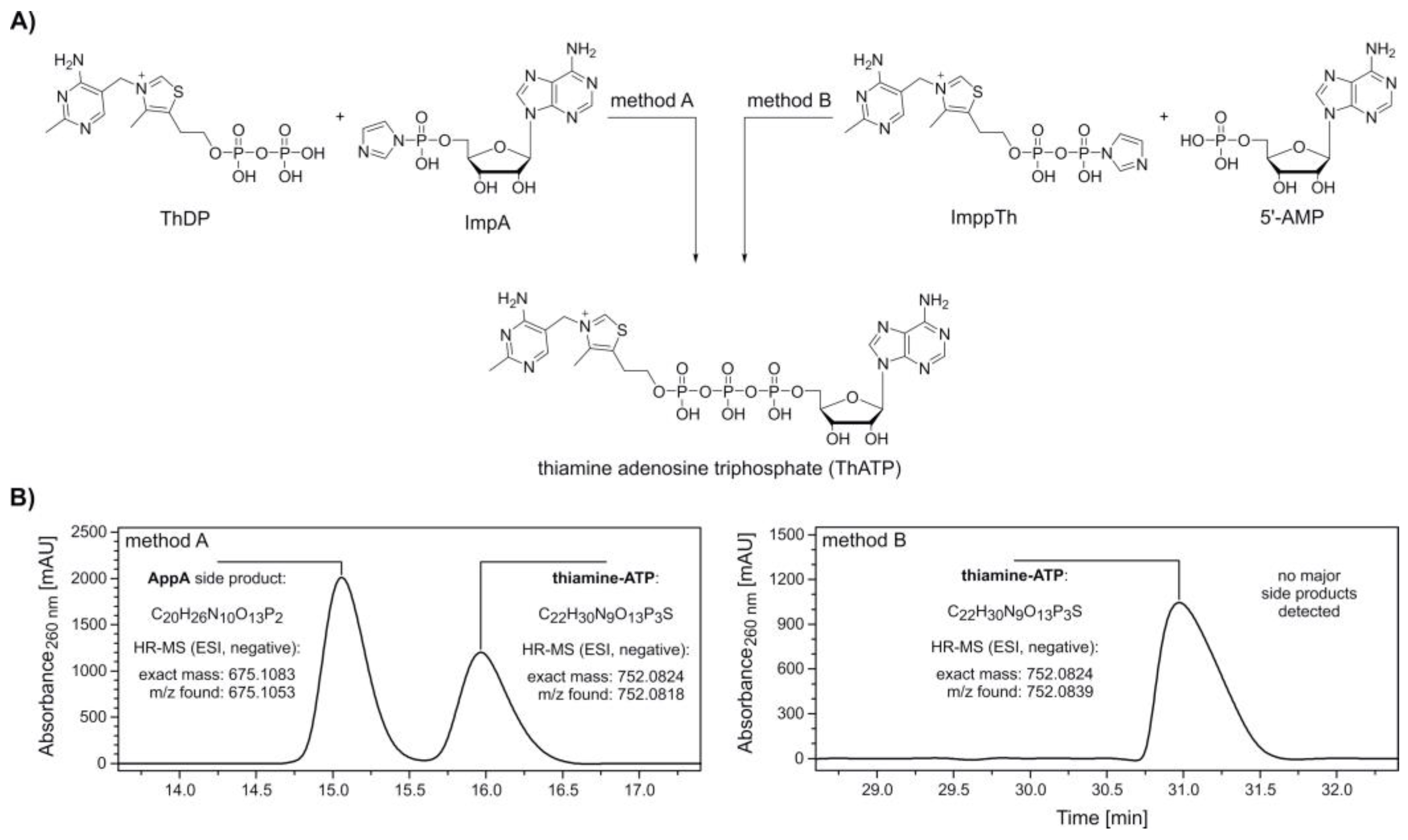
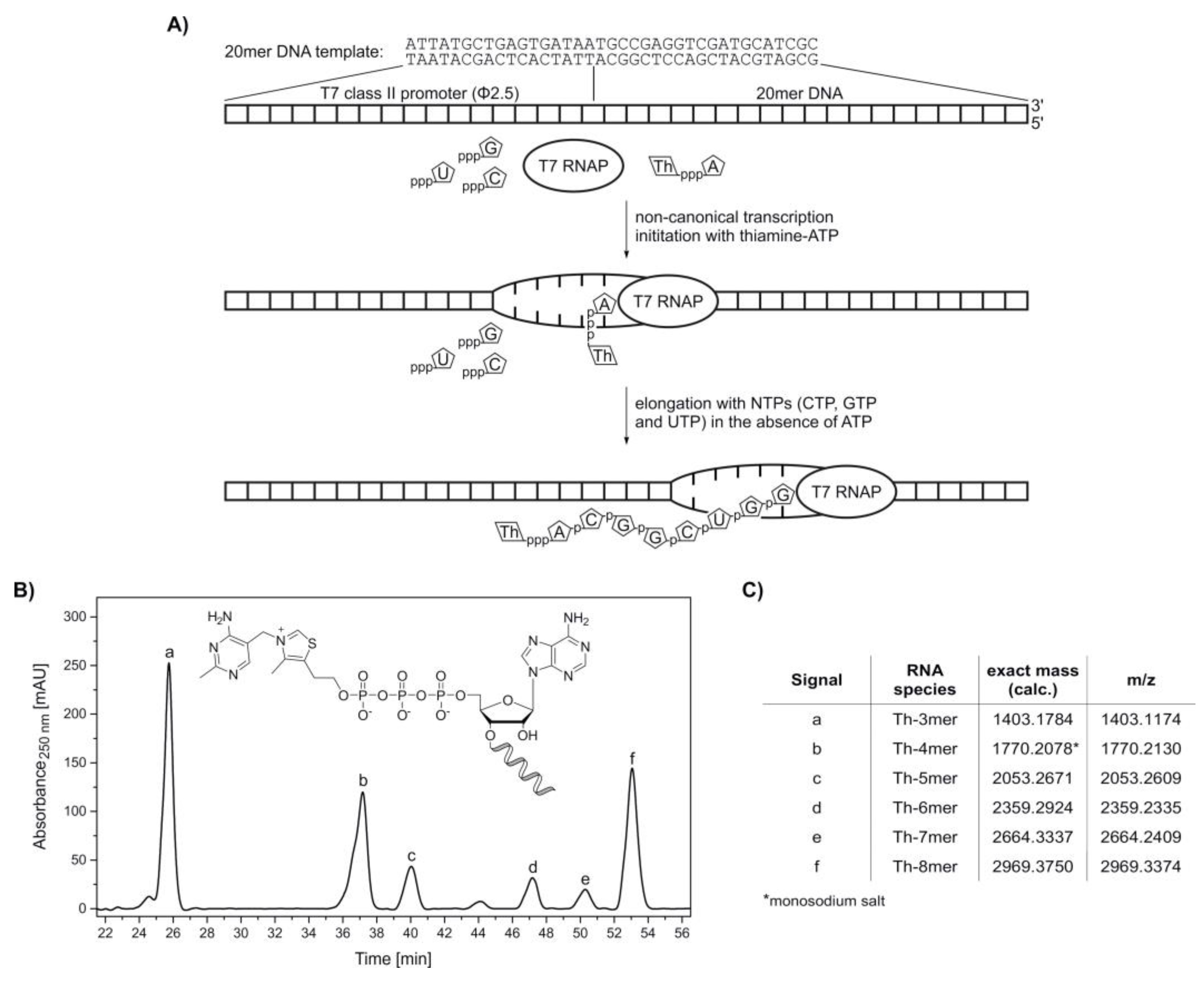
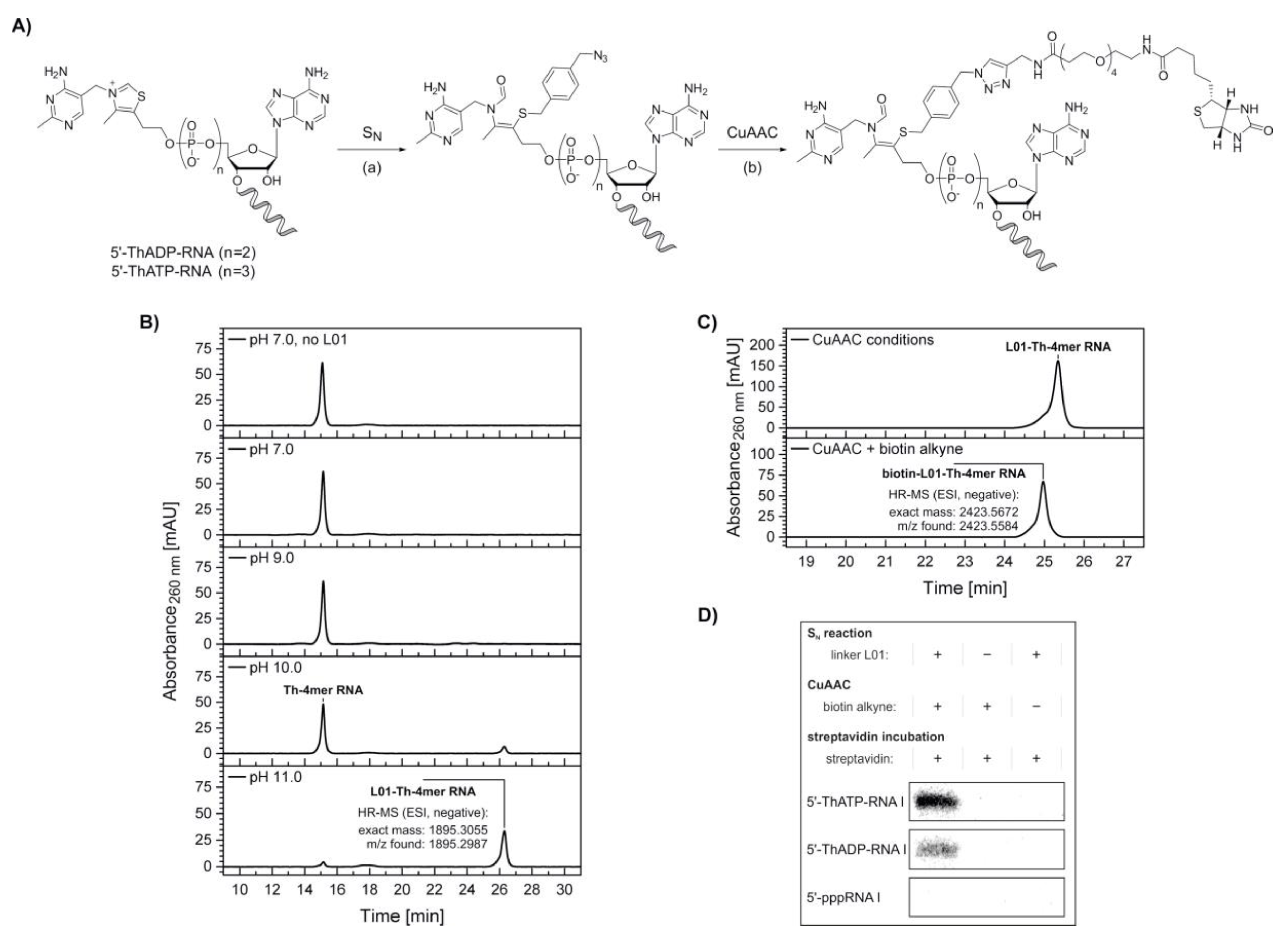
2. Results
3. Discussion
4. Materials and Methods
4.1. General
4.2. Chemical Synthesis
4.3. Preparation, Purification, Modification and Analysis of Ribonucleic Acids
4.3.1. Preparation of 5′-Thiamine-Capped RNA via ImppTh Reaction and Xrn1 Digest
4.3.2. Preparation of DNA Templates for In Vitro Transcription
4.3.3. In Vitro Transcription
4.3.4. Modification of the 5′-Thiamine Cap via Nucleophilic Substitution and Copper-Catalyzed Azide-Alkyne Cycloaddition
4.3.5. Streptavidin Retardation Assay of 5′-Thiamine-Capped RNA
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Doudna, J.A.; Cech, T.R. The chemical repertoire of natural ribozymes. Nature 2002, 418, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Helm, M.; Alfonzo, J.D. Posttranscriptional RNA Modifications: Playing metabolic games in a cell’s chemical Legoland. Chem. Biol. 2014, 21, 174–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yi, C.; Pan, T. Cellular dynamics of RNA modification. Acc. Chem. Res. 2011, 44, 1380–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccaletto, P.; Machnicka, M.A.; Purta, E.; Piątkowski, P.; Bagiński, B.; Wirecki, T.K.; de Crécy-Lagard, V.; Ross, R.; Limbach, P.A.; Kotter, A. MODOMICS: A database of RNA modification pathways. 2017 update. Nucleic Acids Res. 2018, 46, D303–D307. [Google Scholar] [CrossRef]
- Lewis, C.J.; Pan, T.; Kalsotra, A. RNA modifications and structures cooperate to guide RNA—Protein interactions. Nat. Rev. Mol. Cell Biol. 2017, 18, 202. [Google Scholar] [CrossRef] [Green Version]
- Mao, X.; Schwer, B.; Shuman, S. Yeast mRNA cap methyltransferase is a 50-kilodalton protein encoded by an essential gene. Mol. Cell. Biol. 1995, 15, 4167–4174. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.-M.; Gershowitz, A.; Moss, B. Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 1975, 4, 379–386. [Google Scholar] [CrossRef]
- Furuichi, Y.; LaFiandra, A.; Shatkin, A.J. 5′-Terminal structure and mRNA stability. Nature 1977, 266, 235–239. [Google Scholar] [CrossRef]
