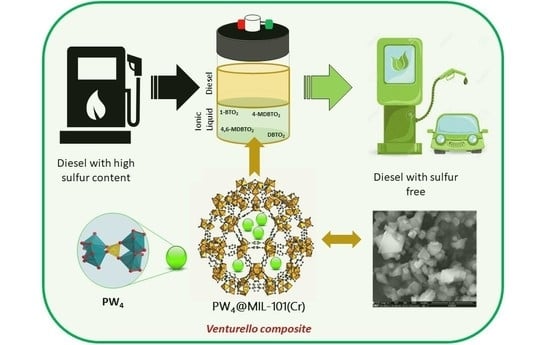
An Effective Hybrid Heterogeneous Catalyst to Desulfurize Diesel: Peroxotungstate@Metal–Organic Framework
Abstract
1. Introduction
2. Results and Discussion
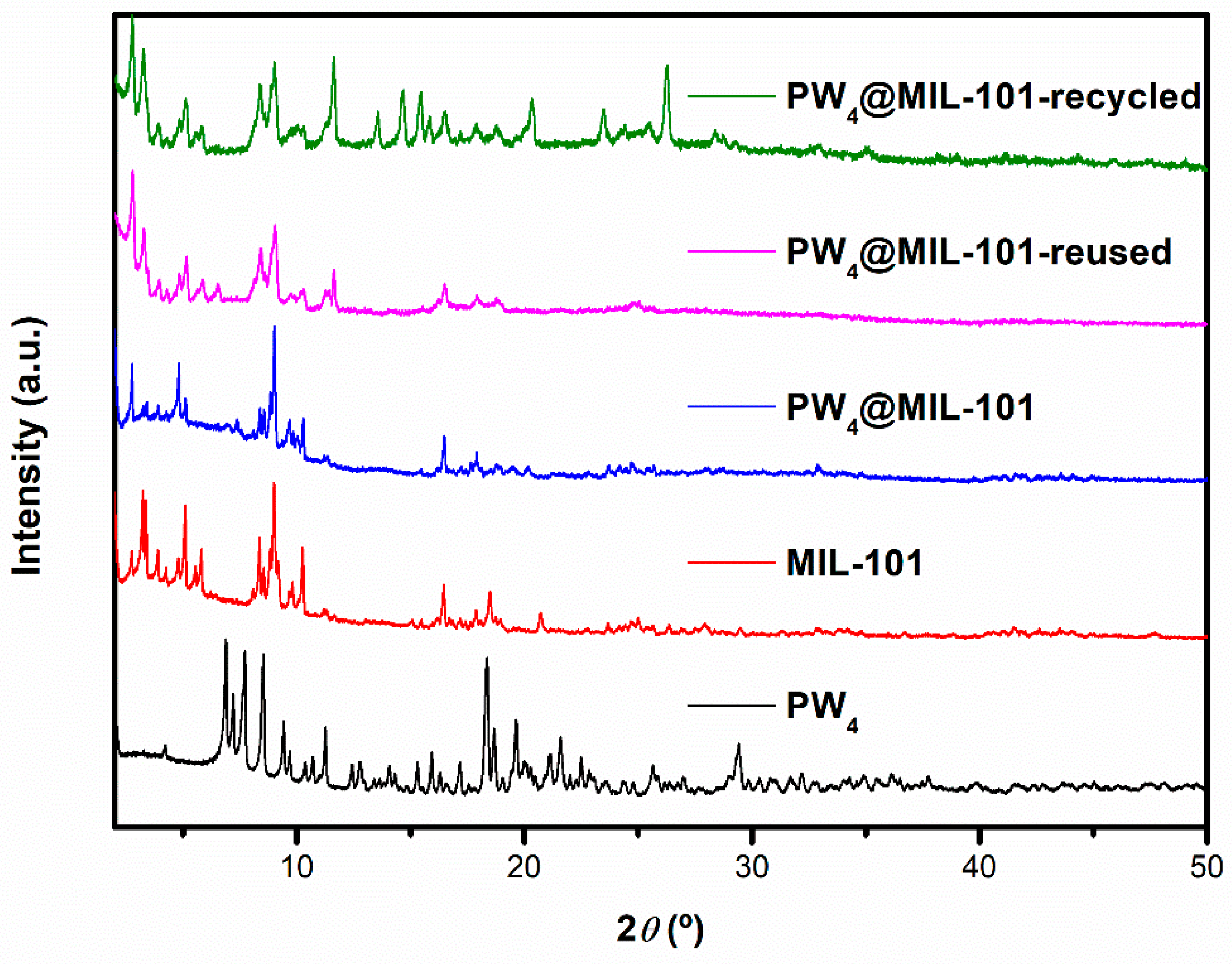
2.1. Characterization
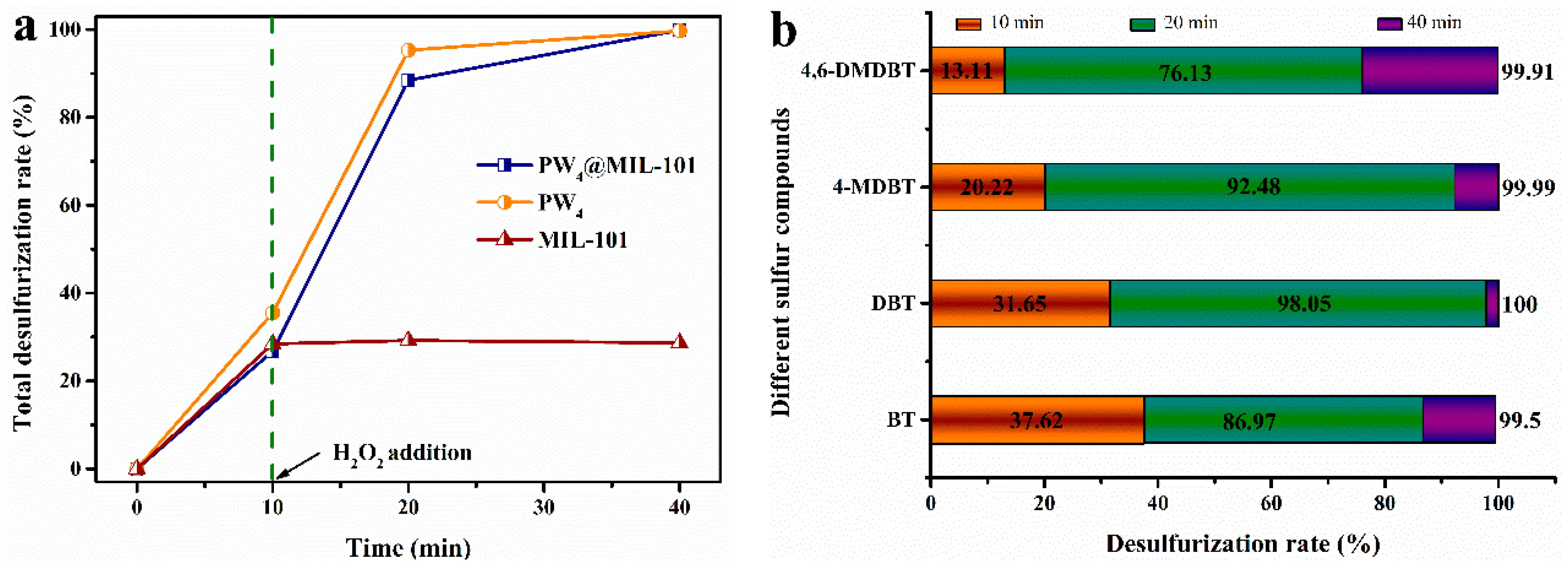
2.2. Extraction and Catalytic Oxidative Desulfurization (ECODS)
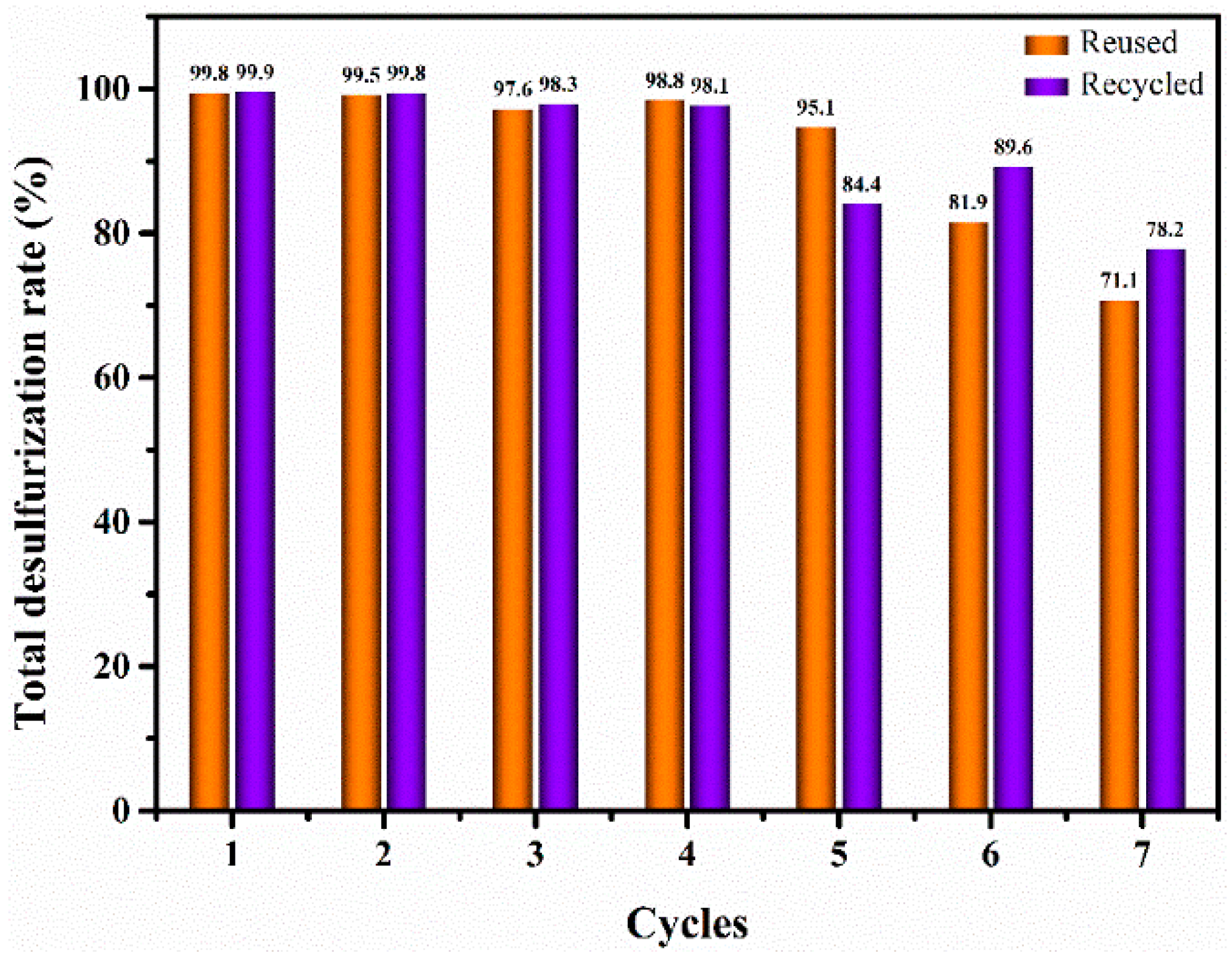
2.3. Reusability and Recyclability of PW4@MIL-101(Cr) Catalyst
2.4. Stability of the Catalyst
2.5. Comparison of Catalytic Efficiency
3. Experimental Section
3.1. Materials and Methods
3.2. Preparation of Catalysts
3.2.1. Synthesis of Peroxotungstate
3.2.2. Preparation of Porous MIL-101(Cr)
3.2.3. Preparation of PW4@MIL-101(Cr) Composite
3.3. Desulfurization of a Multicomponent Model Diesel
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoang, A.T. A review on fuels used for marine diesel engines. J. Mech. Eng. Res. Dev. 2018, 41, 22–32. [Google Scholar] [CrossRef]
- Li, Y.G.; Ma, J.; Zhang, Q.Q.; Wang, C.S.; Chen, Q. Sulfur-Selective Desulfurization of Dibenzothiophene and Diesel Oil by Newly Isolated Rhodococcus erythropolisNCC-1. Chin. J. Chem. 2007, 25, 400–405. [Google Scholar] [CrossRef]
- Dnv, G.; Safer, S.; Greener, D. Compliance options and implications for shipping–focus on scrubbers. In Global Sulphur Cap 2020; Extended and Updated in 2018; DNV-GL: Oslo, Norway, 2018. [Google Scholar]
- Zis, T.; Psaraftis, H.N. The implications of the new sulphur limits on the European Ro-Ro sector. Transp. Res. Part D Transp. Environ. 2017, 52, 185–201. [Google Scholar] [CrossRef]
- Song, C.; Ma, X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Han, F.; Wei, L. Photocatalytic oxidative desulfurization and denitrogenation for fuels in ambient air over Ti3C2/g-C3N4 composites under visible light irradiation. Appl. Catal. B Environ. 2020, 269, 118845. [Google Scholar] [CrossRef]
- Bodin, A.; Christoffersen, A.-L.N.; Elkjær, C.F.; Brorson, M.; Kibsgaard, J.; Helveg, S.; Chorkendorff, I. Engineering Ni–Mo–S Nanoparticles for Hydrodesulfurization. Nano Lett. 2018, 18, 3454–3460. [Google Scholar] [CrossRef]
