Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization and In Vitro Diffusion Study of PEs
2.1.1. Characterization of SNPs
2.1.2. GC Analysis of Melaleuca Alternifolia EO
2.1.3. Characterization of PEs
2.1.4. In Vitro Diffusion Studies through Artificial Membranes
2.2. Microbiological Tests Using Candida albicans and Trichophyton rubrum
3. Materials and Methods
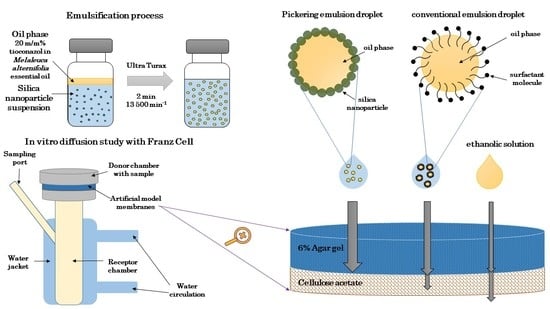
3.1. Preparation and Characterization of Silica Nanoparticle-Stabilized Pickering Emulsions
3.1.1. Synthesis, Surface Modification, and Characterization of Silica Nanoparticles
3.1.2. Gas Chromatography Analysis of Melaleuca Alternifolia Essential Oil
Solid-Phase Microextraction (SPME) Conditions
GC-MS Conditions
3.1.3. Determination of Solubility of Tioconazole in Melaleuca Alternifolia Essential Oil
Solubility Calculations by Hansen Solubility Parameters (HSPs)
Determination of Kinetic Solubility
3.1.4. Preparation and Characterization of Pickering Emulsions
3.1.5. In Vitro Diffusion Studies—Static Franz Diffusion Cell Method
3.2. Microbiological Tests against Candida albicans and Trichophyton rubrum
3.2.1. Instruments Used in the Microbiological Experiments
3.2.2. Materials Used in the Microbiological Experiments
3.2.3. Fungal Cultures and Inoculum Preparation
3.2.4. Determination of Antifungal Activities
3.2.5. Determination of Minimum Inhibitory Concentration of T. rubrum
3.2.6. Determination of Minimum Inhibitory Concentration of C. albicans
3.2.7. Determination of the Minimum Fungicidal Concentration (MFC)
3.2.8. Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sigurgeirsson, B.; Baran, R. The prevalence of onychomycosis in the global population—A literature study. J. Eur. Acad. Dermatol. Venereol. 2013, 28, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Scher, R. Onychomycosis is more than a cosmetic problem. Br. J. Dermatol. 1994, 130, 15. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Tu, L.Q. Therapies for Onychomycosis: A Review. Dermatol. Clin. 2006, 24, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Ameen, M.; Lear, J.T.; Madan, V.; Mohd, M.F.; Richardson, M. British Association of Dermatologists’ guidelines for the management of onychomycosis. Br. J. Dermatol. 2014, 171, 937–958. [Google Scholar] [CrossRef] [PubMed]
