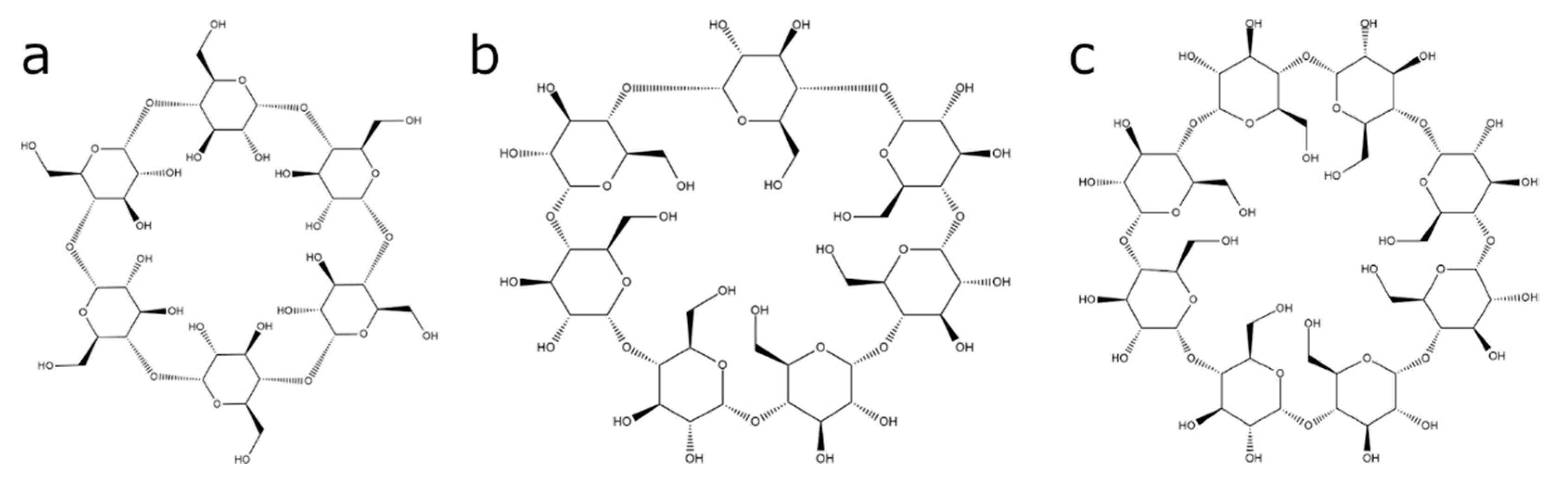
Cyclodextrin-Based Contrast Agents for Medical Imaging
Abstract
1. Introduction
2. CD-Based Contrast Agents for MRI
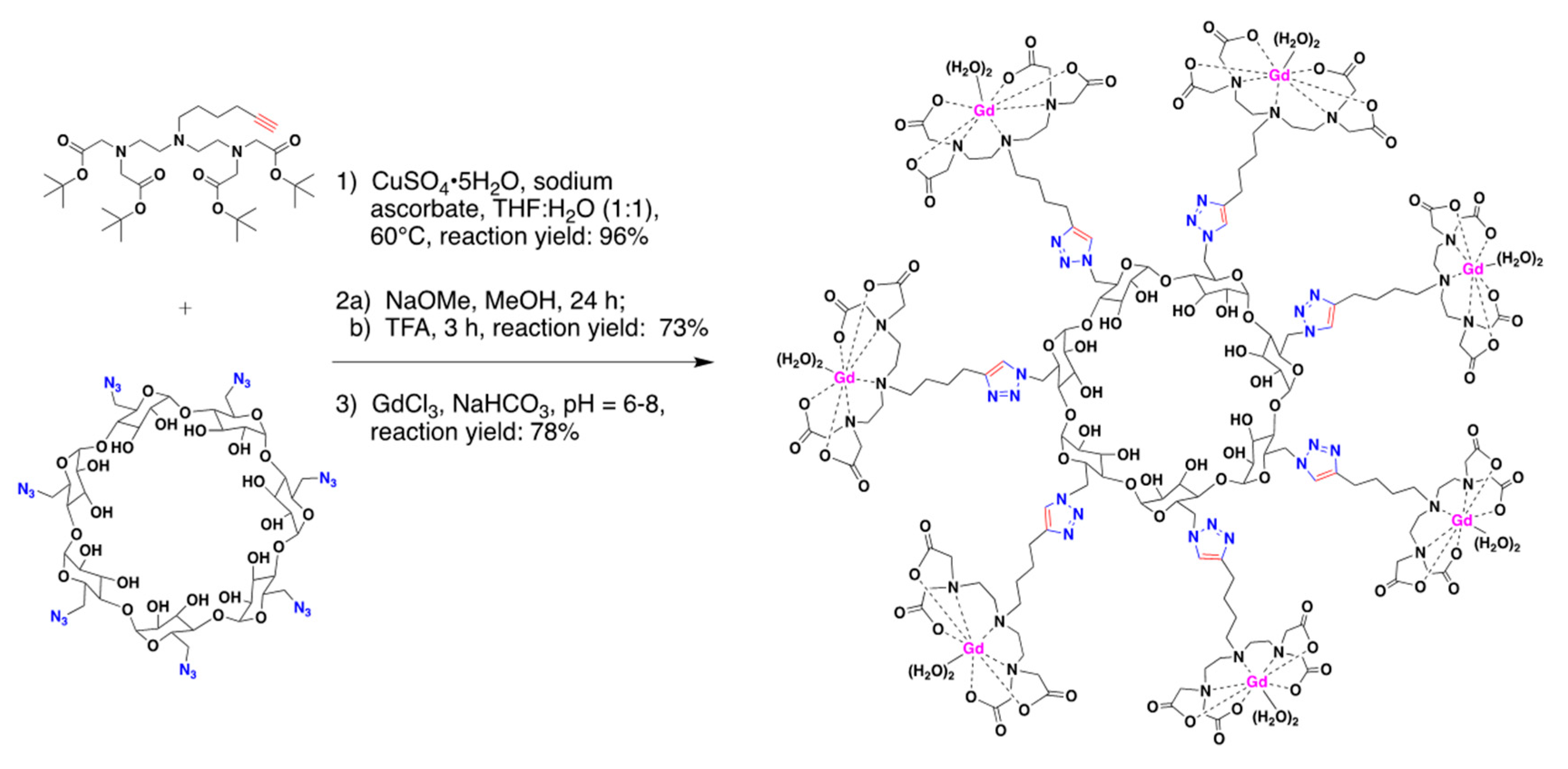
2.1. Contrast Agents Based on Host–Guest Complexation between CDs and Metal–Organic Chelates
2.1.1. In Vitro Studies of the Host–Guest CD-Based MRI Contrast Agents
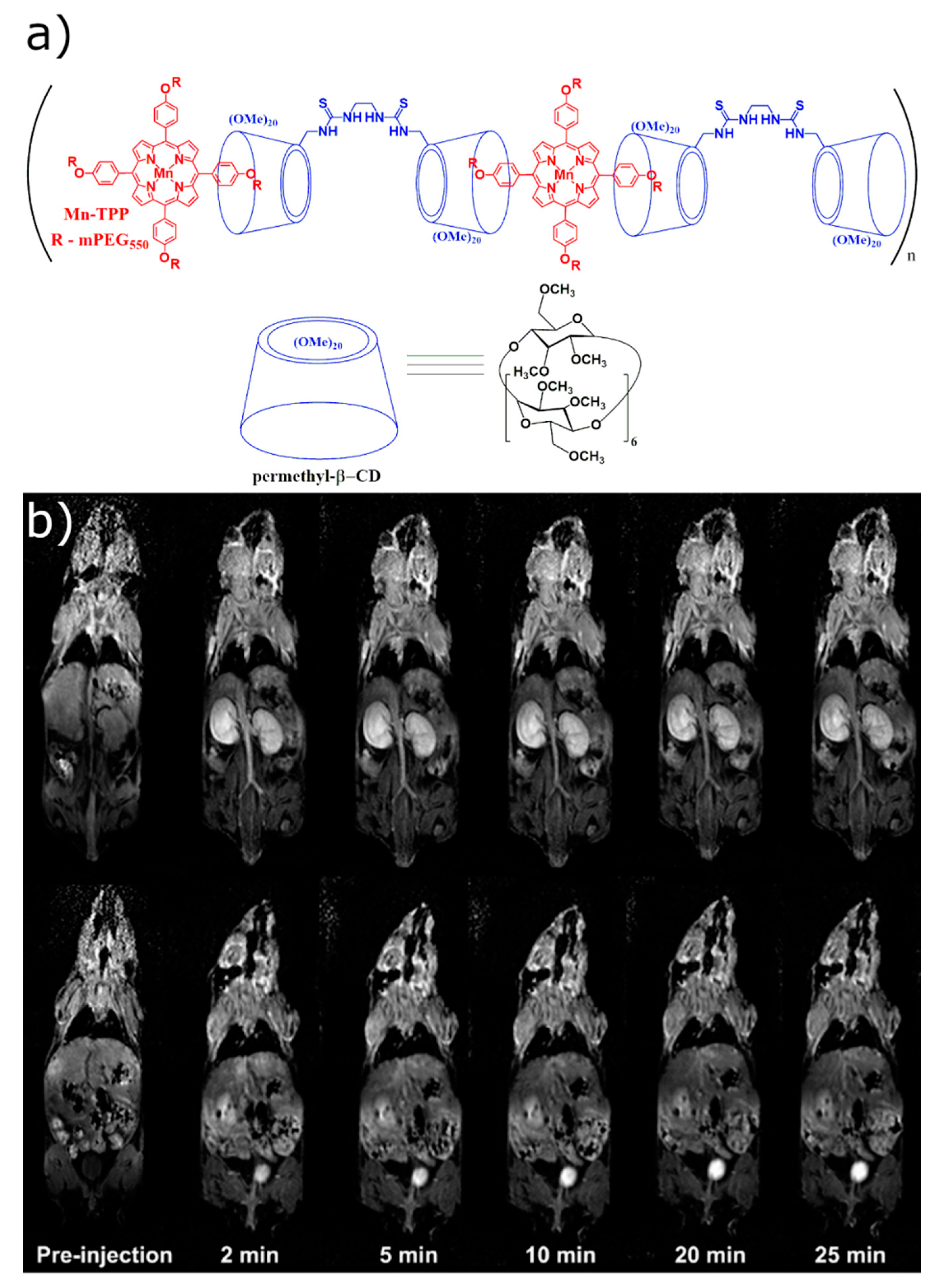
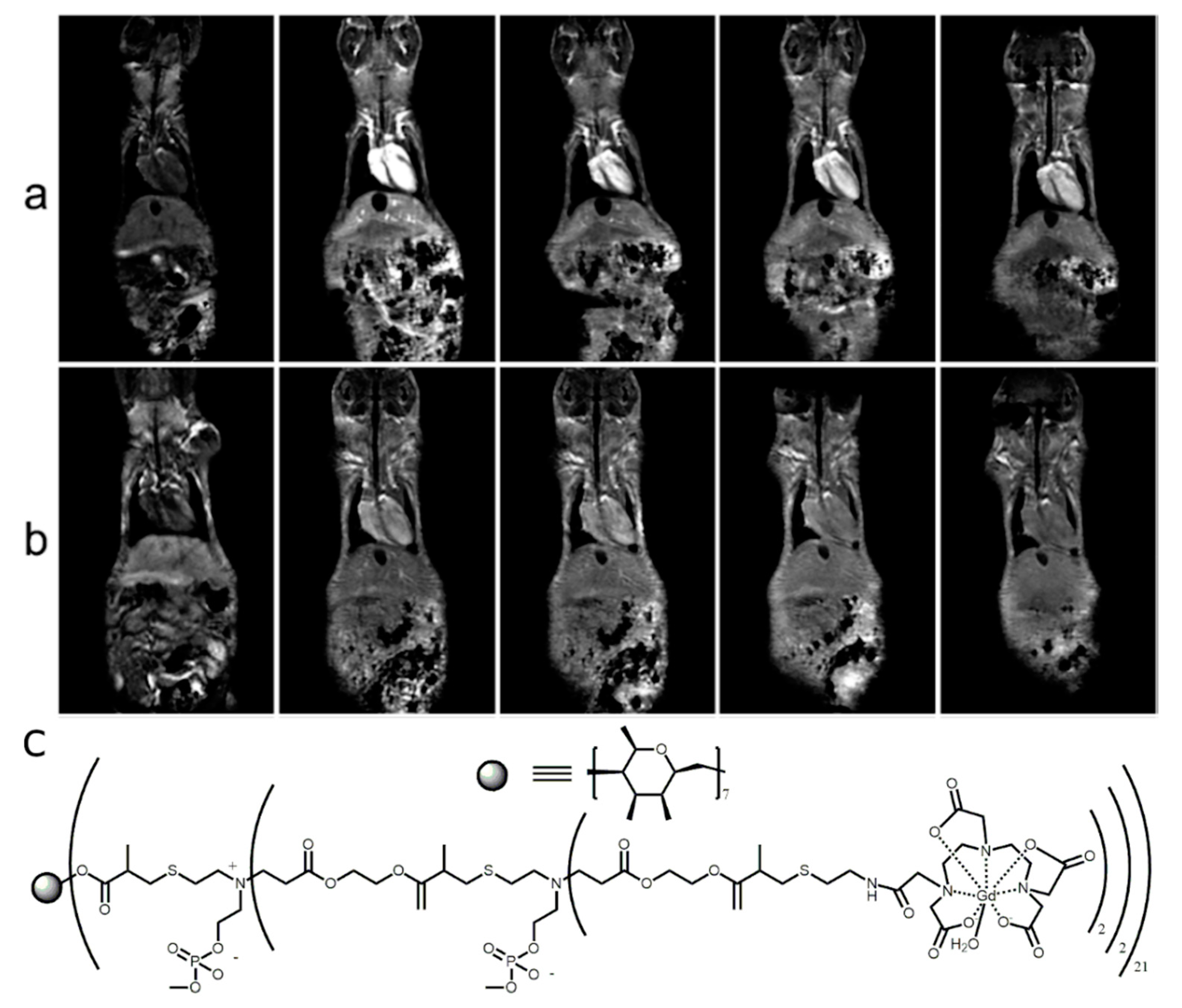
2.1.2. In Vivo Imaging of the Host–Guest CD-Based Contrast Agents
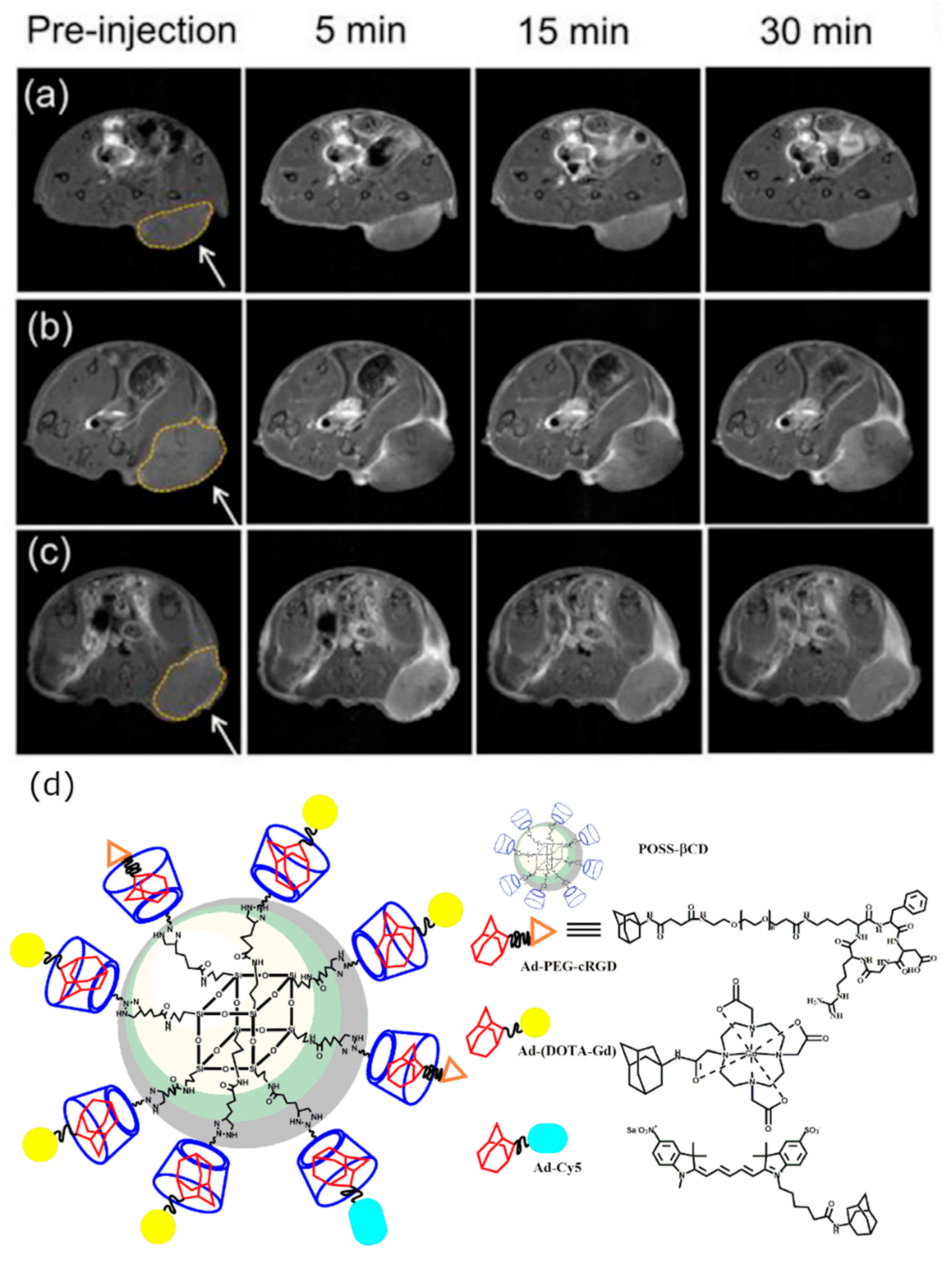
2.1.3. In Vivo Tumor Imaging
2.2. Direct Labeling of the CD Molecules
2.2.1. In Vitro Development
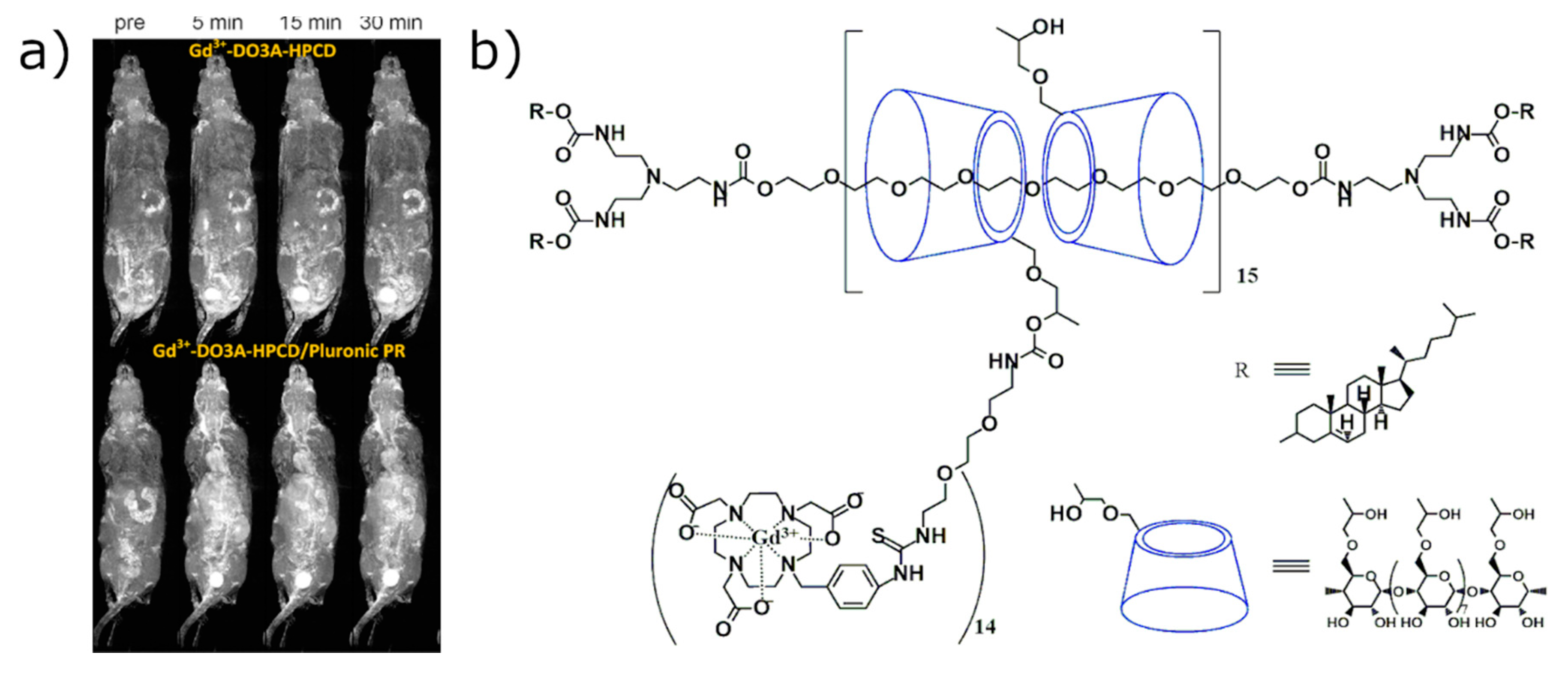
2.2.2. In Vivo Imaging of CD-Based MRI Contrast Agents Based on the Direct Labeling of CD Cavity
2.2.3. In Vivo Imaging of Cancer
2.3. Cyclodextrin-Based Contrast Agents for Hyperpolarized MRI
2.3.1. Hyperpolarized 13C CD-Based Contrast Agents
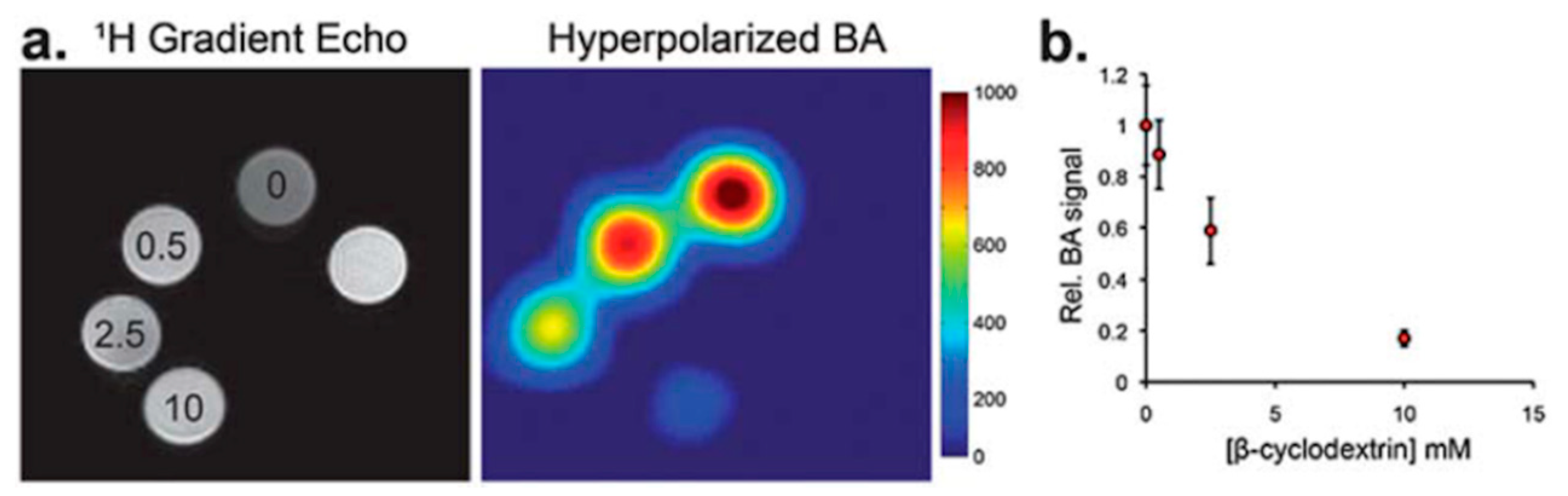
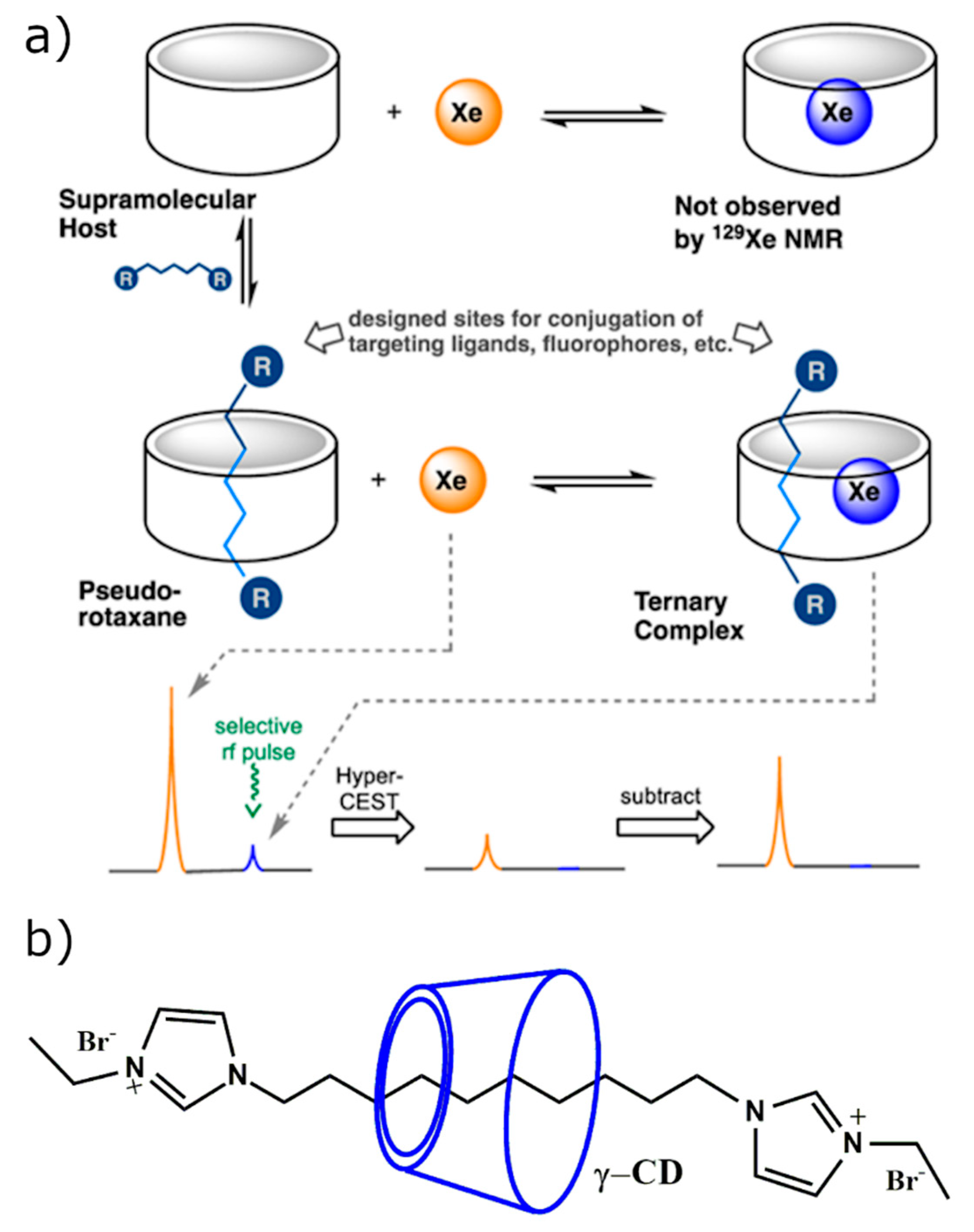
2.3.2. CD-Based Molecular Probes for Hyperpolarized 129Xe MRI
3. CDs-Based Contrast Agent for Ultrasound Imaging and Photoacoustic Imaging
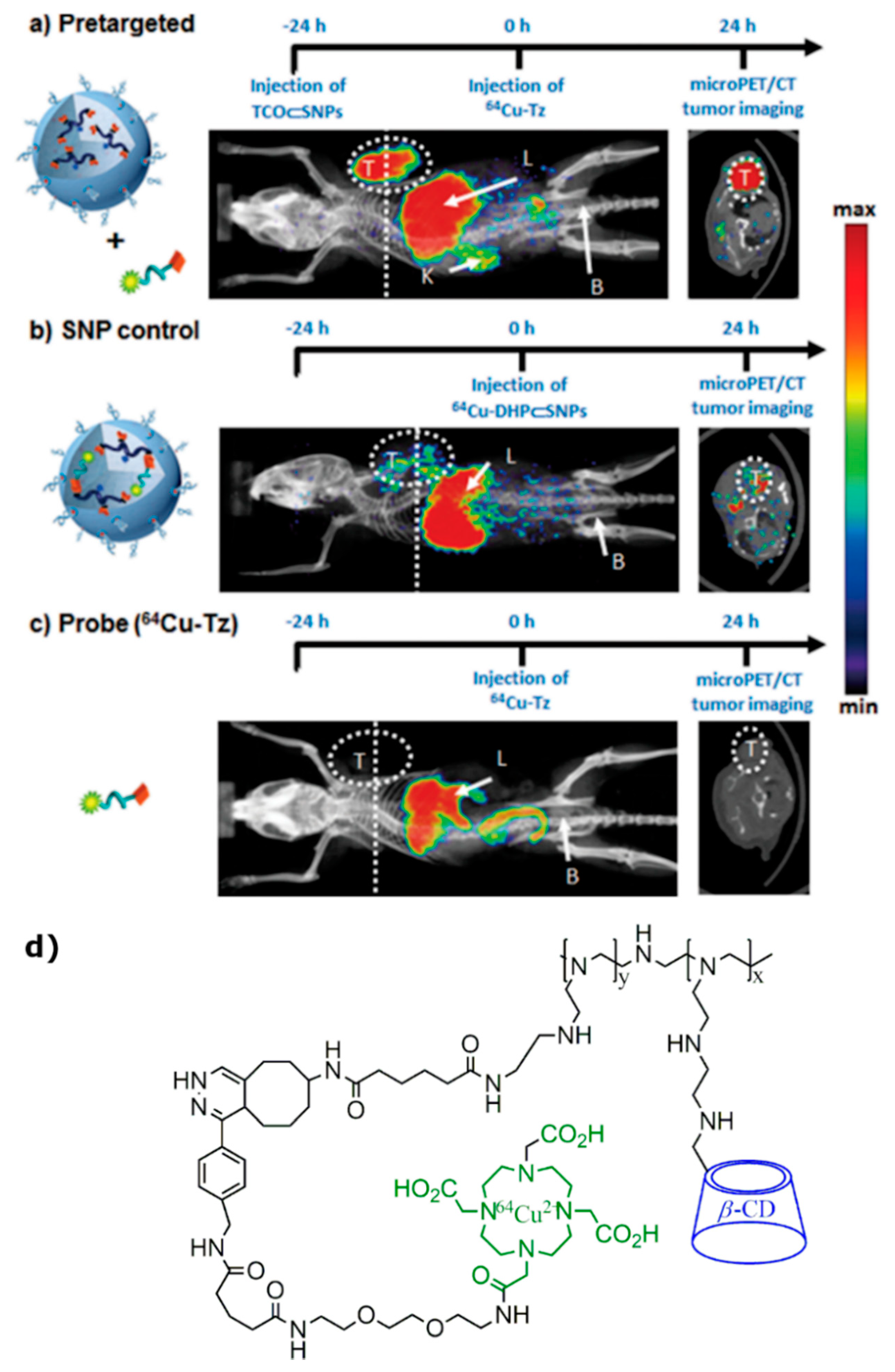
4. Radiolabeled CD-Based Contrast Agents
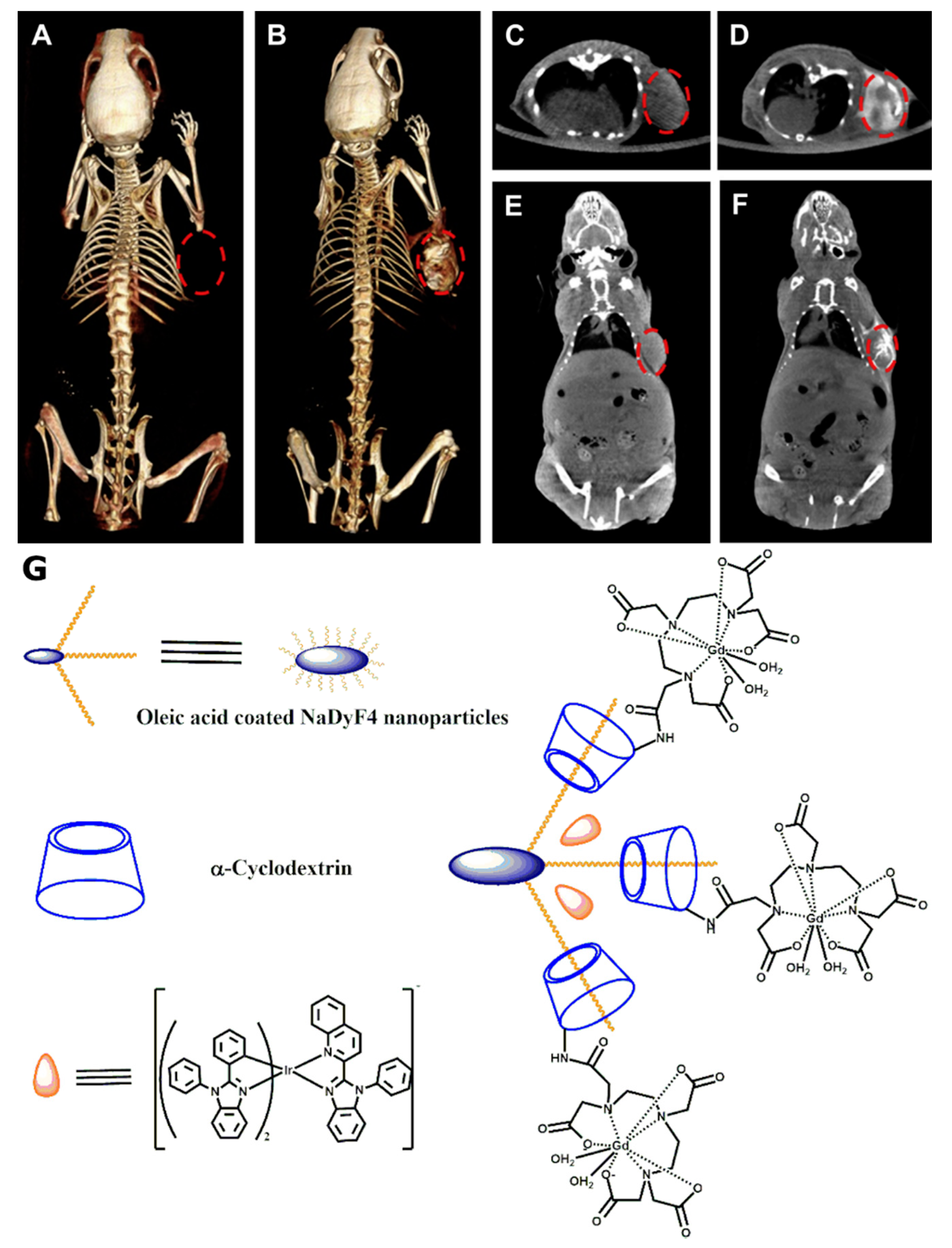
5. CD-Based CT Contrast Agents
6. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Stella, V.J.; He, Q. Cyclodextrins. Toxicol. Pathol. 2008, 36, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2017, 535, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process. Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Lai, W.-F. Cyclodextrins in non-viral gene delivery. Biomaterials 2014, 35, 401–411. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical Applications of Cyclodextrins. III. Toxicological Issues and Safety Evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Bellringer, M.; Smith, T.; Read, R.; Gopinath, C.; Olivier, P. β-Cyclodextrin: 52-Week toxicity studies in the rat and dog. Food Chem. Toxicol. 1995, 33, 367–376. [Google Scholar] [CrossRef]
- Loftsson, T.; Hreinsdóttir, D.; Másson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A Comprehensive Review on Cyclodextrin-Based Carriers for Delivery of Chemotherapeutic Cytotoxic Anticancer Drugs. BioMed Res. Int. 2015, 2015, 198268. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef]
- Schönbeck, C.; Gaardahl, K.; Houston, B. Drug Solubilization by Mixtures of Cyclodextrins: Additive and Synergistic Effects. Mol. Pharm. 2019, 16, 648–654. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations II: Solubilization, binding constant, and complexation efficiency. Drug Discov. Today 2016, 21, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T. Drug solubilization by complexation. Int. J. Pharm. 2017, 531, 276–280. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Cabral-Marques, H. Cyclodextrin nanosystems in oral drug delivery: A mini review. Int. J. Pharm. 2017, 531, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. Toxicol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Lamprou, D.A.; Vavia, P. Synthesis, Characterization, and Drug Delivery Application of Self-assembling Amphiphilic Cyclodextrin. AAPS PharmSciTech 2020, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Doan, V.T.H.; Lee, J.H.; Takahashi, R.; Nguyen, P.T.M.; Nguyen, V.A.T.; Pham, H.T.T.; Fujii, S.; Sakurai, K. Cyclodextrin-based nanoparticles encapsulating α-mangostin and their drug release behavior: Potential carriers of α-mangostin for cancer therapy. Polym. J. 2020, 52, 457–466. [Google Scholar] [CrossRef]
