Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Plant and Essential Oils
3.2. Antimicrobial Assays
3.3. Antikinetoplastid Assays
3.4. Antiproliferative and Cytotoxicity Screening on Malignant and Non-Malignant Cells
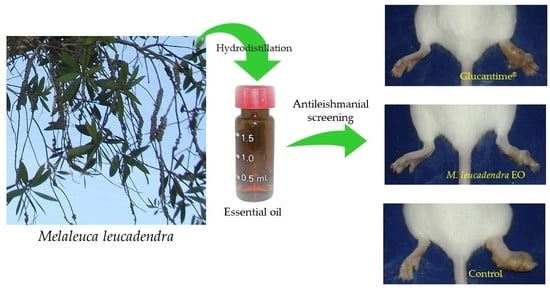
3.5. In Vivo Evaluation on Cutaneous Leishmaniasis Caused by L. amazonensis
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mickymaray, S. Efficacy and mechanism of traditional medicinal plants and bioactive compounds against clinically important pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaban, H.A.E.; El-Ghorab, A.H.; Shibamoto, T. Bioactivity of essential oils and their volatile aroma components: Review. J. Essent. Oil Res. 2012, 24, 203–212. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; de Moura, R.O.; de Araújo, R.S.A.; Monteiro, K.L.C.; de Aquino, T.M.; Ribeiro, F.F.; et al. Active essential oils and their components in use against neglected diseases and arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 6587150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blowman, K.; Magalhães, M.; Lemos, M.F.L.; Cabral, C.; Pires, I.M. Anticancer properties of essential oils and other natural products. Evidence-Based Complement. Altern. Med. 2018, 2018, 3149362. [Google Scholar] [CrossRef]
- Pavithra, P.S.; Mehta, A.; Verma, R.S. Essential oils: From prevention to treatment of skin cancer. Drug Discov. Today 2019, 24, 644–655. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.R.; Johns, J.E.; Rudolph, V. Steam distillation of tea tree (Melaleuca alternifolia) oil. J. Sci. Food Agric. 1992, 58, 49–53. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Varoni, E.M.; Sharopov, F.; Yousaf, Z.; Ayatollahi, S.A.; Kobarfard, F.; Sharifi-Rad, M.; Afdjei, M.H.; Sharifi-Rad, M.; et al. Plants of the Melaleuca genus as antimicrobial agents: From farm to pharmacy. Phyther. Res. 2017, 31, 1475–1494. [Google Scholar] [CrossRef]
- Gan, W.S. Manual of Medicinal Plants in Taiwan, Volume 3; National Research Institute of Chinese Medicine: Taipei City, Taiwan, 1965. [Google Scholar]
- Tsuruga, T.; Chun, Y.-T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Melaleuca leucadendron: Inhibitors of induced histamine release from rat mast cells. Chem. Pharm. Bull. 1991, 39, 3276–3278. [Google Scholar] [CrossRef] [Green Version]
- Surh, J.; Yun, J.-M. Antioxidant and anti-inflammatory activities of butanol extract of Melaleuca leucadendron L. Prev. Nutr. Food Sci. 2012, 17, 22–28. [Google Scholar] [CrossRef]
- Lohakachornpan, P.; Rangsipanuratn, W. Chemical compositions and antimicrobial activities of essential oil from Melaleuca leucadendron var. minor. Thai J. Pharm. Sci. 2001, 25, 133–139. [Google Scholar]
- Farag, R.S.; Shalaby, A.S.; El-Baroty, G.A.; Ibrahim, N.A.; Ali, M.A.; Hassan, E.M. Chemical and biological evaluation of the essential oils of different Melaleuca species. Phyther. Res. 2004, 18, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Calienes Valdés, A.; Mendiola Martínez, J.; Scull Lizama, R.; Vermeersch, M.; Cos, P.; Maes, L. In vitro anti-microbial activity of the Cuban medicinal plants Simarouba glauca DC, Melaleuca leucadendron L and Artemisia absinthium L. Mem. Inst. Oswaldo Cruz 2008, 103, 615–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García, M.; Monzote, L.; Scull, R.; Herrera, P. Activity of Cuban plants extracts against Leishmania amazonensis. ISRN Pharmacol. 2012, 2012, 104540. [Google Scholar] [CrossRef] [Green Version]
- Brophy, J.J. Potentially commercial Melaleucas. In The Genus Melaleuca; Southwell, I., Lowe, R., Eds.; Harwood: Amsterdam, The Netherlands, 1999; pp. 247–274. [Google Scholar]