- Gauwerky, K.; Borelli, C.; Korting, H.C. Targeting virulence: A new paradigm for antifungals. Drug Discov. Today 2009, 14, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Stuttgen, G.; Bauer, E. Bioavailability, skin- and nail-penetration of topically applied antimycotics. Mykosen 1982, 25, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Di Chiacchio, N.; Kaduc, B.V.; De Almeida, A.R.T.; Madeira, C.L. Nail abrasion. J. Cosmet. Dermatol. 2003, 2, 150–152. [Google Scholar] [CrossRef]
- Repka, M.A.; Mididoddi, P.K.; Stodghill, S.P. Influence of human nail etching for the assessment of topical onychomycosis therapies. Int. J. Pharm. 2004, 282, 95–106. [Google Scholar] [CrossRef]
- Mohorčič, M.; Torkar, A.; Friedrich, J.; Kristl, J.; Murdan, S. An investigation into keratinolytic enzymes to enhance ungual drug delivery. Int. J. Pharm. 2007, 332, 196–201. [Google Scholar] [CrossRef]
- Fromtling, R.A. Overview of medically important antifungal azole derivatives. Clin. Microbiol. Rev. 1988, 1, 187–217. [Google Scholar] [CrossRef]
- Smith, K.A.; Hao, J.; Li, S.K. Effects of Organic Solvents on the Barrier Properties of Human Nail. J. Pharm. Sci. 2011, 100, 4244–4257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohn, M.; Kraemer, K.T. Dermatopharmacology of ciclopirox nail lacquer topical solution 8% in the treatment of onychomycosis. J. Am. Acad. Dermatol. 2000, 43, S57–S69. [Google Scholar] [CrossRef]
- Walters, K.A.; Flynn, G.L.; Marvel, J.R. Physicochemical characterization of the human nail: Permeation pattern for water and the homologous alcohols and differences with respect to the stratum corneum*. J. Pharm. Pharmacol. 1983, 35, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.; Tampucci, S.; Chetoni, P.; Burgalassi, S.; Mailland, F. Ciclopirox vs amorolfine: In vitro penetration into and permeation through human healthy nails of commercial nail lacquers. J. Drugs Dermatol. 2014, 13, 143–147. [Google Scholar] [PubMed]
- Canuto, M.M.; Gutiérrez, F. Antifungal drug resistance to azoles and polyenes. Lancet Infect. Dis. 2002, 2, 550–563. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; De Feo, V. Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosato, A.; Vitali, C.; Piarulli, M.; Mazzotta, E.; Argentieri, M.P.; Mallamaci, R. In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgare and Pelargonium graveolens against some Candida species. Phytomedicine 2009, 16, 972–975. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wiederhold, N.P.; O Williams, R. Drug delivery strategies for improved azole antifungal action. Expert Opin. Drug Deliv. 2008, 5, 1199–1216. [Google Scholar] [CrossRef]
