Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin
Abstract
:1. Introduction
2. Results and Discussion
2.1. Yield of Encapsulation
2.2. Water Absorption Index (WAI)
2.3. Water Solubility Index (WSI)
2.4. Glass Transition Temperature (Tg)
2.5. Total Betalains Content (TB)
2.6. Total Polyphenols Content (TP)
2.7. Antioxidant Activity (AA)
2.8. Total Protein Concentration (TPC)
2.9. Correlation between Variables
3. Materials and Methods
3.1. Materials
3.2. Reagents
3.3. Preparation of Beet Juice Powders
3.3.1. Obtaining Beet Juice, Encapsulation, and Lyophilization
3.3.2. Yield of Encapsulation
3.3.3. Aqueous Extract
3.4. Characterization of Beet Juice Powders
3.4.1. Water Absorption and Solubility Index
3.4.2. Glass Transition Temperature
3.4.3. Extraction of Betalains
3.4.4. Total Betalains Content
3.4.5. Total Polyphenols Content
3.4.6. Antioxidant Activity
- AA by ABTS methodologyIt was carried out as established by Thaipong et al., 2006 [80]. A solution of ABTS (2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)) 7.4 mM was prepared, dissolving 38.8 mg of crystallized ammonium salt of ABTS in 10 mL of distilled water. Then, a potassium persulfate solution 2.6 mM was prepared by dissolving 6.6 mg in 10 mL with distilled water. To generate the ABTS radical, these two solutions were mixed and allowed to stand in the dark at room temperature for 12 h. For the ABTS working solution, 1 mL of the ABTS free radical solution was mixed with 60 mL of methanol to reach an absorbance of 1.1 + 0.02. Subsequently, 150 µL of a sample (or standard: Trolox) and 2850 µL of ABTS working solution were placed in a 3 mL plastic cell and allowed to stand for 2 h in the dark at room temperature, then the absorbance was read at 734 nm in a UV spectrophotometer (UV-1800. Shimadzu, Japan). Measurements were made in triplicate. The antioxidant capacity was reported as equivalent mM Trolox (mM TE/100 g). For this, the absorbance obtained was substituted in the regression equation (y = −1.0726x + 0.9863; r² = 0.9967) obtained from the Trolox calibration curve.
- AA by DPPH methodologyThe antioxidant capacity was determined by the DPPH (2,2-diphenyl-1-picrylhydrazyl) method established by Thaipong et al., 2006 [80] with slight modifications. First, a stock solution of DPPH was prepared by dissolving 0.0240 g of DPPH in 100 mL of methanol to obtain a concentration of 0.6 mM. The solution was stored in an amber bottle and frozen at −20 °C until used. From this solution, the working solution was prepared, for which 10 mL of the stock solution were taken and mixed with 45 mL of methanol to obtain a final concentration of 0.1 mM and an absorbance of 1.1 + 0.02. Subsequently, 150 µL of sample (or standard: Trolox) and 2850 µL of the DPPH working solution was placed in a 3 mL quartz cell. It was allowed to stand for 3 h in the dark at room temperature, and then the absorbance was read at 515 nm on a UV spectrophotometer (UV-1800. Shimadzu, Japan). Measurements were made in triplicate. The antioxidant capacity was reported as equivalent mM Trolox (mM TE/100 g) using the absorbance obtained and substituting in the regression equation (y = −1.3055x + 1.1077; r² = 0.9994) obtained from the Trolox calibration curve.