- An, N.T.G.; Huong, L.T.; Satyal, P.; Tai, T.A.; Dai, D.N.; Hung, N.H.; Ngoc, N.T.B.; Setzer, W.N. Mosquito larvicidal activity, antimicrobial activity, and chemical compositions of essential oils from four species of Myrtaceae from central Vietnam. Plants 2020, 9, 544. [Google Scholar] [CrossRef] [Green Version]
- Pino, J.A.; Regalado, E.L.; Rodríguez, J.L.; Fernández, M.D. Phytochemical analysis and in vitro free-radical-scavenging activities of the essential oils from leaf and fruit of Melaleuca leucadendra L. Chem. Biodivers. 2010, 7, 2281–2288. [Google Scholar] [CrossRef]
- Pino, J.; Bello, A.; Urquiola, A.; Aguero, J.; Marbot, R. Chemical composition of cajuput oil (Melaleuca leucadendra L.) from Cuba. J. Essent. Oil Res. 2002, 14, 10–11. [Google Scholar] [CrossRef]
- Siani, A.C.; Nakamura, M.J.; das Neves, G.P.; da Monteiro, S.S.; Ramos, M.F.S. Leaf essential oil from three exotic Mytaceae species growing in the botanical garden of Rio de Janeiro, Brazil. Am. J. Plant Sci. 2016, 7, 834–840. [Google Scholar] [CrossRef] [Green Version]
- De da Silva, C.E.L.; Oyama, J.; Ferreira, F.B.P.; de Lalucci-Silva, M.P.P.; Lordani, T.V.A.; de da Silva, R.C.L.; de Monich, M.S.T.; Teixeira, J.J.V.; Lonardoni, M.V.C. Effect of essential oils on Leishmania amazonensis: A systematic review. Parasitology 2020. first view. [Google Scholar] [CrossRef]
- Suffness, M.; Pezzuto, J.M. Assay related to cancer drug discovery. In Methods in Plant Biochemistry: Assays for Bioactivity, Volume 6; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; pp. 71–133. [Google Scholar]
- Jones, V.S.; Huang, R.-Y.; Chen, L.-P.; Chen, Z.-S.; Fu, L.; Huang, R.-P. Cytokines in cancer drug resistance: Cues to new therapeutic strategies. Biochim. Biophys. Acta 2016, 1865, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Pereira, P.S.; Maia, A.J.; Duarte, A.E.; Oliveira-tintino, C.D.M.; Tintino, S.R.; Barros, L.M.; Vega-Gomez, M.C.; Rolón, M.; Coronel, C.; Coutinho, H.D.M.; et al. Cytotoxic and anti-kinetoplastid potential of the essential oil of Alpinia speciosa K. Schum. Food Chem. Toxicol. 2018, 119, 387–391. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Trypanocidal and antileukaemic effects of the essential oils of Hagenia abyssinica, Leonotis ocymifolia, Moringa stenopetala, and their main individual constituents. Phytomedicine 2010, 17, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.U.; Prates, F.V.; Machado, P.R.L. Disseminated leishmaniasis: Clinical, pathogenic, and therapeutic aspects. An. Bras. Dermatol. 2019, 94, 9–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santana, R.C.; dos Rosa, A.S.; da Mateus, M.H.S.; Soares, D.C.; Atella, G.; Guimarães, A.C.; Siani, A.C.; Ramos, M.F.S.; Saraiva, E.M.; Pinto-da-Silva, L.H. In vitro leishmanicidal activity of monoterpenes present in two species of Protium (Burseraceae) on Leishmania amazonensis. J. Ethnopharmacol. 2020, 259, 112981. [Google Scholar] [CrossRef] [PubMed]
- Camargos, H.S.; Moreira, R.A.; Mendanha, S.A.; Fernandes, K.S.; Dorta, M.L.; Alonso, A. Terpenes increase the lipid dynamics in the Leishmania plasma membrane at concentrations similar to their IC50 values. PLoS ONE 2014, 9, e104429. [Google Scholar] [CrossRef] [PubMed]
- Kamte, S.L.N.; Ranjbarian, F.; Cianfaglione, K.; Sut, S.; Dall’Acqua, S.; Bruno, M.; Afshar, F.H.; Iannarelli, R.; Benelli, G.; Cappellacci, L.; et al. Identification of highly effective antitrypanosomal compounds in essential oils from the Apiaceae family. Ecotoxicol. Environ. Saf. 2018, 156, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Kpoviessi, S.; Bero, J.; Agbani, P.; Gbaguidi, F.; Kpadonou-Kpoviessi, B.; Sinsin, B.; Accrombessi, G.; Frédérich, M.; Moudachirou, M.; Quetin-Leclercq, J. Chemical composition, cytotoxicity and in vitro antitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogon species from Benin. J. Ethnopharmacol. 2014, 151, 652–659. [Google Scholar] [CrossRef]