- Crini, G.; Fourmentin, S.; Fenyvesi, É.; Torri, G.; Fourmentin, M.; Morin-Crini, N. Cyclodextrins, from molecules to applications. Environ. Chem. Lett. 2018, 16, 1361–1375. [Google Scholar] [CrossRef]
- Lai, W.-F. Design of cyclodextrin-based systems for intervention execution. In Delivery of Therapeutics for Biogerontological Interventions; Elsevier BV: Amsterdam, The Netherlands, 2019; pp. 49–59. [Google Scholar]
- Davis, M.E. The First Targeted Delivery of siRNA in Humans via a Self-Assembling, Cyclodextrin Polymer-Based Nanoparticle: From Concept to Clinic. Mol. Pharm. 2009, 6, 659–668. [Google Scholar] [CrossRef]
- Malhotra, M.; Gooding, M.; Evans, J.C.; O’Driscoll, D.; Darcy, R.; O’Driscoll, C.M. Cyclodextrin-siRNA conjugates as versatile gene silencing agents. Eur. J. Pharm. Sci. 2018, 114, 30–37. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Van De Manakker, F.; Vermonden, T.; Van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xiao, Q.; Zhang, Y.; Li, F.; Li, Y.; Li, C.; Wang, Q.; Shi, L.; Lin, H. Neodymium-doped NaHoF4 nanoparticles as near-infrared luminescent/T2-weighted MR dual-modal imaging agents in vivo. J. Mater. Chem. B 2017, 5, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Qian, Y.; Shen, Y.; Hu, H.; Sui, M.; Tang, J. Facile synthesis and in vivo evaluation of biodegradable dendritic MRI contrast agents. J. Mater. Chem. 2012, 22, 14369–14377. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549–15554. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Parvathaneni, V.; Shukla, S.K.; Kanabar, D.D.; Muth, A.; Gupta, V. Cyclodextrin Complexation for Enhanced Stability and Non-invasive Pulmonary Delivery of Resveratrol—Applications in Non-small Cell Lung Cancer Treatment. AAPS PharmSciTech 2020, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef] [PubMed]
- Hădărugă, N.G.; Bandur, G.N.; David, I.; Hădărugă, D.I. A review on thermal analyses of cyclodextrins and cyclodextrin complexes. Environ. Chem. Lett. 2018, 17, 349–373. [Google Scholar] [CrossRef]
- Liu, L.; Guo, Q.-X. The Driving Forces in the Inclusion Complexation of Cyclodextrins. J. Incl. Phenom. Macrocycl. Chem. 2002, 42, 1–14. [Google Scholar] [CrossRef]
- Hashidzume, A.; Yamaguchi, H.; Harada, A. Cyclodextrin-Based Rotaxanes: From Rotaxanes to Polyrotaxanes and Further to Functional Materials. Eur. J. Org. Chem. 2019, 2019, 3344–3357. [Google Scholar] [CrossRef]
- Hashidzume, A.; Yamaguchi, H.; Harada, A. Cyclodextrin-based molecular machines. In Topics in Current Chemistry; Springer: Berlin, Germany, 2014; Volume 354, pp. 71–110. [Google Scholar]
- Lai, W.-F.; Rogach, A.L.; Wong, W.-Y. Chemistry and engineering of cyclodextrins for molecular imaging. Chem. Soc. Rev. 2017, 46, 6379–6419. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Panero, M.; Grandi, M.; Uggeri, F. Inclusion complexes between β-cyclodextrin and β-benzyloxy-α-propionic derivatives of paramagnetic DOTA- and DPTA-Gd(III) complexes. Magn. Reson. Chem. 1991, 29, 923–927. [Google Scholar] [CrossRef]
- Aime, S.; Benetollo, F.; Bombieri, G.; Colla, S.; Fasano, M.; Paoletti, S. Non-ionic Ln(III) chelates as MRI contrast agents: Synthesis, characterisation and 1H NMR relaxometric investigations of bis(benzylamide)diethylenetriaminepentaacetic acid Lu(III) and Gd(III) complexes. Inorg. Chim. Acta 1997, 254, 63–70. [Google Scholar] [CrossRef]
- Bryson, J.M.; Chu, W.-J.; Lee, J.-H.; Reineke, T.M. A β-Cyclodextrin “Click Cluster” Decorated with Seven Paramagnetic Chelates Containing Two Water Exchange Sites. Bioconj. Chem. 2008, 19, 1505–1509. [Google Scholar] [CrossRef]