3.4.7. Total Protein Concentration
3.4.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kapadia, G.J.; Subba, R.G. Red Beet Biotechnology; Neelwarne, B., Ed.; Springer: New York, NY, USA, 2013; ISBN 9781461434573. [Google Scholar]
- Ninfali, P.; Angelino, D. Nutritional and functional potential of Beta vulgaris cicla and rubra. Fitoterapia 2013, 89, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Guadarrama-Lezana, A.Y.; Cruz-Olivares, J.; Martínez-Vargas, S.L.; Carrillo-Navas, H.; Román-Guerrero, A.; Pérez-Alonso, C. Determination of the minimum integral entropy, water sorption and glass transition temperature to establishing critical storage conditions of beetroot juice microcapsules by spray drying. Rev. Mex. Ing. Química 2014, 13, 405–416. [Google Scholar]
- Kanner, J.; Harel, S.; Granit, R. BetalainsA New Class of Dietary Cationized Antioxidants Betalains s A New Class of Dietary Cationized Antioxidants. J. Agric. Food Chem 2001, 49, 5178–5185. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Wang, Y.; Zhang, Z. Radioprotective activity of betalains from red beets in mice exposed to gamma irradiation. Eur. J. Pharmacol. 2009, 615, 223–227. [Google Scholar] [CrossRef]
- Szaefer, H.; Krajka-Kuźniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female sprague-dawley rats. Phyther. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef]
- Kapadia, G.J.; Azuine, M.A.; Sridhar, R.; Okuda, Y.; Tsuruta, A.; Ichiishi, E.; Mukainake, T.; Takasaki, M.; Konoshima, T.; Nishino, H.; et al. Chemoprevention of DMBA-induced UV-B promoted, NOR-1-induced TPA promoted skin carcinogenesis, and DEN-induced phenobarbital promoted liver tumors in mice by extract of beetroot. Pharmacol. Res. 2003, 47, 141–148. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Enhanced chemical stability, chromatic properties and regeneration of betalains in Rivina humilis L. berry juice. LWT-Food Sci. Technol. 2014, 58, 649–657. [Google Scholar] [CrossRef]
- Khan, M.I.; Giridhar, P. Phytochemistry Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267–295. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.G.; Weber, J.; Kneschke, E.M.; Denev, P.N.; Bley, T.; Pavlov, A.I. Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit Dark Red. Plant Foods Hum. Nutr. 2010, 65, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Basulto, J.; Moñino, M.; Farran, A.; Baladia, E.; Manera, M.; Bonany, J.; Gelabert, V.; Ballesteros, J.M.; Martínez, A.; Alonso, M.; et al. Human Nutrition and Diet. Spanish J. Hum. Nutr. Diet. 2014, 18, 100–115. [Google Scholar] [CrossRef]
- Güneşer, O. Pigment and color stability of beetroot betalains in cow milk during thermal treatment. Food Chem. 2016, 196, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Mereddy, R.; Chan, A.; Fanning, K.; Nirmal, N.; Sultanbawa, Y. Betalain rich functional extract with reduced salts and nitrate content from red beetroot (Beta vulgaris L.) using membrane separation technology. Food Chem. 2017, 215, 311–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vulić, J.J.; Ćebović, T.N.; Ćanadanović-Brunet, J.M.; Ćetković, G.S.; Čanadanović, V.M.; Djilas, S.M.; Tumbas Šaponjac, V.T. In vivo and in vitro antioxidant effects of beetroot pomace extracts. J. Funct. Foods 2014, 6, 168–175. [Google Scholar] [CrossRef]
- Netzel, M.; Stintzing, F.C.; Quaas, D.; Straß, G.; Carle, R.; Bitsch, R.; Bitsch, I.; Frank, T. Renal excretion of antioxidative constituents from red beet in humans. Food Res. Int. 2005, 38, 1051–1058. [Google Scholar] [CrossRef]
- Mikołajczyk-Bator, K.; Pawlak, S. The effect of thermal treatment on antioxidant capacity and pigment contents in separated betalain fractions. Acta Sci. Pol. Technol. Aliment. 2016, 15, 257–265. [Google Scholar] [CrossRef] [Green Version]
- PASCH, J.H.; von ELBE, J.H. Betanine Stability in Buffered Solutions Containing Organic Acids, Metal Cations, Antioxidants, or Sequestrants. J. Food Sci. 1979, 44, 72–75. [Google Scholar] [CrossRef]
- von Elbe, J.; Schwartz, S. Colorants. Food Chem. 1996, 3, 651–722. [Google Scholar]
- Stintzing, F.C.; Trichterborn, J.; Carle, R. Characterisation of anthocyanin-betalain mixtures for food colouring by chromatic and HPLC-DAD-MS analyses. Food Chem. 2006, 94, 296–309. [Google Scholar] [CrossRef]