- Chandran, D.; Khalid, M.; Walvekar, R.; Mubarak, N.M.; Dharaskar, S.; Wong, W.Y.; Gupta, T.C.S.M. Deep eutectic solvents for extraction-desulphurization: A review. J. Mol. Liq. 2019, 275, 312–322. [Google Scholar] [CrossRef]
- Huang, L.; Ge, H.; Yan, L.; Wang, G.; Qin, Z.; Wang, J. Competitive reactive adsorption desulphurization of dibenzothiophene and hydrogenation of naphthalene over Ni/ZnO. Can. J. Chem. Eng. 2018, 96, 865–872. [Google Scholar] [CrossRef]
- Serefentse, R.; Ruwona, W.; Danha, G.; Muzenda, E. A review of the desulphurization methods used for pyrolysis oil. Procedia Manuf. 2019, 35, 762–768. [Google Scholar] [CrossRef]
- Chandra Srivastava, V. An evaluation of desulfurization technologies for sulfur removal from liquid fuels. RSC Adv. 2012, 2, 759–783. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, W.; Li, H.; Wang, X.; Yin, S.; Chang, Y.; Li, H. Temperature-responsive ionic liquid extraction and separation of the aromatic sulfur compounds. Fuel 2015, 140, 590–596. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Subhan, S.; Muhammad, Y.; Sahibzada, M.; Subhan, F.; Tong, Z. Studies on the selection of a catalyst–oxidant system for the energy-efficient desulfurization and denitrogenation of fuel oil at mild operating conditions. Energy Fuels 2019, 33, 8423–8439. [Google Scholar] [CrossRef]
- Julião, D.; Mirante, F.; Ribeiro, S.O.; Gomes, A.C.; Valença, R.; Ribeiro, J.C.; Pillinger, M.; de Castro, B.; Gonçalves, I.S.; Balula, S.S. Deep oxidative desulfurization of diesel fuels using homogeneous and SBA-15-supported peroxophosphotungstate catalysts. Fuel 2019, 241, 616–624. [Google Scholar] [CrossRef]
- Julião, D.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Efficient eco-sustainable ionic liquid-polyoxometalate desulfurization processes for model and real diesel. Appl. Catal. A Gen. 2017, 537, 93–99. [Google Scholar] [CrossRef]