- Hsu, C.L.; Stevens, A. Yeast cells lacking 5′→3′ exoribonuclease 1 contain mRNA species that are poly(A) deficient and partially lack the 5′ cap structure. Mol. Cell. Biol. 1993, 13, 4826–4835. [Google Scholar] [CrossRef] [Green Version]
- Shatkin, A. Capping of eucaryotic mRNAs. Cell 1976, 9, 645–653. [Google Scholar] [CrossRef]
- Shatkin, A.J.; Manley, J.L. The ends of the affair: Capping and polyadenylation. Nat. Struct. Biol. 2000, 7, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Marbaniang, C.N.; Vogel, J. Emerging roles of RNA modifications in bacteria. Curr. Opin. Microbiol. 2016, 30, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.G.; Kowtoniuk, W.E.; Agarwal, I.; Shen, Y.; Liu, D.R. LC/MS analysis of cellular RNA reveals NAD-linked RNA. Nat. Chem. Biol. 2009, 5, 879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F. Efficient incorporation of CoA, NAD and FAD into RNA by in vitro transcription. Nucleic Acids Res. 2003, 31, e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, J.G.; Zhang, Y.; Tian, Y.; Panova, N.; Barvík, I.; Greene, L.; Liu, M.; Buckley, B.; Krásný, L.; Lee, J.K. The mechanism of RNA 5′ capping with NAD+, NADH and desphospho-CoA. Nature 2016, 535, 444. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Alvin Chew, B.L.; Lai, Y.; Dong, H.; Xu, L.; Balamkundu, S.; Cai, W.M.; Cui, L.; Liu, C.F.; Fu, X.-Y.; et al. Quantifying the RNA cap epitranscriptome reveals novel caps in cellular and viral RNA. Nucleic Acids Res. 2019, 47, e130. [Google Scholar] [CrossRef] [Green Version]
- Cahová, H.; Winz, M.-L.; Höfer, K.; Nübel, G.; Jäschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374. [Google Scholar] [CrossRef]
- Winz, M.-L.; Cahová, H.; Nübel, G.; Frindert, J.; Höfer, K.; Jäschke, A. Capture and sequencing of NAD-capped RNA sequences with NAD captureSeq. Nat. Protoc. 2017, 12, 122. [Google Scholar] [CrossRef]
- Frindert, J.; Zhang, Y.; Nübel, G.; Kahloon, M.; Kolmar, L.; Hotz-Wagenblatt, A.; Burhenne, J.; Haefeli, W.E.; Jäschke, A. Identification, biosynthesis, and decapping of NAD-capped RNAs in B. subtilis. Cell Rep. 2018, 24, 1890–1901.e1898. [Google Scholar] [CrossRef] [Green Version]