- Kujawska, M.; Ignatowicz, E.; Murias, M.; Miko, K.; Jodynis-liebert, J. Protective Effect of Red Beetroot against Carbon Tetrachloride- and N-Nitrosodiethylamine-Induced Oxidative Stress in Rats. J. Agric. Food Chem. 2009, 2570–2575. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Natural food pigments and colorants. Curr. Opin. Food Sci. 2016, 7, 20–26. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Natural food additives: Quo vadis? Trends Food Sci. Technol. 2015, 45, 284–295. [Google Scholar] [CrossRef]
- Huang, A.; von Elbe, J. Kinetics of the degradation and regeneration of betanine. J. Food Sci. 1985, 50, 1115–1120. [Google Scholar] [CrossRef]
- Penfield, M.; Campbell, A. Experimental Food Science. Fruits and Vegetables; Academic Press: San Diego, CA, USA, 1990; pp. 294–330. [Google Scholar]
- Slimen, I.B.; Najar, T.; Abderrabba, M. Chemical and antioxidant properties of betalains. J. Agric. Food Chem. 2017, 65, 675–689. [Google Scholar] [CrossRef] [PubMed]
- Von Elbe, J.H.; Young Maing, I.; Amundson, C.H. Color stability of betanin. J. Food Sci. 1974, 39, 334–337. [Google Scholar] [CrossRef]
- Wong, Y.M.; Siow, L.F. Effects of heat, pH, antioxidant, agitation and light on betacyanin stability using red-fleshed dragon fruit (Hylocereus polyrhizus) juice and concentrate as models. J. Food Sci. Technol. 2015, 52, 3086–3092. [Google Scholar] [CrossRef] [Green Version]
- Serris, G.S.; Biliaderis, C.G. Degradation kinetics of beetroot pigment encapsulated in polymeric matrices. J. Sci. Food Agric. 2001, 700, 691–700. [Google Scholar] [CrossRef]
- Herbach, K.M.; Stintzing, F.C.; Carle, R. Stability and color changes of thermally treated betanin, phyllocactin, and hylocerenin solutions. J. Agric. Food Chem. 2006, 54, 390–398. [Google Scholar] [CrossRef]
- Herbach, K.; Stintzing, F.; Carle, R. Betalain Stability and Degradation—Structural and Chromatic Aspects. J. Food Sci. 2006, 71, 41–50. [Google Scholar] [CrossRef]
- Herbach, K.M.; Rohe, M.; Stintzing, F.C.; Carle, R. Structural and chromatic stability of purple pitaya (Hylocereus polyrhizus [Weber] Britton & Rose) betacyanins as affected by the juice matrix and selected additives. Food Res. Int. 2006, 39, 667–677. [Google Scholar] [CrossRef]
- Martins, N.; Roriz, C.; Morales, P.; Barrosa, L.; Ferreira, I. Coloring attributes of betalains: A key emphasis on stability and future applications. Food Funct. 2017, 8, 1357–1372. [Google Scholar] [CrossRef]
- Flores-Mancha, M.A.; Renteria-Monterubio, A.L.; Sanchez-Vega, R.; Chavez-Martinez, A. Structure and stability of betalains. Investig. Cienc. 2019, 44, 318–325. [Google Scholar]
- López, A.; Deladino, L.; Navarro, S.; Martino, M. Encapsulation of bioactive compounds with alginates for the food industry. Aliment. Cienc. y Tecnol. Aliment. 2012, 10, 18–27. [Google Scholar]
- Vergara, C.; Saavedra, J.; Sáenz, C.; García, P.; Robert, P. Microencapsulation of pulp and ultrafiltered cactus pear (Opuntia ficus-indica) extracts and betanin stability during storage. Food Chem. 2014, 157, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Xue, F.; Li, C.; Liu, Y.; Zhu, X.; Pan, S.; Wang, L. Encapsulation of tomato oleoresin with zein prepared from corn gluten meal. J. Food Eng. 2013, 119, 439–445. [Google Scholar] [CrossRef]
- Luna-Guevara, J.; López-Fuentes, J.; Jiménez-Gónzalez, O. Microencapsulation of some bioactive compounds through spray drying. Rev. Iberoam. las Ciencias Biológicas y Agropecu. 2016, 5, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Parra-Huertas, R. Food Microencapsulation: A Review. Rev. Fac. Nac. Agric. Medellín 2011, 63, 5669–5684. [Google Scholar]
- Flores-Belmont, I.; Jiménez-Munguía, M. Microencapsulation of active compounds with chitosan. Temas Sel. Ing. Aliment. 2013, 7, 48–56. [Google Scholar]
- Arrazola, G.; Herazo, I.; Alvis, A. Microencapsulation of Eggplant Anthocyanins (Solanum melongena L.) by Spray Drying and Evaluation of its Color Stability and Antioxidant Capacity. Inf. Tecnológica 2014, 25, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Gallo-Nunura, M.; Cevallos-Vera, M. Comparative study of the dehydration of aguaymanto (Physalis peruvianum) by atomization and lyophilization using encapsulating agents in the retention of vitamin C. Repos. Univ. Nac. Pedro Ruiz Gallo. 2014. Lambayeque. Available online: http://repositorio.unprg.edu.pe/bitstream/handle/UNPRG/115/BC-TES-3840.pdf?sequence=1&isAllowed=y (accessed on 23 November 2020).