- Mikus, J.; Harkenthal, M.; Steverding, D.; Reichling, J. In vitro effect of essential oils and isolated mono- and sesquiterpenes on Leishmania major and Trypanosoma brucei. Planta Med. 2000, 66, 366–368. [Google Scholar] [CrossRef]
- Do Carmo, D.F.M.; Amaral, A.C.F.F.; Machado, G.M.C.; Leon, L.L.; Rocha, J.; de Silva, J.R.A. Chemical and biological analyses of the essential oils and main constituents of Piper species. Molecules 2012, 17, 1819–1829. [Google Scholar] [CrossRef]
- Cseke, L.J.; Setzer, W.N.; Vogler, B.; Kirakosyan, A.; Kaufman, P.B. Traditional, analytical and preparative separations of natural products. In Natural Products from Plants; Cseke, L.J., Kirakosyan, A., Kaufman, P.B., Warber, S.L., Duke, J.A., Brielmann, H.L., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 263–317. ISBN 0-8493-2976-0. [Google Scholar]
- Caridha, D.; Vesely, B.; van Bocxlaer, K.; Arana, B.; Mowbray, C.E.; Rafati, S.; Uliana, S.; Reguera, R.; Kreishman-Deitrick, M.; Sciotti, R.; et al. Route map for the discovery and pre-clinical development of new drugs and treatments for cutaneous leishmaniasis. IJP Drugs Drug Resist. 2019, 11, 106–117. [Google Scholar] [CrossRef]
- Pinart, M.; Rueda, J.-R.; Romero, G.A.S.; Pinzón-Flórez, C.E.; Osorio-Arango, K.; Silveira Maia-Elkhoury, A.N.; Reveiz, L.; Elias, V.M.; Tweed, J.A. Interventions for American cutaneous and mucocutaneous leishmaniasis. Cochrane Database Syst. Rev. 2020. [Google Scholar] [CrossRef]
- Heras-Mosteiro, J.; Monge-Maillo, B.; Pinart, M.; Lopez Pereira, P.; Garcia-Carrasco, E.; Campuzano Cuadrado, P.; Royuela, A.; Mendez Roman, I.; López-Vélez, R. Interventions for Old World cutaneous leishmaniasis. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef]
- De Lima, J.P.S.; Pinheiro, M.L.B.; Santos, A.M.G.; Pereira, J.L.S.; Santos, D.M.F.; Silva-Jardim, I.; Costa, E.V. In vitro antileishmanial and cytotoxic activities of Annona mucosa (Annonaceae). Rev. Virtual Quim. 2012, 4, 692–702. [Google Scholar]
- Costa, J.M.L.; Uthant, A.A.; CostaML, E.A.; Bezerril, A.C.; Barral, A. Diffuse cutaneous leishmaniasis (DCL) in Brazil after 60 years of your first description. Gaz. Médica Bahia 2009, 79, 19–24. [Google Scholar]
- dos Santos, A.O.; Costa, M.A.; Ueda-Nakamura, T.; Dias-Filho, B.P.; da Veiga-Júnior, V.F.; de Souza Lima, M.M.; Nakamura, C.V. Leishmania amazonensis: Effects of oral treatment with copaiba oil in mice. Exp. Parasitol. 2011, 129, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Moraes, A.R.D.P.; Tavares, G.D.; Rocha, F.J.S.; de Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Microbial-modulating activities of essential oils: Implications in colonic pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef] [PubMed]
- Da Rodrigues, K.A.F.; Amorim, L.V.; Dias, C.N.; Moraes, D.F.C.; Carneiro, S.M.P.; de Carvalho, F.A.A. Syzygium cumini (L.) Skeels essential oil and its major constituent α-pinene exhibit anti-Leishmania activity through immunomodulation in vitro. J. Ethnopharmacol. 2015, 160, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Satyal, P. Development of GC-MS Database of Essential Oil Components by the Analysis of Natural Essential Oils and Synthetic Compounds and Discovery of Biologically Active Novel Chemotypes in Essential Oils. Ph.D. Thesis, University of Alabama in Huntsville, Huntsville, AL, USA, 2015. [Google Scholar]