- Yu, D.; Wang, J.; Shao, X.; Xu, F.; Wang, H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015, 119, 1253–1262. [Google Scholar] [CrossRef] [Green Version]
- Pape, W.J.; Pfannenbecker, U.; Hoppe, U. Validation of the red blood cell test system as in vitro assay for the rapid screening of irritation potential of surfactants. Mol. Toxicol. 1987, 1, 525–536. [Google Scholar]
- Yang, Y.; Fang, Z.; Chen, X.; Zhang, W.; Xie, Y.; Chen, Y.; Liu, Z.G.; Yuan, W. An Overview of Pickering Emulsions: Solid-Particle Materials, Classification, Morphology, and Applications. Front. Pharmacol. 2017, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chevalier, Y.; Bolzinger, M.-A. Emulsions stabilized with solid nanoparticles: Pickering emulsions. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 439, 23–34. [Google Scholar] [CrossRef]
- Binks, B.P. Particles as surfactants–similarities and differences. Curr. Opin. Colloid. Interface Sci. 2002, 7, 21–41. [Google Scholar] [CrossRef]
- Horváth, B.; Pál, S.; Széchenyi, A. Preparation and in vitro diffusion study of essential oil Pickering emulsions stabilized by silica nanoparticles. Flavour Fragr. J. 2018, 33, 385–396. [Google Scholar] [CrossRef]
- Scherer, W.P.; Scherer, M.D. Scanning Electron Microscope Imaging of Onychomycosis. J. Am. Podiatr. Med. Assoc. 2004, 94, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Baraldi, A.; Jones, S.A.; Guesné, S.; Traynor, M.J.; McAuley, W.J.; Brown, M.B.; Murdan, S. Human Nail Plate Modifications Induced by Onychomycosis: Implications for Topical Therapy. Pharm. Res. 2015, 32, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Elewski, B.E. Onychomycosis: Pathogenesis, Diagnosis, and Management. Clin. Microbiol. Rev. 1998, 11, 415–429. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, F.; Nonell, S.; Morales, J. Silica-based nanosystems for therapeutic applications in the skin. Nanomedicine 2019, 14, 2243–2267. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pickering emulsions. Int. J. Pharm. 2009, 371, 56–63. [Google Scholar] [CrossRef]