- Manjunatha, S.S.; Raju, P.S. Rheological characteristics of reconstituted spray dried beetroot (Beta vulgaris L.) juice powder at different solid content, temperatures and carrier materials. Int. Food Res. J. 2015, 22, 2333–2345. [Google Scholar]
- Diaz, Y.L.; Torres, L.S.; Serna, J.A. Effect of Encapsulation in Drying by Atomization of Biocomponents of Yellow Pitahaya with Functional Interest. Inf. Tecnológica 2017, 28, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Azeredo, H.; Santos, A.; Souza, A.; Mendes, K.; Andrade, M.I. Betacyanin stability during processing and storage of a microencapsulated red beetroot extract. Am. J. Food Technol. 2007, 2, 307–312. [Google Scholar] [CrossRef]
- Pitalua, E.; Jimenez, M.; Vernon-Carter, E.J.; Beristain, C.I. Antioxidative activity of microcapsules with beetroot juice using gum Arabic as wall material. Food Bioprod. Process. 2010, 88, 253–258. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Cabanes, J.; Escribano, J.; García-Carmona, F.; Jiménez-Atiénzar, M. Encapsulation of the most potent antioxidant betalains in edible matrixes as powders of different colors. J. Agric. Food Chem. 2013, 61, 4294–4302. [Google Scholar] [CrossRef]
- Janiszewska, E. Microencapsulated beetroot juice as a potential source of betalain. Powder Technol. 2014, 264, 190–196. [Google Scholar] [CrossRef]
- Ravichandran, K.; Palaniraj, R.; Min, N.; Thaw, M.; Gabr, A.M.M.; Ahmed, A.R.; Knorr, D.; Smetanska, I. Effects of different encapsulation agents and drying process on stability of betalains extract. J. Food Sci. Technol. 2014, 51, 2216–2221. [Google Scholar] [CrossRef] [Green Version]
- Antigo, J.L.D.; Bergamasco, R.D.C.; Madrona, G.S. Effect of ph on the stability of red beet extract (Beta vulgaris L.) microcapsules produced by spray drying or freeze drying. Food Sci. Technol. 2017. [Google Scholar] [CrossRef] [Green Version]
- Omae, J.M.; Goto, P.A.; Rodrigues, L.M.; Santos, S.; Paraiso, C.M.; Madrona, G.S.; Bergamasco, D.C. Beetroot Extract Encapsulated in Inulin: Storage Stability and Incorporation in Sorbet. Chem. Eng. Trans. 2017, 57, 1843–1848. [Google Scholar] [CrossRef]
- Guevara-Breton, N.A.; Jimenez-Munguia, M.T. Materiales utilizados en la encapsulacion. Temas Sel. Ing. Aliment. 2008, 2, 22–27. [Google Scholar]
- Araujo-Diaz, S.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Alvarez-Salas, C. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Encapsulação: Aplicação a tecnologia de alimentos. Aliment. e Nutr. 2005, 16, 89–97. [Google Scholar] [CrossRef]
- Madene, A.; Jacquot, M.; Scher, J.; Desobry, S. Flavour encapsulation and controlled release—A review. Int. J. Food Sci. Technol. 2006, 41, 1–21. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Barragán-Huerta, B.; Yáñez-Fernández, J. Use of gelatin-maltodextrin composite as an encapsulation support for clari fi ed juice from purple cactus pear (Opuntia stricta). LWT-Food Sci. Technol. 2015, 62, 242–248. [Google Scholar] [CrossRef]
- Obón, J.M.; Castellar, M.R.; Alacid, M.; Fernández-lópez, J.A. Production of a red-purple food colorant from Opuntia stricta fruits by spray drying and its application in food model systems. J. Food Eng. 2009, 90, 471–479. [Google Scholar] [CrossRef]