- Morales-Filloy, H.G.; Zhang, Y.; Nübel, G.; George, S.E.; Korn, N.; Wolz, C.; Jäschke, A. The 5′ NAD Cap of RNAIII Modulates Toxin Production in Staphylococcus aureus Isolates. J. Bacteriol. 2020, 202. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.W.; Matheny, T.; Mizoue, L.S.; Rao, B.S.; Muhlrad, D.; Parker, R. Identification of NAD+ capped mRNAs in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 2017, 114, 480–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, S.; Zhao, Y.; You, C.; Le, B.; Gong, Z.; Mo, B.; Xia, Y.; Chen, X. NAD+-capped RNAs are widespread in the Arabidopsis transcriptome and can probably be translated. Proc. Natl. Acad. Sci. USA 2019, 116, 12094–12102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, X.; Doamekpor, S.K.; Bird, J.G.; Nickels, B.E.; Tong, L.; Hart, R.P.; Kiledjian, M. 5′ end nicotinamide adenine dinucleotide cap in human cells promotes RNA decay through DXO-mediated deNADding. Cell 2017, 168, 1015–1027.e1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, J.G.; Basu, U.; Kuster, D.; Ramachandran, A.; Grudzien-Nogalska, E.; Towheed, A.; Wallace, D.C.; Kiledjian, M.; Temiakov, D.; Patel, S.S. Highly efficient 5′ capping of mitochondrial RNA with NAD+ and NADH by yeast and human mitochondrial RNA polymerase. Elife 2018, 7, e42179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kuster, D.; Schmidt, T.; Kirrmaier, D.; Nübel, G.; Ibberson, D.; Benes, V.; Hombauer, H.; Knop, M.; Jäschke, A. Extensive 5′-Surveillance Guards Against Non-Canonical NAD-Caps of Nuclear mRNAs in Yeast. bioRxiv 2020. [Google Scholar] [CrossRef] [PubMed]
- Doamekpor, S.K.; Grudzien-Nogalska, E.; Mlynarska-Cieslak, A.; Kowalska, J.; Kiledjian, M.; Tong, L. DXO/Rai1 enzymes remove 5′-end FAD and dephospho-CoA caps on RNAs. Nucleic Acids Res. 2020, 48, 6136–6148. [Google Scholar] [CrossRef] [PubMed]
- White, H.B. Coenzymes as fossils of an earlier metabolic state. J. Mol. Evol. 1976, 7, 101–104. [Google Scholar] [CrossRef]
- Monteverde, D.; Gómez-Consarnau, L.; Suffridge, C.; Sañudo-Wilhelmy, S.A. Life’s utilization of B vitamins on early Earth. Geobiology 2017, 15, 3–18. [Google Scholar] [CrossRef]
- Bettendorff, L.; Wirtzfeld, B.; Makarchikov, A.F.; Mazzucchelli, G.; Frédérich, M.; Gigliobianco, T.; Gangolf, M.; De Pauw, E.; Angenot, L.; Wins, P. Discovery of a natural thiamine adenine nucleotide. Nat. Chem. Biol. 2007, 3, 211. [Google Scholar] [CrossRef]