- An, S.S.A.; Ryu, H.J.; Seong, N.-W.; So, B.J.; Seo, H.-S.; Kim, J.-H.; Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Kim, Y.-R.; et al. Evaluation of silica nanoparticle toxicity after topical exposure for 90 days. Int. J. Nanomed. 2014, 9, 127–136. [Google Scholar] [CrossRef] [Green Version]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions stabilised solely by colloidal particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Leung, K.; Nielsen, I.M.B.; Criscenti, L.J. Elucidating the Bimodal Acid−Base Behavior of the Water−Silica Interface from First Principles. J. Am. Chem. Soc. 2009, 131, 18358–18365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jevons, S.; Gymer, G.E.; Brammer, K.W.; Cox, D.A.; Leeming, M.R.G. Antifungal Activity of Tioconazole (UK-20,349), a New Imidazole Derivative. Antimicrob. Agents Chemother. 1979, 15, 597–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nenoff, P.; Haustein, U.-F.; Brandt, W. Antifungal Activity of the Essential Oil of Melaleuca alternifolia (Tea Tree Oil) against Pathogenic Fungi in vitro. Ski. Pharmacol. Physiol. 1996, 9, 388–394. [Google Scholar] [CrossRef]
- Horváth, B.; Balázs, V.L.; Varga, A.; Böszörményi, A.; Kocsis, B.; Horváth, G.; Széchenyi, A. Preparation, characterisation and microbiological examination of Pickering nano-emulsions containing essential oils, and their effect on Streptococcus mutans biofilm treatment. Sci. Rep. 2019, 9, 16611. [Google Scholar]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Horváth, B.; Salem, A.; Šafarik, T.; Pál, S.; Széchenyi, A. Systematic study of reaction conditions for size controlled synthesis of silica nanoparticles. In Proceedings of the 6th Nano Today Conference, Lissbon, Portugal, 16–20 June 2019; p. 4.12. [Google Scholar]
- Hansen, C.M. The Three Dimensional Solubility Parameter and Solvent Diffusion Coefficient; Danish Technical Press: Copenhagen, Denmark, 1967. [Google Scholar]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef]
- Reus, M.A.; Van Der Heijden, A.E.D.M.; Ter Horst, J.H. Solubility Determination from Clear Points upon Solvent Addition. Org. Process. Res. Dev. 2015, 19, 1004–1011. [Google Scholar] [CrossRef] [Green Version]
- Shivakumar, H.N.; Juluri, A.; Desai, B.G.; Murthy, S.N. Ungual and Transungual drug delivery. Drug Dev. Ind. Pharm. 2011, 38, 901–911. [Google Scholar] [CrossRef]