- Barge, A.; Cravotto, G.; Robaldo, B.; Gianolio, E.; Aime, S. New CD derivatives as self-assembling contrast agents for magnetic resonance imaging (MRI). J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 489–495. [Google Scholar] [CrossRef]
- Carrera, C.; Digilio, G.; Baroni, S.; Burgio, D.; Consol, S.; Fedeli, F.; Longo, D.L.; Mortillaro, A.; Aime, S. Synthesis and characterization of a Gd(iii) based contrast agent responsive to thiol containing compounds. Dalton Trans. 2007, 4980–4987. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.M.O.; Silva, A.M.; Silva, V.L. Pyrazoles as Key Scaffolds for the Development of Fluorine-18-Labeled Radiotracers for Positron Emission Tomography (PET). Molecules 2020, 25, 1722. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, M.; Sun, Y.; Chen, G.; Yang, T.; Gao, Y.; Zhang, X.; Li, F. Multifunctional rare-earth self-assembled nanosystem for tri-modal upconversion luminescence /fluorescence /positron emission tomography imaging. Biomaterials 2011, 32, 8243–8253. [Google Scholar] [CrossRef]
- Hajdu, I.; Angyal, J.; Szikra, D.; Kertész, I.; Malanga, M.; Fenyvesi, É.; Szente, L.; Vecsernyés, M.; Bácskay, I.; Váradi, J.; et al. Radiochemical synthesis and preclinical evaluation of 68Ga-labeled NODAGA-hydroxypropyl-beta-cyclodextrin (68Ga-NODAGA-HPBCD). Eur. J. Pharm. Sci. 2019, 128, 202–208. [Google Scholar] [CrossRef]
- Trencsényi, G.; Kis, A.; Szabó, J.P.; Ráti, Á.; Csige, K.; Fenyvesi, É.; Szente, L.; Malanga, M.; Méhes, G.; Emri, M.; et al. In vivo preclinical evaluation of the new 68Ga-labeled beta-cyclodextrin in prostaglandin E2 (PGE2) positive tumor model using positron emission tomography. Int. J. Pharm. 2020, 576, 118954. [Google Scholar] [CrossRef]
- Areses, P.; Agüeros, M.T.; Quincoces, G.; Collantes, M.; Richter, J.Á.; López-Sánchez, L.M.; Sánchez-Martínez, M.; Irache, J.M.; Peñuelas, I. Molecular Imaging Techniques to Study the Biodistribution of Orally Administered 99mTc-Labelled Naive and Ligand-Tagged Nanoparticles. Mol. Imaging Biol. 2011, 13, 1215–1223. [Google Scholar] [CrossRef]
- Perret, P.; Bacot, S.; Gèze, A.; Maurin, A.G.D.; Debiossat, M.; Soubies, A.; Blanc-Marquis, V.; Choisnard, L.; Boutonnat, J.; Ghezzi, C.; et al. Biodistribution and preliminary toxicity studies of nanoparticles made of Biotransesterified β–cyclodextrins and PEGylated phospholipids. Mater. Sci. Eng. C 2018, 85, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Liu, X.; Liu, T.; Zhou, J.; Zhu, J.; Sun, G.; He, D. Preparation of inclusion complex of perfluorocarbon compound with β-cyclodextrin for ultrasound contrast agent. RSC Adv. 2015, 5, 6305–6310. [Google Scholar] [CrossRef]
- Weber, J.; Beard, P.C.; Bohndiek, S.E. Contrast agents for molecular photoacoustic imaging. Nat. Methods 2016, 13, 639–650. [Google Scholar] [CrossRef] [PubMed]
- Laramie, M.D.; Smith, M.K.; Marmarchi, F.; McNally, L.R.; Henary, M. Small Molecule Optoacoustic Contrast Agents: An Unexplored Avenue for Enhancing In Vivo Imaging. Molecules 2018, 23, 2766. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, M.; Pan, W.; Wang, H.; Li, N.; Tang, B. Tumor microenvironment-triggered fabrication of gold nanomachines for tumor-specific photoacoustic imaging and photothermal therapy. Chem. Sci. 2017, 8, 4896–4903. [Google Scholar] [CrossRef]
- Wang, Y.; Thompson, J.M.; Ashbaugh, A.G.; Khodakivskyi, P.; Budin, G.; Sinisi, R.; Heinmiller, A.; Van Oosten, M.; Van Dijl, J.M.; Van Dam, G.M.; et al. Preclinical Evaluation of Photoacoustic Imaging as a Novel Noninvasive Approach to Detect an Orthopaedic Implant Infection. J. Am. Acad. Orthop. Surg. 2017, 25, S7–S12. [Google Scholar] [CrossRef]