- Kluger, R.; Tittmann, K. Thiamin diphosphate catalysis: Enzymic and nonenzymic covalent intermediates. Chem. Rev. 2008, 108, 1797–1833. [Google Scholar] [CrossRef] [PubMed]
- Edwards, K.A.; Tu-Maung, N.; Cheng, K.; Wang, B.; Baeumner, A.J.; Kraft, C.E. Thiamine assays—Advances, challenges, and caveats. ChemistryOpen 2017, 6, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Makarchikov, A.F.; Lakaye, B.; Gulyai, I.; Czerniecki, J.; Coumans, B.; Wins, P.; Grisar, T.; Bettendorff, L. Thiamine triphosphate and thiamine triphosphatase activities: From bacteria to mammals. Cell. Mol. Life Sci. 2003, 60, 1477–1488. [Google Scholar] [CrossRef] [PubMed]
- Gigliobianco, T.; Lakaye, B.; Makarchikov, A.F.; Wins, P.; Bettendorff, L. Adenylate kinase-independent thiamine triphosphate accumulation under severe energy stress in Escherichia coli. BMC Microbiol. 2008, 8, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarchikov, A.F.; Brans, A.; Bettendorff, L. Thiamine diphosphate adenylyl transferase from E. coli: Functional characterization of the enzyme synthesizing adenosine thiamine triphosphate. BMC Biochem. 2007, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makarchikov, A.F.; Saroka, T.V.; Kudyrka, T.G.; Kolas, I.K.; Luchko, T.A.; Rusina, I.M.; Gurinovich, V.A. Adenosine thiamine triphosphate and adenosine thiamine triphosphate hydrolase activity in animal tissues. Ukr. Biochem. J. 2018, 90, 52–63. [Google Scholar] [CrossRef]
- Luciano, D.J.; Levenson-Palmer, R.; Belasco, J.G. Stresses that raise Np4A levels induce protective nucleoside tetraphosphate capping of bacterial RNA. Mol. Cell 2019, 75, 957–966. [Google Scholar] [CrossRef]
- Luciano, D.J.; Belasco, J.G. Np4A alarmones function in bacteria as precursors to RNA caps. Proc. Natl. Acad. Sci. USA 2020. [Google Scholar] [CrossRef]
- Frédérich, M.; Delvaux, D.; Gigliobianco, T.; Gangolf, M.; Dive, G.; Mazzucchelli, G.; Elias, B.; De Pauw, E.; Angenot, L.; Wins, P. Thiaminylated adenine nucleotides. Chemical synthesis, structural characterization and natural occurrence. FEBS J. 2009, 276, 3256–3268. [Google Scholar] [CrossRef]