- Lusiana; Reichl, S.; Müller-Goymann, C.C. Keratin film made of human hair as a nail plate model for studying drug permeation. Eur. J. Pharm. Biopharm. 2011, 78, 432–440. [Google Scholar] [CrossRef]
- Van Hoogdalem, E.; Hoven, W.V.D.; Terpstra, I.; Van Zijtveld, J.; Verschoor, J.; Visser, J. Nail penetration of the antifungal agent oxiconazole after repeated topical application in healthy volunteers, and the effect of acetylcysteine. Eur. J. Pharm. Sci. 1997, 5, 119–127. [Google Scholar] [CrossRef]
- Hui, X.; Baker, S.J.; Wester, R.C.; Barbadillo, S.; Cashmore, A.K.; Sanders, V.; Hold, K.M.; Akama, T.; Zhang, Y.; Plattner, J.J.; et al. In Vitro Penetration of a Novel Oxaborole Antifungal (AN2690) Into the Human Nail Plate. J. Pharm. Sci. 2007, 96, 2622–2631. [Google Scholar] [CrossRef] [PubMed]
- Tiwary, A.K.; Sapra, B. High failure rate of transungal drug delivery: Need for new strategies. Ther. Deliv. 2017, 8, 239–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercer, D.; Stewart, C.S.; Miller, L.; Robertson, J.; Duncan, V.M.S.; O’Neil, D. Improved Methods for Assessing Therapeutic Potential of Antifungal Agents against Dermatophytes and Their Application in the Development of NP213, a Novel Onychomycosis Therapy Candidate. Antimicrob. Agents Chemother. 2019, 63, e02117-18. [Google Scholar] [CrossRef] [Green Version]
- Lahaye, M.; Rochas, C. Chemical structure and physico-chemical properties of agar. Hydrobiologia 1991, 221, 137–148. [Google Scholar] [CrossRef]
- Meulemans, A.; Paycha, F.; Hannoun, P.; Vulpillat, M. Measurement and clinical and pharmacokinetic implications of diffusion coefficients of antibiotics in tissues. Antimicrob. Agents Chemother. 1989, 33, 1286–1290. [Google Scholar] [CrossRef] [Green Version]
- Mertin, D.; Lippold, B.C. In-vitro Permeability of the Human Nail and of a Keratin Membrane from Bovine Hooves: Penetration of Chloramphenicol from Lipophilic Vehicles and a Nail Lacquer. J. Pharm. Pharmacol. 1997, 49, 241–245. [Google Scholar] [CrossRef]
- Baran, R. Agache’s Measuring the Skin; Springer: Cham, Switzerlands, 2017. [Google Scholar]
- Berker, D. Nail Anatomy. Clin. Dermatol. 2013, 31, 509–515. [Google Scholar] [CrossRef]
- Haigh, J.M.; Smith, E.W. The selection and use of natural and synthetic membranes for in vitro diffusion experiments. Eur. J. Pharm. Sci. 1994, 2, 311–330. [Google Scholar] [CrossRef]