- Maji, S.K.; Sreejith, S.; Joseph, J.; Lin, M.; He, T.; Tong, Y.; Sun, H.; Yu, S.W.-K.; Zhao, Y. Upconversion Nanoparticles as a Contrast Agent for Photoacoustic Imaging in Live Mice. Adv. Mater. 2014, 26, 5633–5638. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Tian, G.; Ren, W.; Yan, L.; Jin, S.; Gu, Z.; Zhou, L.; Li, J.; Zhao, Y. Design of multifunctional alkali ion doped CaF2 upconversion nanoparticles for simultaneous bioimaging and therapy. Dalton Trans. 2014, 43, 3861–3870. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, X.; Qian, L.; Yao, N.; Pan, Y.; Zhang, L. Doxorubicin-Loaded Unimolecular Micelle-Stabilized Gold Nanoparticles as a Theranostic Nanoplatform for Tumor-Targeted Chemotherapy and Computed Tomography Imaging. Biomacromolecules 2017, 18, 3869–3880. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, S.-B.; Chen, Z.-X.; Fan, J.-X.; Zhong, Z.; Zhang, X.-Z. A tungsten nitride-based degradable nanoplatform for dual-modal image-guided combinatorial chemo-photothermal therapy of tumors. Nanoscale 2019, 11, 2027–2036. [Google Scholar] [CrossRef]
- Zhou, J.; Lu, Z.; Shan, G.; Wang, S.; Liao, Y. Gadolinium complex and phosphorescent probe-modified NaDyF4 nanorods for T1- and T2-weighted MRI/CT/phosphorescence multimodality imaging. Biomaterials 2014, 35, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Champagne, P.-L.; Barbot, C.; Zhang, P.; Han, X.; Gaamoussi, I.; Hubert-Roux, M.; Bertolesi, G.E.; Gouhier, G.; Ling, C.-C. Synthesis and Unprecedented Complexation Properties of β-Cyclodextrin-Based Ligand for Lanthanide Ions. Inorg. Chem. 2018, 57, 8964–8977. [Google Scholar] [CrossRef] [PubMed]
- Kotková, Z.; Kotek, J.; Jirák, D.; Jendelová, P.; Herynek, V.; Berková, Z.; Hermann, P.; Lukeš, I. Cyclodextrin-Based Bimodal Fluorescence/MRI Contrast Agents: An Efficient Approach to Cellular Imaging. Chem. A Eur. J. 2010, 16, 10094–10102. [Google Scholar] [CrossRef] [PubMed]
- Kotková, Z.; Helm, L.; Kotek, J.; Hermann, P.; Lukeš, I. Gadolinium complexes of monophosphinic acid DOTA derivatives conjugated to cyclodextrin scaffolds: Efficient MRI contrast agents for higher magnetic fields. Dalton Trans. 2012, 41, 13509–13519. [Google Scholar] [CrossRef]
- Freed, J.H. Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. II. Finite jumps and independent T1 processes. J. Chem. Phys. 1978, 68, 4034–4037. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Frullano, L.; Crich, S.G.; Giovenzana, G.B.; Pagliarin, R.; Palmisano, G.; Sisti, M. Contrast Agents for Magnetic Resonance Imaging: A Novel Route to Enhanced Relaxivities Based on the Interaction of a Gd III Chelate with Poly-b-cyclodextrins. Chem. Eur. J. 1999, 5, 1254–1260. [Google Scholar] [CrossRef]
- Aime, S.; Botta, M.; Fedeli, F.; Gianolio, E.; Terreno, E.; Anelli, P. High-Relaxivity Contrast Agents for Magnetic Resonance Imaging Based on Multisite Interactions between a β-Cyclodextrin Oligomer and Suitably Functionalized Gd III Chelates. Chem. Eur. J. 2001, 7, 5262–5269. [Google Scholar] [CrossRef]
- Battistini, E.; Gianolio, E.; Gref, R.; Couvreur, P.; Füzerová, S.; Othman, M.; Aime, S.; Badet, B.; Durand, P.; Patrick, C. High-Relaxivity Magnetic Resonance Imaging (MRI) Contrast Agent Based on Supramolecular Assembly between a Gadolinium Chelate, a Modified Dextran, and Poly-β-Cyclodextrin. Chem. A Eur. J. 2008, 14, 4551–4561. [Google Scholar] [CrossRef]
- Martinelli, J.; Fekete, M.; Tei, L.; Botta, M. Cleavable β-cyclodextrin nanocapsules incorporating GdIII-chelates as bioresponsive MRI probes. Chem. Commun. 2011, 47, 3144–3146. [Google Scholar] [CrossRef]