- Yu, X.; Shi, M.; Yan, S.; Wang, H.; Wang, X.; Yang, W. Designation of choline functionalized polyoxometalates as highly active catalysts in aerobic desulfurization on a combined oxidation and extraction procedure. Fuel 2017, 207, 13–21. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Nogueira, L.S.; Julião, D.; Mirante, F.; Ananias, D.; Balula, S.S.; Cunha-Silva, L. Influence of a porous MOF support on the catalytic performance of Eu-polyoxometalate based materials: Desulfurization of a model diesel. Catal. Sci. Technol. 2016, 6, 1515–1522. [Google Scholar] [CrossRef]
- Mirante, F.; Dias, L.; Silva, M.; Ribeiro, S.O.; Corvo, M.C.; de Castro, B.; Granadeiro, C.M.; Balula, S.S. Efficient heterogeneous polyoxometalate-hybrid catalysts for the oxidative desulfurization of fuels. Catal. Commun. 2018, 104, 1–8. [Google Scholar] [CrossRef]
- Chi, M.; Zhu, Z.; Sun, L.; Su, T.; Liao, W.; Deng, C.; Zhao, Y.; Ren, W.; Lü, H. Construction of biomimetic catalysis system coupling polyoxometalates with deep eutectic solvents for selective aerobic oxidation desulfurization. Appl. Catal. B Environ. 2019, 259, 118089. [Google Scholar] [CrossRef]
- Ribeiro, S.O.; Julião, D.; Cunha-Silva, L.; Domingues, V.F.; Valença, R.; Ribeiro, J.C.; de Castro, B.; Balula, S.S. Catalytic oxidative/extractive desulfurization of model and untreated diesel using hybrid based zinc-substituted polyoxometalates. Fuel 2016, 166, 268–275. [Google Scholar] [CrossRef]
- Bertleff, B.; Claußnitzer, J.; Korth, W.; Wasserscheid, P.; Jess, A.; Albert, J. Extraction coupled oxidative desulfurization of fuels to sulfate and water-soluble sulfur compounds using polyoxometalate catalysts and molecular oxygen. ACS Sustain. Chem. Eng. 2017, 5, 4110–4118. [Google Scholar] [CrossRef]
- Zhang, S.; Gao, S.; Xi, Z.; Xu, J. Solvent-free oxidation of alcohols catalyzed by an efficient and reusable heteropolyphosphatotungstate. Catal. Commun. 2007, 8, 531–534. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, G.; Gao, S.; Xi, Z.; Xu, J. Secondary alcohols oxidation with hydrogen peroxide catalyzed by [n-C16H33N(CH3)3]3PW12O40: Transform-and-retransform process between catalytic precursor and catalytic activity species. J. Mol. Catal. A Chem. 2008, 289, 22–27. [Google Scholar] [CrossRef]