- Bagary, R.I.E.; Elkady, E.F.; Tammam, M.H.; El-maaty, A.A. Simultaneous HPLC and derivative spectrophotometry detrmination of tioconazole and benzyl alcohol in bulk and cream with tioconazole forced degradation study. Anal. Chem. Ind. J. 2014, 14, 462–473. [Google Scholar]
- Hammer, K.A.; Carson, C.F.; Riley, T.V. Antifungal effects of Melaleuca alternifolia (tea tree) oil and its components on Candida albicans, Candida glabrata and Saccharomyces cerevisiae. J. Antimicrob. Chemother. 2004, 53, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Barros, L.; Calhelha, R.C.; Vaz, J.A.; Ferreira, I.; Baptista, P.; Estevinho, L.M. Antimicrobial activity and bioactive compounds of Portuguese wild edible mushrooms methanolic extracts. Eur. Food Res. Technol. 2006, 225, 151–156. [Google Scholar] [CrossRef]
- Araújo, C.R.; Miranda, K.C.; Fernandes, O.D.F.L.; Soares, A.J.; Silva, M.D.R.R. In vitro susceptibility testing of dermatophytes isolated in Goiania, Brazil, against five antifungal agents by broth microdilution method. Revista do Instituto de Medicina Tropical de São Paulo 2009, 51, 9–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cantón, E.; Pemán, J.; Viudes, A.; Quindós, G.; Gobernado, M.; Espinel-Ingroff, A. Minimum fungicidal concentrations of amphotericin B for bloodstream Candida species. Diagn. Microbiol. Infect. Dis. 2003, 45, 203–206. [Google Scholar] [CrossRef]
- Das, S.; Czuni, L.; Báló, V.; Papp, G.; Gazdag, Z.; Papp, N.; Kőszegi, T. Papp Cytotoxic Action of Artemisinin and Scopoletin on Planktonic Forms and on Biofilms of Candida Species. Molecules 2020, 25, 476. [Google Scholar] [CrossRef] [Green Version]
- Santos, D.; Hamdan, J.S. In vitro activities of four antifungal drugs against Trichophyton rubrum isolates exhibiting resistance to fluconazole. Mycoses 2007, 50, 286–289. [Google Scholar] [CrossRef]
- Majoros, L.; Kardos, G.; Feiszt, P.; Szabó, B. Efficacy of amphotericin B and flucytosine against fluconazole-resistant Candida inconspicua clinical isolates. J. Antimicrob. Chemother. 2005, 56, 253–254. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the Pickering emulsions are available from the authors. |



| Samples | dDLS (nm) | PDIDLS | dTEM (nm) | PDITEM |
|---|---|---|---|---|
| 20HS | 20.1 ± 0.2 | 0.008 | 20.0 | 0.011 |
| 50HS | 52.7 ± 0.9 | 0.017 | 53.0 | 0.037 |
| 100HS | 105.2 ± 3.6 | 0.021 | 103.0 | 0.083 |