- Saénz, C.; Tapia, S.; Chávez, J.; Robert, P. Microencapsulation by spray drying of bioactive compounds from cactus pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Sánchez, F.D.; Maria, E.; López, S.; Kerstupp, S.F.; Ibarra, R.V.; Scheinvar, L. Colorant extract from red prickly pear (Opuntia lasiacantha) for food application. J. Environ. Agric. Food Chem. 2006, 5, 1130–1337. [Google Scholar]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, V.; Hinrichs, W.L.J.; Polymers, C. Inulin, a flexible oligosaccharide I: 2 Review of its physicochemical characteristics. Carbohydr. Polym. 2015, 130, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Gutiérrez, M.G.; Amaya-Guerra, C.A.; Quintero-Ramos, A.; Pérez-Carrillo, E. Physicochemical properties of cereal pigmented with red prickly pear powder. Investig. y Desarro. en Cienc. y Tecnol. Aliment. 2016, 1, 710–716. [Google Scholar]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion cooking on functional properties and in vitro starch digestibility of barley-based extrudates from fruit and vegetable by-products. J. Food Sci. 2009, 74. [Google Scholar] [CrossRef]
- Rodriguez-Sandoval, E.; Lascano, A.; Sandoval, G. Influence of Partial Substitution of Wheat Flour by Quinoa and Potato Flour on Thermomechanical and Dough Baking Properties. Artic. Cient. 2012, 15, 199–207. [Google Scholar]
- Cui, S.W.; Nie, S.; Roberts, K.T.; Food, G. Functional Properties of Dietary Fiber, 2nd ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; Volume 1. [Google Scholar]
- Ferreira-Cardoso, F.; Ramirez-Ascheri, P.D.; Piler De Carvalho, C.W. Rheological and water adsorption properties of extruded rice flour and barley bagasse. Rev. Ceres Viçosa 2014, 61, 313–322. [Google Scholar]
- Rashid, S.; Rakha, A.; Butt, M.S.; Asgher, M. Physicochemical and techno-functional characterization of inulin extracted from chicory roots and Jerusalem artichoke tubers and exploring their ability to replace the fat in cakes. Prog. Nutr. 2018, 20, 191–202. [Google Scholar] [CrossRef]
- Ruiz-Gutiérrez, M.G.; Amaya-Guerra, C.A.; Quintero-Ramos, A.; Ruiz-Anchondo, T.D.J.; Gutiérrez-Uribe, J.A.; Baez-González, J.G.; Lardizabal-Gutiérrez, D.; Campos-Venegas, K. Effect of Soluble Fiber on the Physicochemical Properties of Cactus Pear (Opuntia ficus indica) Encapsulated Using Spray Drying. 2014, 23, 755–763. [Google Scholar] [CrossRef]
- Cai, Y.; Corke, H. Production and Properties of Spray - dried Amaranthus Betacyanin Pigments. J. Food Sci. 2000, 65, 1248–1252. [Google Scholar] [CrossRef]
- von Elbe, J.H.; SY, S.H.; Maing, I.Y.; Gabelman, W.H. Quantitative analysis of fetacyanins in red table beets (Beta vulgaris). J. Food Sci. 1972, 37, 932–934. [Google Scholar] [CrossRef]
- von Elbe, J.H. The Betalaines; Furia, E.T., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1977; pp. 29–39. [Google Scholar]
- Delgado-Vargas, F.; Jiménez, A.R.; Paredes-López, O. Natural Pigments: Carotenoids, Anthocyanins, and Betalains—Characteristics, Biosynthesis, Processing, and Stability. Crit. Rev. Food Sci. Nutr. 2000, 40, 173–289. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Garrido, I.C. Stability of betalains in a dry mix for refreshing beverages, based on microencapsulated pulp and extract of purple prickly pear (Opuntia ficus-indica). Repos. Univ. Chile 2013. Available online: http://repositorio.uchile.cl/bitstream/handle/2250/113994/castillo_ic.pdf?sequence=1&isAllowed=y (accessed on 23 November 2020).