- Lahrech, H.; Perles-Barbacaru, A.-T.; Aous, S.; Le Bas, J.-F.; Debouzy, J.-C.; Gadelle, A.; Fries, P.H. Cerebral Blood Volume Quantification in a C6 Tumor Model Using Gadolinium per (3,6-Anhydro) α-Cyclodextrin as a New Magnetic Resonance Imaging Preclinical Contrast Agent. J. Cereb. Blood Flow Metab. 2008, 28, 1017–1029. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.-Y.; Liu, B.-W.; Liu, Y. Construction of a Supramolecular Polymer by Bridged Bis(permethyl-β-cyclodextrin)s with Porphyrins and Its Highly Efficient Magnetic Resonance Imaging. Macromolecules 2013, 46, 4268–4275. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, H.; Hu, X.; Liu, B.; Liu, Y. Hyperbranched Supramolecular Polymer of Tris(permethyl-β-cyclodextrin)s with Porphyrins: Characterization and Magnetic Resonance Imaging. Chin. J. Chem. 2014, 32, 771–776. [Google Scholar] [CrossRef]
- Gomori, J.M.; I Grossman, R.; Yu-Ip, C.; Asakura, T. NMR relaxation times of blood: Dependence on field strength, oxidation state, and cell integrity. J. Comput. Assist. Tomogr. 1987, 11, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.M.; Brookes, M.J.; Hoogenraad, F.G.; Gowland, P.A.; Francis, S.T.; Morris, P.G.; Bowtell, R. T2* measurements in human brain at 1.5, 3 and 7 T. Magn. Reson. Imaging 2007, 25, 748–753. [Google Scholar] [CrossRef]
- Zhou, Z.; Han, Z.; Lu, Z.-R. A targeted nanoglobular contrast agent from host–guest self-assembly for MR cancer molecular imaging. Biomaterials 2016, 85, 168–179. [Google Scholar] [CrossRef]
- Liu, T.; Li, X.; Qian, Y.; Hu, X.; Liu, S. Multifunctional pH-Disintegrable micellar nanoparticles of asymmetrically functionalized β-cyclodextrin-Based star copolymer covalently conjugated with doxorubicin and DOTA-Gd moieties. Biomaterials 2012, 33, 2521–2531. [Google Scholar] [CrossRef]
- Skinner, P.J.; Beeby, A.; Dickins, R.S.; Parker, D.; Aime, S.; Botta, M. Conjugates of cyclodextrins with charged and neutral macrocyclic europium, terbium and gadolinium complexes: Sensitised luminescence and relaxometric investigations and an example of supramolecular relaxivity enhancement. J. Chem. Soc. Perkin Trans. 2 2000, 1329–1338. [Google Scholar] [CrossRef]
- Fredy, J.W.; Scelle, J.; Guenet, A.; Morel, E.; De Beaumais, S.A.; Ménand, M.; Marvaud, V.; Bonnet, C.S.; Tóth, É.; Sollogoub, M.; et al. Cyclodextrin Polyrotaxanes as a Highly Modular Platform for the Development of Imaging Agents. Chem. A Eur. J. 2014, 20, 10915–10920. [Google Scholar] [CrossRef]
- Su, H.; Liu, Y.; Wang, D.; Wu, C.; Xia, C.; Gong, Q.; Song, B.; Ai, H. Amphiphilic starlike dextran wrapped superparamagnetic iron oxide nanoparticle clsuters as effective magnetic resonance imaging probes. Biomaterials 2013, 34, 1193–1203. [Google Scholar] [CrossRef]
- Oroujeni, M.; Kaboudin, B.; Xia, W.; Jönsson, P.; Ossipov, D.A. Conjugation of cyclodextrin to magnetic Fe3O4 nanoparticles via polydopamine coating for drug delivery. Prog. Org. Coat. 2018, 114, 154–161. [Google Scholar] [CrossRef]
- Zhou, Z.; Mondjinou, Y.; Hyun, S.-H.; Kulkarni, A.; Lu, Z.-R.; Thompson, D.H. Gd3+-1,4,7,10-Tetraazacyclododecane-1,4,7-triacetic-2-hydroxypropyl-β-cyclodextrin/Pluronic Polyrotaxane as a Long Circulating High Relaxivity MRI Contrast Agent. ACS Appl. Mater. Interfaces 2015, 7, 22272–22276. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, M.; Han, Y.; Tang, J.; Qian, Y.; Hu, H.; Shen, Y. Enhancing MRI of liver metastases with a zwitterionized biodegradable dendritic contrast agent. Biomater. Sci. 2017, 5, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lu, Y.; Xu, X.; Li, S.; Du, Y.; Yu, R.-S. MR molecular imaging of HCC employing a regulated ferritin gene carried by a modified polycation vector. Int. J. Nanomed. 