| 20ET | 20.1 ± 0.8 | 0.158 | 22.0 | 0.210 |
| 50ET | 54.2 ± 2.7 | 0.178 | 55.0 | 0.337 |
| 100ET | 110.7 ± 4.1 | 0.231 | 112.0 | 0.349 |
| Compounds | Retention Time tR (min) | Percentage Ratio of Compounds (%) |
|---|---|---|
| α-thujene | 5 | 1.7 |
| α-pinene | 5.2 | 4.6 |
| β-phellandrene | 5.2 | 0.6 |
| β-pinene | 6.2 | 1.2 |
| β-mycrene | 6.4 | 0.8 |
| α-terpinene | 7.0 | 1.4 |
| p-cymene | 7.3 | 35.2 |
| terpinyl-acetate | 7.3 | 2.1 |
| cineole | 7.4 | 5.8 |
| γ-terpinene | 8 | 7.6 |
| terpinolene | 8.6 | 1.7 |
| terpinene-4-ol | 10.7 | 32.5 |
| α-terpineol | 11 | 2.6 |
| aromadendrene | 15.5 | 0.7 |
| epiglobulol | 16.4 | 1.2 |
| PE 20ET | PE 50ET | PE 100ET | ||||
|---|---|---|---|---|---|---|
| Coil Phase (mg/mL) | Droplet Size (nm) | Stability | Droplet Size (nm) | Stability | Droplet Size (nm) | Stability |
| 0.90 | 4306 ± 39.6 | 10 min | 1280 ± 62.8 | 1 day | 1070 ± 438.5 | 30 min |
| 1.79 | 615 ± 22.2 | 30 min | 1320 ± 95.9 | 1 day | 1350 ± 531.8 | 30 min |
| 2.69 | 890 ± 103.3 | 30 min | 1440 ± 83.5 | 1 day | 1630 ± 464.5 | 30 min |
| 3.58 | 1250 ± 94.5 | 1 day | 1650 ± 51.5 | 2 day | 1730 ± 514.5 | 10 min |
| 4.48 | 1320 ± 32.5 | 8 weeks | 1670 ± 216.8 | 2 day | 1850 ± 107.9 | 10 min |
| 5.37 | 1440 ± 100.2 | 8 weeks | 1620 ± 79.7 | 8 weeks | 1890 ± 333.8 | 10 min |
| 6.27 | 1470 ± 35.2 | 8 weeks | 1610 ± 34.4 | 8 weeks | 1950 ± 95.0 | 10 min |
| 7.16 | 1470 ± 62.5 | 8 weeks | 1670 ± 62.8 | 8 weeks | 1940 ± 20.1 | 1 week * |
| 8.96 | 1660 ± 56.7 | 8 weeks | 1690 ± 70.4 | 8 weeks | 2070 ± 51.2 | 1 week * |
| 11.19 | 1890 ± 41.2 | 20 weeks | 2200 ± 188.9 | 8 weeks | 2200 ± 59.5 | 1 week * |
| 13.43 | 1840 ± 141.0 | 20 weeks | 2250 ± 170.8 | 2 weeks * | 2800.0 ± 85.7 | 1 week |
| 16.12 | 1820.0 ± 99.6 | 20 weeks | 2080 ± 160.1 | 2 weeks * | 2850 ± 184.3 | 1 week |
| 17.91 | 1850 ± 496.6 | 8 weeks | 2380 ± 157.0 | 2 weeks * | 3090 ± 116.6 | 1 week |
| Coil Phase (mg/mL) | Appearance PET 20ET | Appearance PET 50ET | Appearance PET 100ET | |||
| 0.90 | creaming | sedimentation | creaming | |||
| 1.79 | creaming | sedimentation | creaming | |||
| 2.69 | creaming | sedimentation | creaming | |||
| 3.58 | opalescent | opalescent | creaming | |||
| 4.48 | opalescent | opalescent | creaming | |||
| 5.37 | opalescent | milky | creaming | |||
| 6.27 | opalescent | milky | creaming | |||