- Jin, M.; Guo, Z.; Lv, Z. Synthesis of Convertible {PO4[WO3⇆(O)2(O2)]4}-DMA16 in SBA-15 Nanochannels and Its Catalytic Oxidation Activity. Catal. Lett. 2019, 149, 2794–2806. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Alavijeh, M.K.; Hosseini, M.-S. Venturello anion immobilized on the surface of porous activated carbon as heterogeneous catalyst for the epoxidation of olefins. React. Kinet. Mech. Catal. 2020, 130, 1–13. [Google Scholar] [CrossRef]
- Xie, D.; He, Q.; Su, Y.; Wang, T.; Xu, R.; Hu, B. Oxidative desulfurization of dibenzothiophene catalyzed by peroxotungstate on functionalized MCM-41 materials using hydrogen peroxide as oxidant. Chin. J. Catal. 2015, 36, 1205–1213. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Z.; Chen, X.; Zhao, Q.; Wang, X.; Jiang, Z. A micro reaction-controlled phase-transfer catalyst for oxidative desulfurization based on polyoxometalate modified silica. Appl. Catal. A Gen. 2013, 467, 26–32. [Google Scholar] [CrossRef]
- Zhu, W.; Zhu, G.; Li, H.; Chao, Y.; Zhang, M.; Du, D.; Wang, Q.; Zhao, Z. Catalytic kinetics of oxidative desulfurization with surfactant-type polyoxometalate-based ionic liquids. Fuel Process. Technol. 2013, 106, 70–76. [Google Scholar] [CrossRef]
- Julião, D.; Ribeiro, S.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Polyoxometalates-Based Nanocatalysts and Their Efficiency for Production of Sulfur-Free Diesel. In Nanocomposites for the Desulfurization of Fuels; Tawfik Abdo, S., Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 92–133. [Google Scholar]
- Canioni, R.; Roch-Marchal, C.; Haouas, M.; Vimont, A.; Horcajada, P.; Sécheresse, F.; Serre, C. MIL-101 (Cr) MOF as a Support for Reactive Polyoxometalates (POMs) Clusters. Principles of POMs Encapsulation and Chemistry of POMs Inside MIL-101 Cavities. Curr. Inorg. Chem. 2017, 7, 145–156. [Google Scholar] [CrossRef]
- Sun, J.; Abednatanzi, S.; Voort, P.V.D.; Liu, Y.-Y.; Leus, K. POM@ MOF Hybrids: Synthesis and Applications. Catalysts 2020, 10, 578. [Google Scholar] [CrossRef]