2019, 14, 3189–3201. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, T.; Gholibegloo, E.; Alam, N.R.; Dehghani, S.; Haghgoo, S.; Ghanaati, H.; Khoobi, M. Gadolinium (III) oxide nanoparticles coated with folic acid-functionalized poly(β-cyclodextrin-co-pentetic acid) as a biocompatible targeted nano-contrast agent for cancer diagnostic: In vitro and in vivo studies. MAGMA Magn. Reson. Mater. Phys. Biol. Med. 2019, 32, 487–500. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, X.; Qian, Y.; Hu, H.; Zhou, Z.; Liu, X.; Tang, J.; Shen, Y. Hypoxia-targeting dendritic MRI contrast agent based on internally hydroxy dendrimer for tumor imaging. Biomaterials 2019, 213, 119195. [Google Scholar] [CrossRef]
- Hane, F.T.; Robinson, M.; Lee, B.Y.; Bai, O.; Leonenko, Z.; Albert, M.S. Recent Progress in Alzheimer’s Disease Research, Part 3: Diagnosis and Treatment. J. Alzheimers Dis. 2017, 57, 645–665. [Google Scholar] [CrossRef]
- Schröder, L.; Lowery, T.J.; Hilty, C.; Wemmer, D.E.; Pines, A. Molecular Imaging Using a Targeted Magnetic Resonance Hyperpolarized Biosensor. Science 2006, 314, 446–449. [Google Scholar] [CrossRef]
- Hane, F.T.; Li, T.; Smylie, P.; Pellizzari, R.M.; Plata, J.A.; DeBoef, B.; Albert, M.S. In vivo detection of cucurbit[6]uril, a hyperpolarized xenon contrast agent for a xenon magnetic resonance imaging biosensor. Sci. Rep. 2017, 7, 41027. [Google Scholar] [CrossRef]
- Albert, M.S.; Cates, G.D.; Driehuys, B.; Happer, W.; Saam, B.; Springer, C.S.; Wishnia, A. Biological magnetic resonance imaging using laser-polarized 129Xe. Nat. Cell Biol. 1994, 370, 199–201. [Google Scholar] [CrossRef]
- Norquay, G.; Collier, G.J.; Rao, M.; Stewart, N.J.; Wild, J.M. Xe129 -Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Phys. Rev. Lett. 2018, 121, 153201. [Google Scholar] [CrossRef]
- Nikolaou, P.; Coffey, A.M.; Walkup, L.L.; Gust, B.M.; Whiting, N.; Newton, H.; Barcus, S.; Muradyan, I.; Dabaghyan, M.; Moroz, G.D.; et al. Near-unity nuclear polarization with an open-source 129Xe hyperpolarizer for NMR and MRI. Proc. Natl. Acad. Sci. USA 2013, 110, 14150–14155. [Google Scholar] [CrossRef] [PubMed]
- Comment, A.; Jannin, S.; Hyacinthe, J.-N.; Miéville, P.; Sarkar, R.; Ahuja, P.; Vasos, P.R.; Montet, X.; Lazeyras, F.; Vallee, J.-P.; et al. Hyperpolarizing Gases via Dynamic Nuclear Polarization and Sublimation. Phys. Rev. Lett. 2010, 105, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Keshari, K.R.; Kurhanewicz, J.; Macdonald, J.M.; Wilson, D.M. Generating contrast in hyperpolarized 13C MRI using ligand–receptor interactions. Analyst 2012, 137, 3427–3429. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, F.; Carretta, P.; Filibian, M.; Melone, L. Dynamic Nuclear Polarization of β-Cyclodextrin Macromolecules. J. Phys. Chem. B 2017, 121, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Caracciolo, F.; Paioni, A.L.; Filibian, M.; Melone, L.; Carretta, P. Proton and Carbon-13 Dynamic Nuclear Polarization of Methylated β-Cyclodextrins. J. Phys. Chem. B 2018, 122, 1836–1845. [Google Scholar] [CrossRef]
- Wang, Y.; Dmochowski, I.J. An Expanded Palette of Xenon-129 NMR Biosensors. Acc. Chem. Res. 2016, 49, 2179–2187. [Google Scholar] [CrossRef]
- Shapiro, M.G.; Ramirez, R.M.; Sperling, L.J.; Sun, G.; Sun, J.; Pines, A.; Schaffer, D.V.; Bajaj, V.S. Genetically encoded reporters for hyperpolarized xenon magnetic resonance imaging. Nat. Chem. 2014, 6, 629–634. [Google Scholar] [CrossRef]