| 7.16 | opalescent | milky | milky | |||
| 8.96 | opalescent | milky | milky | |||
| 11.19 | milky | milky | milky | |||
| 13.43 | milky | milky | aggregation, sedimentation | |||
| 16.12 | milky | milky | aggregation, sedimentation | |||
| 17.91 | opalescent | milky | aggregation, sedimentation | |||
| Samples | Stabilizing Agent | Droplet Size (nm) | CA Agar Gel (%) | CA Agar Gel (mg/cm2) | CA Composite Membrane (%) | CA Composite Membrane (mg/cm2) |
|---|---|---|---|---|---|---|
| ES | - | - | 18.33 | 0.26 | 15.90 | 0.22 |
| CE | Tween80 | 2470.0 ± 89.1 | 35.02 | 0.49 | 11.01 | 0.15 |
| PE 20ET | 20ET SNPs | 1850 ± 496.6 | 89.88 | 1.26 | 5.70 | 0.08 |
| PE 50ET | 50ET SNPs | 2380 ± 157.0 | 67.18 | 0.95 | 6.06 | 0.05 |
| PE 100ET | 100ET SNPs | 3090 ± 116.6 | 45.22 | 0.63 | 0.09 | 0.001 |
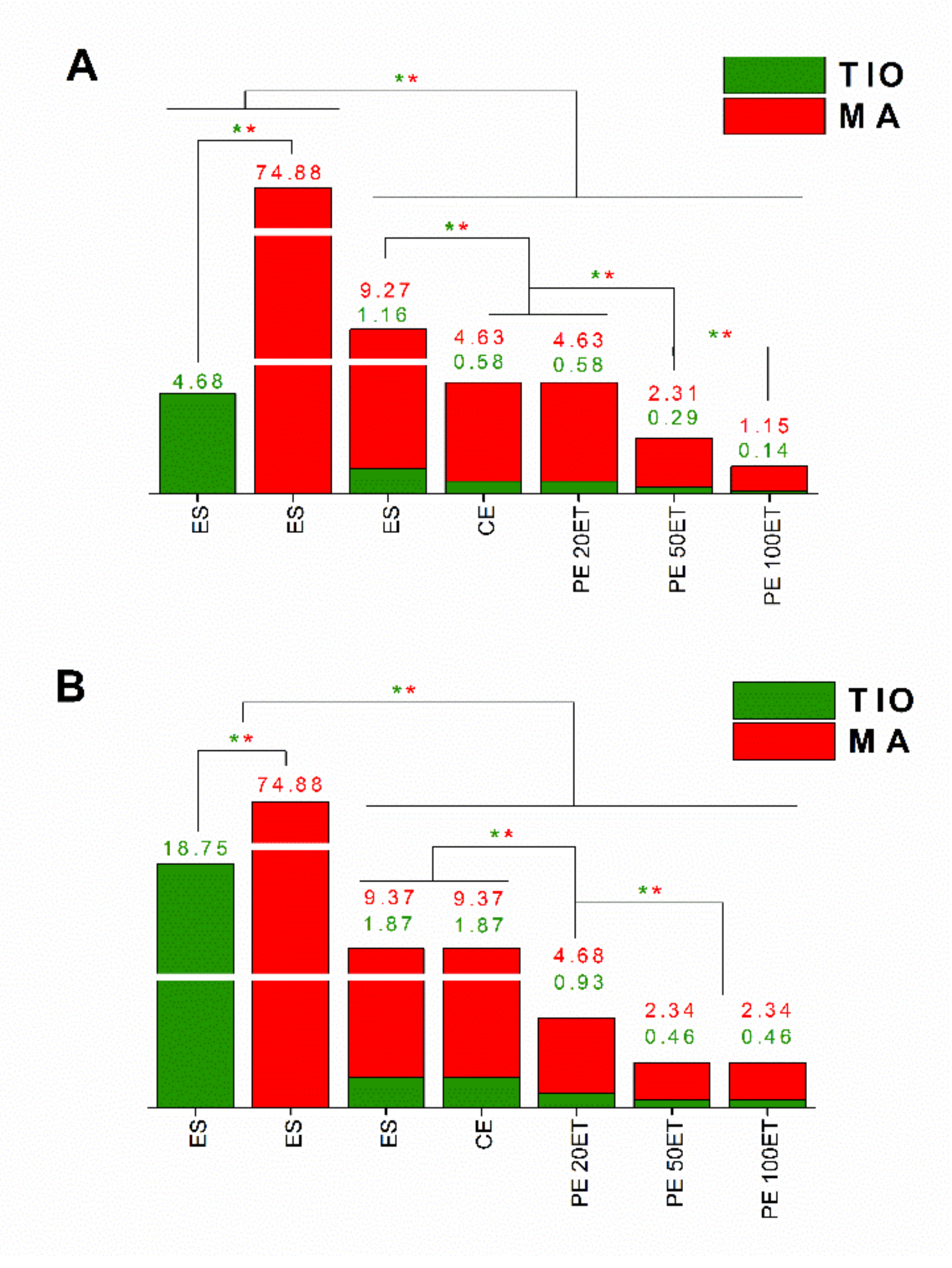
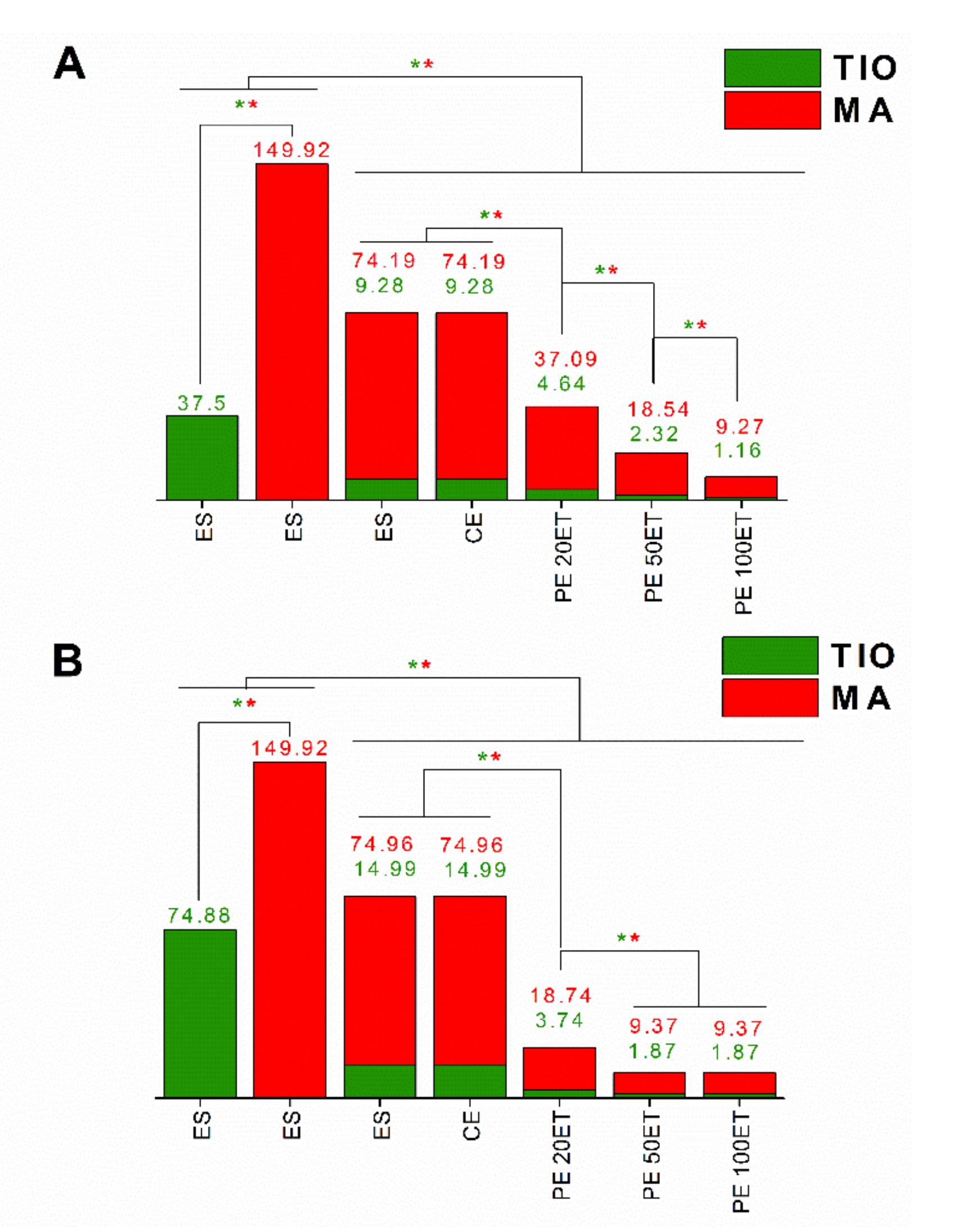
| Sample | T. rubrum | C. albicans | ||
|---|---|---|---|---|
| MIC (µg/mL) | MFC (µg/mL) | MIC (µg/mL) | MFC (µg/mL) | |
| ES-TIO | 4.68 | 37.5 | 18.75 | 74.88 |
| ES-MA | 74.88 | 149.92 | 74.88 | 149.92 |
| ES-TIO-MA | 10.43 | 83.47 | 11.24 | 89.95 |
| CE | 5.21 | 83.47 | 11.24 | 89.95 |
| PE 20ET | 5.21 | 41.73 | 5.61 | 22.48 |
| PE 50ET | 2.6 | 20.86 | 2.8 | 11.24 |
| PE 100ET | 1.29 | 10.43 | 2.8 | 11.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vörös-Horváth, B.; Das, S.; Salem, A.; Nagy, S.; Böszörményi, A.; Kőszegi, T.; Pál, S.; Széchenyi, A. Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules 2020, 25, 5544. https://doi.org/10.3390/molecules25235544
Vörös-Horváth B, Das S, Salem A, Nagy S, Böszörményi A, Kőszegi T, Pál S, Széchenyi A. Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules. 2020; 25(23):5544. https://doi.org/10.3390/molecules25235544
Chicago/Turabian StyleVörös-Horváth, Barbara, Sourav Das, Ala’ Salem, Sándor Nagy, Andrea Böszörményi, Tamás Kőszegi, Szilárd Pál, and Aleksandar Széchenyi. 2020. "Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment" Molecules 25, no. 23: 5544. https://doi.org/10.3390/molecules25235544
APA StyleVörös-Horváth, B., Das, S., Salem, A., Nagy, S., Böszörményi, A., Kőszegi, T., Pál, S., & Széchenyi, A. (2020). Formulation of Tioconazole and Melaleuca alternifolia Essential Oil Pickering Emulsions for Onychomycosis Topical Treatment. Molecules, 25(23), 5544. https://doi.org/10.3390/molecules25235544