- Wang, X.-S.; Huang, Y.-B.; Lin, Z.-J.; Cao, R. Phosphotungstic acid encapsulated in the mesocages of amine-functionalized metal–organic frameworks for catalytic oxidative desulfurization. Dalton Trans. 2014, 43, 11950–11958. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, P.M.P.; Grazina, R.; Barbosa, A.D.S.; de Castro, B.; Moura, J.J.G.; Cunha-Silva, L.; Balula, S.S. Insights into the electrochemical behaviour of composite materials: Monovacant polyoxometalates @ porous metal-organic framework. Electrochim. Acta 2013, 87, 853–859. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Silva, P.; Paz, F.A.A.; Saini, V.K.; Pires, J.; de Castro, B.; Balula, S.S.; Cunha-Silva, L. Monovacant polyoxometalates incorporated into MIL-101(Cr): Novel heterogeneous catalysts for liquid phase oxidation. Appl. Catal. A Gen. 2013, 453, 316–326. [Google Scholar] [CrossRef]
- Mouanni, S.; Amitouche, D.; Mazari, T.; Rabia, C. Transition metal-substituted Keggin-type polyoxometalates as catalysts for adipic acid production. Appl. Petrochem. Res. 2019, 9, 67–75. [Google Scholar] [CrossRef]
- Castillo, K.; Parsons, J.G.; Chavez, D.; Chianelli, R.R. Oxidation of dibenzothiophene to dibenzothiophene-sulfone using silica gel. J. Catal. 2009, 268, 329–334. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Barbosa, A.D.S.; Ribeiro, S.; Santos, I.; de Castro, B.; Cunha-Silva, L.; Balula, S.S. Oxidative catalytic versatility of a trivacant polyoxotungstate incorporated into MIL-101(Cr). Catal. Sci. Technol. 2014, 4, 1416–1425. [Google Scholar] [CrossRef]
- Ribeiro, S.; Granadeiro, C.M.; Silva, P.; Paz, F.A.A.; de Biani, F.F.; Cunha-Silva, L.; Balula, S.S. An efficient oxidative desulfurization process using terbium-polyoxometalate@MIL-101(Cr). Catal. Sci. Technol. 2013, 3, 2404–2414. [Google Scholar] [CrossRef]
- Sun, M.; Chen, W.C.; Zhao, L.; Wang, X.L.; Su, Z.M. A PTA@MIL-101(Cr)-diatomite composite as catalyst for efficient oxidative desulfurization. Inorg. Chem. Commun. 2018, 87, 30–35. [Google Scholar] [CrossRef]