- Hofer, A.; Marques, E.; Kieliger, N.; Gatter, S.-K.N.; Jordi, S.; Ferrari, E.; Hofmann, M.; Fitzpatrick, T.B.; Hottiger, M.O.; Jessen, H.J. Chemoselective dimerization of phosphates. Org. Lett. 2016, 18, 3222–3225. [Google Scholar] [CrossRef]
- Jemielity, J.; Fowler, T.; Zuberek, J.; Stepinski, J.; Lwedorowicz, M.; Niedzwiecka, A.; Stolarski, R.; Darzynkiewicz, E.; Rhoads, R.E. Novel “anti-reverse” cap analogs with superior translational properties. RNA 2003, 9, 1108–1122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rydzik, A.M.; Lukaszewicz, M.; Zuberek, J.; Kowalska, J.; Darzynkiewicz, Z.M.; Darzynkiewicz, E.; Jemielity, J. Synthetic dinucleotide mRNA cap analogs with tetraphosphate 5′, 5′ bridge containing methylenebis(phosphonate) modification. Org. Biomol. Chem. 2009, 7, 4763–4776. [Google Scholar] [CrossRef] [PubMed]
- Lau, N.C.; Lim, L.P.; Weinstein, E.G.; Bartel, D.P. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science 2001, 294, 858–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hafner, M.; Landgraf, P.; Ludwig, J.; Rice, A.; Ojo, T.; Lin, C.; Holoch, D.; Lim, C.; Tuschl, T. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods 2008, 44, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höfer, K.; Abele, F.; Schlotthauer, J.; Jäschke, A. Synthesis of 5′-NAD-capped RNA. Bioconjug. Chem. 2016, 27, 874–877. [Google Scholar] [CrossRef] [PubMed]
- Thillier, Y.; Decroly, E.; Morvan, F.; Canard, B.; Vasseur, J.-J.; Debart, F. Synthesis of 5′ cap-0 and cap-1 RNAs using solid-phase chemistry coupled with enzymatic methylation by human (guanine-N7)-methyl transferase. RNA 2012, 18, 856–868. [Google Scholar] [CrossRef] [Green Version]
- Lacatena, R.; Cesareni, G. Base pairing of RNA I with its complementary sequence in the primer precursor inhibits ColE1 replication. Nature 1981, 294, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, Y.; Tomizawa, J.-I. Complex formed by complementary RNA stem-loops and its stabilization by a protein: Function of ColE1 Rom protein. Cell 1990, 60, 199–209. [Google Scholar] [CrossRef]
- Houseley, J.; Tollervey, D. The many pathways of RNA degradation. Cell 2009, 136, 763–776. [Google Scholar] [CrossRef] [Green Version]