- Yousefi, S.; Emam-djomeh, Z.; Mousavi, S.M. Effect of carrier type and spray drying on the physicochemical properties of powdered and reconstituted pomegranate juice (Punica Granatum L.). J. Food Sci. Technol. 2011, 48, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Valencia-Avilés, E.; Ignacio-Figueroa, I.; Sosa-Martínez, E.; Bartolomé-Camacho, M.C. Polyphenols: Antioxidant and toxicological properties. Rev. la Fac. Ciencias Químicas 2017, 16, 15–29. [Google Scholar]
- Wang, H.Y.; Qian, H.; Yao, W.R. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 2011, 128, 573–584. [Google Scholar] [CrossRef]
- Anderson, R.A.; Conway, H.F.; Peplinski, A.J. Gelatinization of Corn Grits by Roll Cooking, Extrusion Cooking and Steaming. J. Cereal Sci. Today 1970, 22, 130–135. [Google Scholar] [CrossRef]
- Ahmed, M.; Akter, M.S.; Eun, J.B. Impact of α-amylase and maltodextrin on physicochemical, functional and antioxidant capacity of spray-dried purple sweet potato flour. J. Sci. Food Agric. 2010, 90, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Chang, S.K.C. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J. Food Sci. 2007, 72. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic 1965, 16, 144–158. [Google Scholar]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 254, 248–254. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

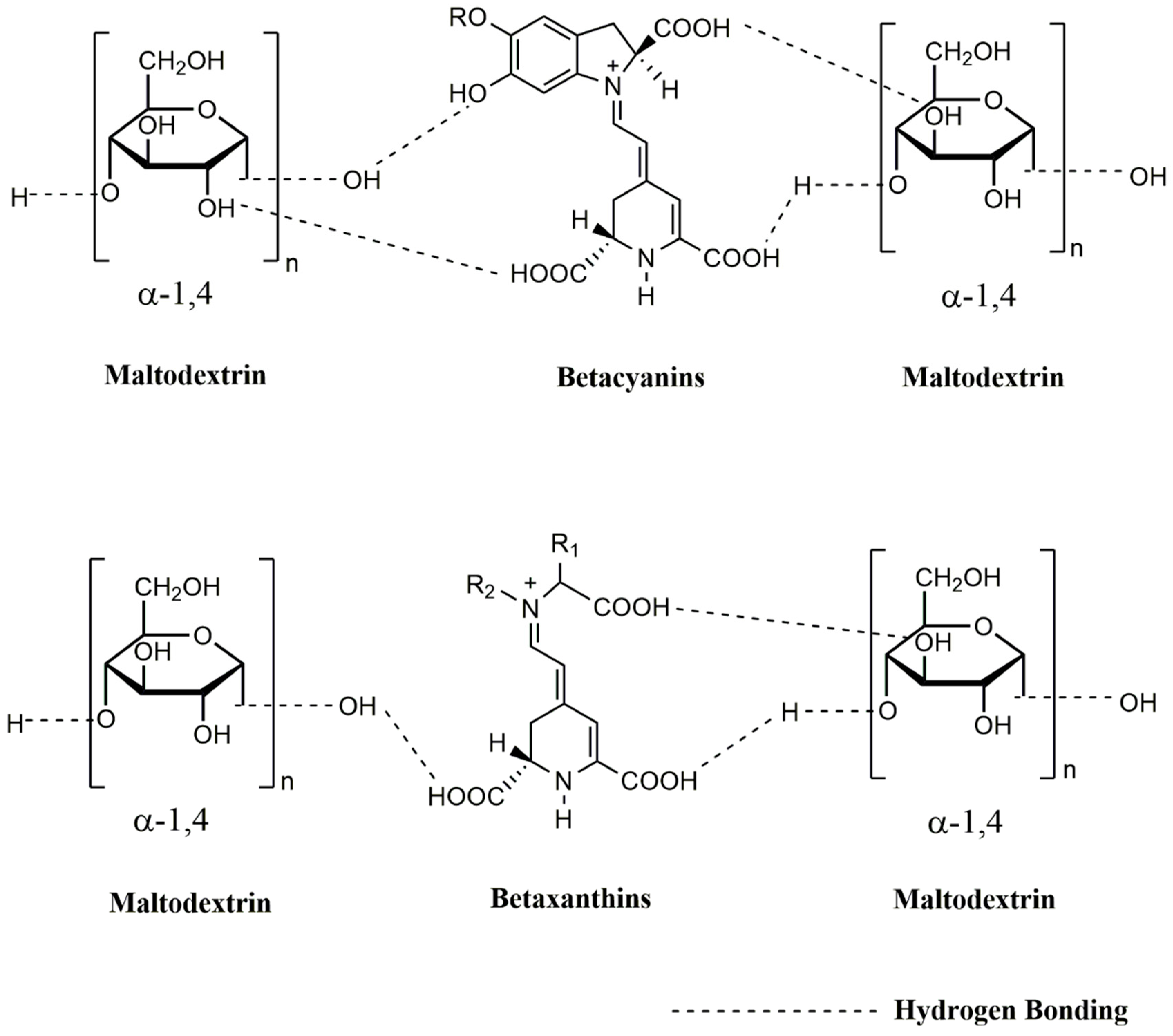
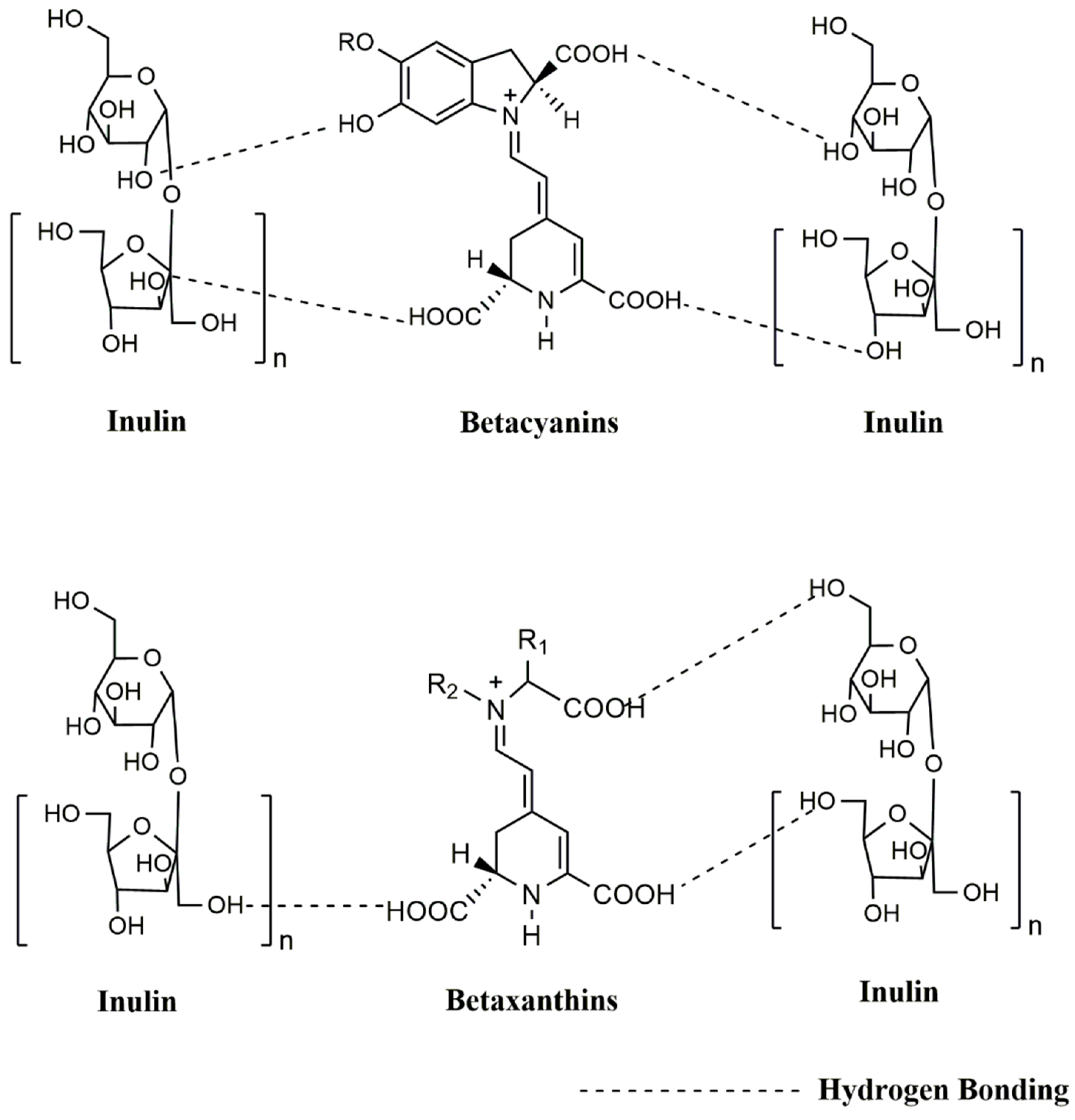