- Granadeiro, C.M.; Ferreira, P.M.C.; Juliao, D.; Ribeiro, L.A.; Valenca, R.; Ribeiro, J.C.; Goncalves, I.S.; de Castro, B.; Pillinger, M.; Cunha-Silva, L.; et al. Efficient Oxidative Desulfurization Processes Using Polyoxomolybdate Based Catalysts. Energies 2018, 11, 1696. [Google Scholar] [CrossRef]
- Juliao, D.; Gomes, A.C.; Pillinger, M.; Cunha-Silva, L.; de Castro, B.; Goncalves, I.S.; Balula, S.S. Desulfurization of model diesel by extraction/oxidation using a zinc-substituted polyoxometalate as catalyst under homogeneous and heterogeneous (MIL-101 (Cr) encapsulated) conditions. Fuel Process. Technol. 2015, 131, 78–86. [Google Scholar] [CrossRef]
- Ishii, Y.; Yamawaki, K.; Ura, T.; Yamada, H.; Yoshida, T.; Ogawa, M. Hydrogen peroxide oxidation catalyzed by heteropoly acids combined with cetylpyridinium chloride. Epoxidation of olefins and allylic alcohols, ketonization of alcohols and diols, and oxidative cleavage of 1,2-diols and olefins. J. Org. Chem. 1988, 53, 3587–3593. [Google Scholar] [CrossRef]




Sample Availability: Samples of the compounds are not available. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Mirante, F.; de Castro, B.; Zhao, J.; Cunha-Silva, L.; Balula, S.S. An Effective Hybrid Heterogeneous Catalyst to Desulfurize Diesel: Peroxotungstate@Metal–Organic Framework. Molecules 2020, 25, 5494. https://doi.org/10.3390/molecules25235494
Gao Y, Mirante F, de Castro B, Zhao J, Cunha-Silva L, Balula SS. An Effective Hybrid Heterogeneous Catalyst to Desulfurize Diesel: Peroxotungstate@Metal–Organic Framework. Molecules. 2020; 25(23):5494. https://doi.org/10.3390/molecules25235494
Chicago/Turabian StyleGao, Yan, Fátima Mirante, Baltazar de Castro, Jianshe Zhao, Luís Cunha-Silva, and Salete S. Balula. 2020. "An Effective Hybrid Heterogeneous Catalyst to Desulfurize Diesel: Peroxotungstate@Metal–Organic Framework" Molecules 25, no. 23: 5494. https://doi.org/10.3390/molecules25235494
APA StyleGao, Y., Mirante, F., de Castro, B., Zhao, J., Cunha-Silva, L., & Balula, S. S. (2020). An Effective Hybrid Heterogeneous Catalyst to Desulfurize Diesel: Peroxotungstate@Metal–Organic Framework. Molecules, 25(23), 5494. https://doi.org/10.3390/molecules25235494