- Chang, J.H.; Xiang, S.; Xiang, K.; Manley, J.L.; Tong, L. Structural and biochemical studies of the 5′→ 3′ exoribonuclease Xrn1. Nat. Struct. Mol. Biol. 2011, 18, 270. [Google Scholar] [CrossRef]
- Nagarajan, V.K.; Jones, C.I.; Newbury, S.F.; Green, P.J. XRN 5′→3′ exoribonucleases: Structure, mechanisms and functions. Biochim. Biophys. Acta Gene Regul. Mech. 2013, 1829, 590–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celesnik, H.; Deana, A.; Belasco, J.G. Initiation of RNA decay in Escherichia coli by 5′ pyrophosphate removal. Mol. Cell 2007, 27, 79–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milligan, J.F.; Groebe, D.R.; Witherell, G.W.; Uhlenbeck, O.C. Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 1987, 15, 8783–8798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, J.J.; Studier, F.W.; Gottesman, M. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J. Mol. Biol. 1983, 166, 477–535. [Google Scholar] [CrossRef]
- Coleman, T.M.; Wang, G.; Huang, F. Superior 5′ homogeneity of RNA from ATP-initiated transcription under the T7 ϕ2.5 promoter. Nucleic Acids Res. 2004, 32, e14. [Google Scholar] [CrossRef] [Green Version]
- Contreras, R.; Cheroutre, H.; Degrave, W.; Fiers, W. Simple, efficient in vitro synthesis of capped RNA useful for direct expression of cloned eukaryotic genes. Nucleic Acids Res. 1982, 10, 6353–6362. [Google Scholar] [CrossRef] [Green Version]
- Breslow, R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Maier, G.D.; Metzler, D.E. Structures of Thiamine in Basic Solution. J. Am. Chem. Soc. 1957, 79, 4386–4391. [Google Scholar] [CrossRef]
- Pérez-Caballero, G.; Pérez-Arévalo, J.F.; Morales-Hipólito, E.A.; Carbajal-Arenas, M.E.; Rojas-Hernández, A. Potentiometric study of acid-base properties of thiamine hydrochloride and thiamine mononitrate in aqueous medium. J. Mex. Chem. Soc. 2011, 55, 126–131. [Google Scholar] [CrossRef]
- Li, Y.; Breaker, R.R. Kinetics of RNA degradation by specific base catalysis of transesterification involving the 2′-hydroxyl group. J. Am. Chem. Soc. 1999, 121, 5364–5372. [Google Scholar] [CrossRef]