| Powder | WAI | WSI | Tg (°C) | ABTS (mM TE/100 g) | DPPH (mM TE/100 g) |
| B | 44.007 ± 1.000 a | 9.051 ± 0.016 b | 18.340 ± 0.953 c | 0.907 ± 0.001 a | 0.447 ± 0.001 a |
| M | 27.095 ± 6.096 b | 9.374 ± 0.014 a | 61.633 ± 1.261 a | 0.741 ± 0.001 b | 0.295 ± 0.001 b |
| I | 30.684 ± 3.427 b | 8.955 ± 0.023 c | 27.593 ± 0.382 b | 0.647 ± 0.001 c | 0.208 ± 0.001 c |
| Powder | TP (mg GAE/g) | TB (mg/100 g) | BC (mg/100 g) | BX (mg/100 g) | TPC (µg/mL) |
| B | 12.354 ± 0.001 a | 382.351 ± 0.092 a | 219.175 ± 0.092 a | 163.176 ± 0.001 a | 5.974 ± 0.001 a |
| M | 6.093 ± 0.001 b | 15.718 ± 0.016 b | 10.001 ± 0.001 b | 5.717 ± 0.006 b | 3.524 ± 0.001 c |
| I | 5.975 ± 0.001 c | 10.110 ± 0.016 c | 6.279 ± 0.001 c | 3.831 ± 0.006 c | 3.655 ± 0.001 b |
| WSI | Tg | ABTS | DPPH | TP | TB | BC | BX | TPC | |
|---|---|---|---|---|---|---|---|---|---|
| WAI | −0.4519 | −0.7410 | 0.8968 | 0.8963 | 0.8933 | 0.8882 | 0.8878 | 0.8887 | 0.8982 |
| p-value | 0.2220 | 0.0224 | 0.0011 | 0.0011 | 0.0012 | 0.0014 | 0.0014 | 0.0014 | 0.0010 |
| WSI | 0.9068 | −0.6166 | −0.6188 | −0.3118 | −0.2838 | −0.2817 | −0.2865 | −0.3412 | |
| p-value | 0.0007 | 0.0770 | 0.0756 | 0.4141 | 0.4593 | 0.4627 | 0.4549 | 0.3689 | |
| Tg | −0.8872 | −0.8885 | −0.6768 | −0.6549 | −0.6533 | −0.6571 | −0.6995 | ||
| p-value | 0.0014 | 0.0014 | 0.0453 | 0.0556 | 0.0564 | 0.0545 | 0.0360 | ||
| ABTS | 1.0000 | 0.9398 | 0.9293 | 0.9285 | 0.9304 | 0.9500 | |||
| p-value | <0.0001 | 0.0002 | 0.0003 | 0.0003 | 0.0003 | <0.0001 | |||