- Hirumi, H.; Hirumi, K. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 1989, 75, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Buckner, F.S.; Verlinde, C.L.M.J.; La Flamme, A.C.; van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing β-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Torres-Santos, E.C.; Moreira, D.L.; Kaplan, M.A.; Meirelles, M.N.; Rossi-Bergmann, B. Selective effect of 2′,6′-dihydroxy-4′-methoxychalcone isolated from Piper aduncum on Leishmania amazonensis. Antimicrob. Agents Chemother. 1999, 43, 1234–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, S.P.C. Rapid chemosensitivity testing of human lung tumor cells using the MTT assay. Cancer Chemother. Pharmacol. 1986, 17, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Komendantova, A.S.; Scherbakov, A.M.; Komkov, A.V.; Chertkova, V.V.; Gudovanniy, A.O.; Chernoburova, E.I.; Sorokin, D.V.; Dzichenka, Y.U.; Shirinian, V.Z.; Volkova, Y.A.; et al. Novel steroidal 1,3,4-thiadiazines: Synthesis and biological evaluation in androgen receptor-positive prostate cancer 22Rv1 cells. Bioorg. Chem. 2019, 91, 103142. [Google Scholar] [CrossRef] [PubMed]
- Geroldinger, G.; Tonner, M.; Quirgst, J.; Walter, M.; De Sarkar, S.; Machín, L.; Monzote, L.; Stolze, K.; Duvigneau, J.C.; Staniek, K.; et al. Activation of artemisinin and heme degradation in Leishmania tarentolae promastigotes: A possible link. Biochem. Pharmacol. 2020, 173, 113737. [Google Scholar] [CrossRef] [PubMed]
- Buffet, P.A.; Sulahian, A.; Garin, Y.J.F.; Nassar, N.; Derouin, F. Culture microtitration: A sensitive method for quantifying Leishmania infantum in tissues of infected mice. Antimicrob. Agents Chemother. 1995, 39, 2167–2168. [Google Scholar] [CrossRef] [Green Version]



| RI | Compound | % | RI | Compound | % |
|---|---|---|---|---|---|
| 858 | (3Z)-Hexen-1-ol | 0.1 | 1167 | Borneol | 0.1 |
| 928 | α-Thujene | tr | 1169 | δ-Terpineol | 0.3 |
| 934 | α-Pinene | 2.7 | 1174 | Ethyl benzoate | tr |
| 947 | Camphene | 0.1 | 1179 | Terpinen-4-ol | 1.2 |
| 957 | Benzaldehyde | 0.1 | 1194 | α-Terpineol | 15.6 |
| 973 | β-Pinene | 1.2 | 1200 | Methyl chavicol (=Estragole) | 0.1 |
| 989 | Myrcene | 0.5 | 1230 | Citronellol | 0.1 |
| 1001 | α-Phellandrene | 0.1 | 1235 | Ascaridole | tr |
| 1007 | δ-3-Carene | 0.1 | 1277 | Safrole | tr |
| 1014 | α-Terpinene | tr | 1280 | Unidentified | 0.1 |
| 1023 | p-Cymene | 0.7 | 1420 | β-Caryophyllene | 0.2 |
| 1027 | Limonene | 4.8 | 1451 | α-Humulene | tr |
| 1031 | 1,8-Cineole | 61.0 | 1458 | allo-Aromadendrene | tr |
| 1049 | (E)-β-Ocimene | tr | 1492 | Viridiflorene (=Ledene) | tr |
| 1059 | γ-Terpinene | 0.2 | 1563 | Palustrol | 0.1 |
| 1090 | Terpinolene | 0.1 | 1589 | Viridiflorol | 7.9 |
| 1096 | Methyl benzoate | 0.1 | 1597 | Guaiol | 0.2 |
| 1103 | Linalool | 0.2 | 1600 | Ledol | 0.8 |
| 1114 | endo-Fenchol | 0.1 | 1606 | Humulene epoxide II | 0.1 |
| 1147 | neo-Isopulegol | 0.4 | 1642 | τ-Cadinol | 0.1 |
| 1149 | Camphene hydrate | tr | 1650 | β-Eudesmol | 0.1 |
| 1158 | iso-Isopulegol | 0.1 | 1653 | α-Eudesmol | 0.1 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monzote, L.; Scherbakov, A.M.; Scull, R.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment. Molecules 2020, 25, 5514. https://doi.org/10.3390/molecules25235514
Monzote L, Scherbakov AM, Scull R, Satyal P, Cos P, Shchekotikhin AE, Gille L, Setzer WN. Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment. Molecules. 2020; 25(23):5514. https://doi.org/10.3390/molecules25235514
Chicago/Turabian StyleMonzote, Lianet, Alexander M. Scherbakov, Ramón Scull, Prabodh Satyal, Paul Cos, Andrey E. Shchekotikhin, Lars Gille, and William N. Setzer. 2020. "Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment" Molecules 25, no. 23: 5514. https://doi.org/10.3390/molecules25235514
APA StyleMonzote, L., Scherbakov, A. M., Scull, R., Satyal, P., Cos, P., Shchekotikhin, A. E., Gille, L., & Setzer, W. N. (2020). Essential Oil from Melaleuca leucadendra: Antimicrobial, Antikinetoplastid, Antiproliferative and Cytotoxic Assessment. Molecules, 25(23), 5514. https://doi.org/10.3390/molecules25235514