- Song, Y.-Q.; Goodson, A.P.B.M.; Taylor, R.E.; Laws, D.D.; Navon, G.; Pines, A. Selective Enhancement of NMR Signals forα-Cyclodextrin with Laser-Polarized Xenon. Angew. Chem. Int. Ed. 1997, 36, 2368–2370. [Google Scholar] [CrossRef]
- Karas, S. The Synthesis of Rotaxane Probes for Magnetic Resonance Imaging (MRI). Master’s Thesis, University of Rhode Island, Kingston, RI, USA, 2016. [Google Scholar]
- Hane, F.T.; Smylie, P.S.; Julia, R.; Ruberto, J.; Dowhos, K.; Ball, I.; Tomanek, B.; DeBoef, B.; Albert, M.S. HyperCEST detection of cucurbit[6]uril in whole blood using an ultrashort saturation Pre-pulse train. Contrast Media Mol. Imaging 2016, 11, 285–290. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Slack, C.C.; Bruns, C.J.; Jeong, K.; Wemmer, D.E.; Pines, A.; Francis, M.B. Rotaxane-mediated suppression and activation of cucurbit[6]uril for molecular detection by 129Xe hyperCEST NMR. Chem. Commun. 2016, 52, 3119–3122. [Google Scholar] [CrossRef] [PubMed]
- Hane, F.T.; Fernando, A.; Prete, B.R.J.; Peloquin, B.; Karas, S.; Chaudhuri, S.; Chahal, S.; Shepelytskyi, Y.; Wade, A.; Li, T.; et al. Cyclodextrin-Based Pseudorotaxanes: Easily Conjugatable Scaffolds for Synthesizing Hyperpolarized Xenon-129 Magnetic Resonance Imaging Agents. ACS Omega 2018, 3, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Cavalieri, F.; El Hamassi, A.; Chiessi, E.; Paradossi, G.; Villa, R.; Zaffaroni, N. Tethering Functional Ligands onto Shell of Ultrasound Active Polymeric Microbubbles. Biomacromolecules 2006, 7, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Yeo, S.K.; Shepelytskyi, Y.; Grynko, V.; Albert, M.S. Molecular Imaging of Fluorinated Probes for Tau Protein and Amyloid-β Detection. Molecules 2020, 25, 3413. [Google Scholar] [CrossRef] [PubMed]
- Schluep, T.; Hwang, J.; Hildebrandt, I.J.; Czernin, J.; Hang, C.; Choi, J.; Alabi, C.A.; Mack, B.C.; Davis, M.E. Pharmacokinetics and tumor dynamics of the nanoparticle IT-101 from PET imaging and tumor histological measurements. Proc. Natl. Acad. Sci. USA 2009, 106, 11394–11399. [Google Scholar] [CrossRef]
- Hou, S.; Choi, J.-S.; Garcia, M.A.; Xing, Y.; Chen, K.-J.; Chen, Y.-M.; Jiang, Z.K.; Ro, T.; Wu, L.; Stout, D.B.; et al. Pretargeted Positron Emission Tomography Imaging That Employs Supramolecular Nanoparticles with in Vivo Bioorthogonal Chemistry. ACS Nano 2016, 10, 1417–1424. [Google Scholar] [CrossRef]
- Yaméogo, J.B.G.; Géze, A.; Choisnard, L.; Putaux, J.-L.; Semdé, R.; Wouessidjewe, D. Progress in developing amphiphilic cyclodextrin-based nanodevices for drug delivery. Curr. Top. Med. Chem. 2014, 14, 526–541. [Google Scholar] [CrossRef]
- Yaméogo, J.B.; Gèze, A.; Choisnard, L.; Putaux, J.-L.; Mazet, R.; Passirani, C.; Keramidas, M.; Coll, J.-L.; Lautram, N.; Bejaud, J.; et al. Self-assembled biotransesterified cyclodextrins as potential Artemisinin nanocarriers. II: In vitro behavior toward the immune system and in vivo biodistribution assessment of unloaded nanoparticles. Eur. J. Pharm. Biopharm. 2014, 88, 683–694. [Google Scholar] [CrossRef]
- Yu, G.; Yang, Z.; Fu, X.; Yung, B.C.; Yang, J.; Mao, Z.; Shao, L.; Hua, B.; Liu, Y.; Zhang, F.; et al. Polyrotaxane-based supramolecular theranostics. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Sauer, R.-S.; Rittner, H.L.; Roewer, N.; Sohajda, T.; Shityakov, S.; Brack, A.; Broscheit, J.-A. A Novel Approach for the Control of Inflammatory Pain: Prostaglandin E2 Complexation by Randomly Methylated β-Cyclodextrins. Anesth. Analg. 2017, 124, 675–685. [Google Scholar] [CrossRef]
- Zhu, C.; Ma, X.; Ma, D.; Zhang, T.; Gu, N. Crosslinked Dextran Gel Microspheres with Computed Tomography Angiography and Drug Release Function. J. Nanosci. Nanotechnol. 2018, 18, 2931–2937. [Google Scholar] [CrossRef]