| DPPH | 0.9388 | 0.9283 | 0.9275 | 0.9293 | 0.9491 | ||||
| p-value | 0.0002 | 0.0003 | 0.0003 | 0.0003 | <0.0001 | ||||
| TP | 0.9996 | 0.9995 | 0.9997 | 0.9995 | |||||
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| TB | 1.0000 | 1.0000 | 0.9982 | ||||||
| p-value | <0.0001 | <0.0001 | <0.0001 | ||||||
| BC | 1.0000 | 0.9980 | |||||||
| p-value | <0.0001 | <0.0001 | |||||||
| BX | 0.9983 | ||||||||
| p-value | <0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Mancha, M.A.; Ruíz-Gutiérrez, M.G.; Sánchez-Vega, R.; Santellano-Estrada, E.; Chávez-Martínez, A. Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules 2020, 25, 5498. https://doi.org/10.3390/molecules25235498
Flores-Mancha MA, Ruíz-Gutiérrez MG, Sánchez-Vega R, Santellano-Estrada E, Chávez-Martínez A. Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules. 2020; 25(23):5498. https://doi.org/10.3390/molecules25235498
Chicago/Turabian StyleFlores-Mancha, Martha A., Martha G. Ruíz-Gutiérrez, Rogelio Sánchez-Vega, Eduardo Santellano-Estrada, and América Chávez-Martínez. 2020. "Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin" Molecules 25, no. 23: 5498. https://doi.org/10.3390/molecules25235498
APA StyleFlores-Mancha, M. A., Ruíz-Gutiérrez, M. G., Sánchez-Vega, R., Santellano-Estrada, E., & Chávez-Martínez, A. (2020). Characterization of Beet Root Extract (Beta vulgaris) Encapsulated with Maltodextrin and Inulin. Molecules, 25(23), 5498. https://doi.org/10.3390/molecules25235498