- Marshall, W.S.; Kaiser, R.J. Recent advances in the high-speed solid phase synthesis of RNA. Curr. Opin. Chem. Biol. 2004, 8, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Lendeckel, W.; Tuschl, T. RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev. 2001, 15, 188–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X. Solid-Phase Synthesis of RNA Analogs Containing Phosphorodithioate Linkages. Curr. Protoc. Nucleic Acid Chem. 2017, 70, 4.77.1–4.77.13. [Google Scholar] [CrossRef] [PubMed]
- Warminski, M.; Kowalska, J.; Jemielity, J. Solid-Phase Synthesis of RNA 5′-Azides and Their Application for Labeling, Ligation, and Cyclization Via Click Chemistry. Curr. Protoc. Nucleic Acid Chem. 2020, 82, e112. [Google Scholar] [CrossRef]
- Leigh, J.A. Levels of water-soluble vitamins in methanogenic and non-methanogenic bacteria. Appl. Environ. Microbiol. 1983, 45, 800–803. [Google Scholar] [CrossRef] [Green Version]
- Cernak, P.; Sen, D. A thiamin-utilizing ribozyme decarboxylates a pyruvate-like substrate. Nat. Chem. 2013, 5, 971. [Google Scholar] [CrossRef]
- Winkler, W.; Nahvi, A.; Breaker, R.R. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. Nature 2002, 419, 952. [Google Scholar] [CrossRef]
- Edwards, K.A.; Seog, W.J.; Han, L.; Feder, S.; Kraft, C.E.; Baeumner, A.J. High-throughput detection of thiamine using periplasmic binding protein-based biorecognition. Anal. Chem. 2016, 88, 8248–8256. [Google Scholar] [CrossRef]
- Abdelhedi-Miladi, I.; Montarnal, D.; Obadia, M.M.; Ben Romdhane, H.; Drockenmuller, E. UV-Patterning of Ion Conducting Negative Tone Photoresists Using Azide-Functionalized Poly(Ionic Liquid)s. ACS Macro Lett. 2014, 3, 1187–1190. [Google Scholar] [CrossRef]

Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möhler, M.; Höfer, K.; Jäschke, A. Synthesis of 5?-Thiamine-Capped RNA. Molecules 2020, 25, 5492. https://doi.org/10.3390/molecules25235492
Möhler M, Höfer K, Jäschke A. Synthesis of 5?-Thiamine-Capped RNA. Molecules. 2020; 25(23):5492. https://doi.org/10.3390/molecules25235492
Chicago/Turabian StyleMöhler, Marvin, Katharina Höfer, and Andres Jäschke. 2020. "Synthesis of 5?-Thiamine-Capped RNA" Molecules 25, no. 23: 5492. https://doi.org/10.3390/molecules25235492
APA StyleMöhler, M., Höfer, K., & Jäschke, A. (2020). Synthesis of 5?-Thiamine-Capped RNA. Molecules, 25(23), 5492. https://doi.org/10.3390/molecules25235492