- Lin, W.; Yao, N.; Qian, L.; Zhang, X.; Chen, Q.; Wang, J.; Zhang, L.-J. pH-responsive unimolecular micelle-gold nanoparticles-drug nanohybrid system for cancer theranostics. Acta Biomater. 2017, 58, 455–465. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Yang, C.; Xue, Z.; Huang, Y.; Luo, H.; Zu, X.; Zhang, L.; Yi, G. Controlled construction of gold nanoparticles in situ from β-cyclodextrin based unimolecular micelles for in vitro computed tomography imaging. J. Colloid Interface Sci. 2018, 528, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.; Choi, S.H.; Hyeon, T. Nano-Sized CT Contrast Agents. Adv. Mater. 2013, 25, 2641–2660. [Google Scholar] [CrossRef]
- Xi, D.; Dong, S.; Meng, X.; Lu, Q.; Meng, L.; Ye, J. Gold nanoparticles as computerized tomography (CT) contrast agents. RSC Adv. 2012, 2, 12515–12524. [Google Scholar] [CrossRef]
- Shilo, M.; Reuveni, T.; Motiei, M.; Popovtzer, R. Nanoparticles as computed tomography contrast agents: Current status and future perspectives. Nanomedicine 2012, 7, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Bednarz, B.; Caracappa, P.F.; Xu, X.G. The development, validation and application of a multi-detector CT (MDCT) scanner model for assessing organ doses to the pregnant patient and the fetus using Monte Carlo simulations. Phys. Med. Biol. 2009, 54, 2699–2717. [Google Scholar] [CrossRef] [PubMed]
- Semeniuk, O.; Grynko, O.; DeCrescenzo, G.; Juska, G.; Wang, K.; Reznik, A. Characterization of polycrystalline lead oxide for application in direct conversion X-ray detectors. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Wolterink, J.M.; Leiner, T.; Viergever, M.A.; Isgum, I. Generative Adversarial Networks for Noise Reduction in Low-Dose CT. IEEE Trans. Med. Imaging 2017, 36, 2536–2545. [Google Scholar] [CrossRef]
- Grynko, O.; Juska, G.; Reznik, A. An Engineering of multilayered lead oxide photoconductor for lag-free X-ray digital detector. In Proceedings of the IEEE Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), Manchester, UK, 26 October–2 November 2019. [Google Scholar] [CrossRef]
- Patino, M.; Prochowski, A.; Agrawal, M.D.; Simeone, F.J.; Gupta, R.; Hahn, P.F.; Sahani, D.V. Material Separation Using Dual-Energy CT: Current and Emerging Applications. Radiographics 2016, 36, 1087–1105. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.H.; Vogl, T.J.; Martin, S.S.; Nance, J.W.; Duguay, T.M.; Wichmann, J.L.; De Cecco, C.N.; Varga-Szemes, A.; Van Assen, M.; Tesche, C.; et al. Review of Clinical Applications for Virtual Monoenergetic Dual-Energy CT. Radiology 2019, 293, 260–271. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shepelytskyi, Y.; Newman, C.J.; Grynko, V.; Seveney, L.E.; DeBoef, B.; Hane, F.T.; Albert, M.S. Cyclodextrin-Based Contrast Agents for Medical Imaging. Molecules 2020, 25, 5576. https://doi.org/10.3390/molecules25235576
Shepelytskyi Y, Newman CJ, Grynko V, Seveney LE, DeBoef B, Hane FT, Albert MS. Cyclodextrin-Based Contrast Agents for Medical Imaging. Molecules. 2020; 25(23):5576. https://doi.org/10.3390/molecules25235576
Chicago/Turabian StyleShepelytskyi, Yurii, Camryn J. Newman, Vira Grynko, Lauren E. Seveney, Brenton DeBoef, Francis T. Hane, and Mitchell S. Albert. 2020. "Cyclodextrin-Based Contrast Agents for Medical Imaging" Molecules 25, no. 23: 5576. https://doi.org/10.3390/molecules25235576
APA StyleShepelytskyi, Y., Newman, C. J., Grynko, V., Seveney, L. E., DeBoef, B., Hane, F. T., & Albert, M. S. (2020). Cyclodextrin-Based Contrast Agents for Medical Imaging. Molecules, 25(23), 5576. https://doi.org/10.3390/molecules25235576






