New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Unidentified Compounds
2.1.1. Structure Elucidation of Compound 38
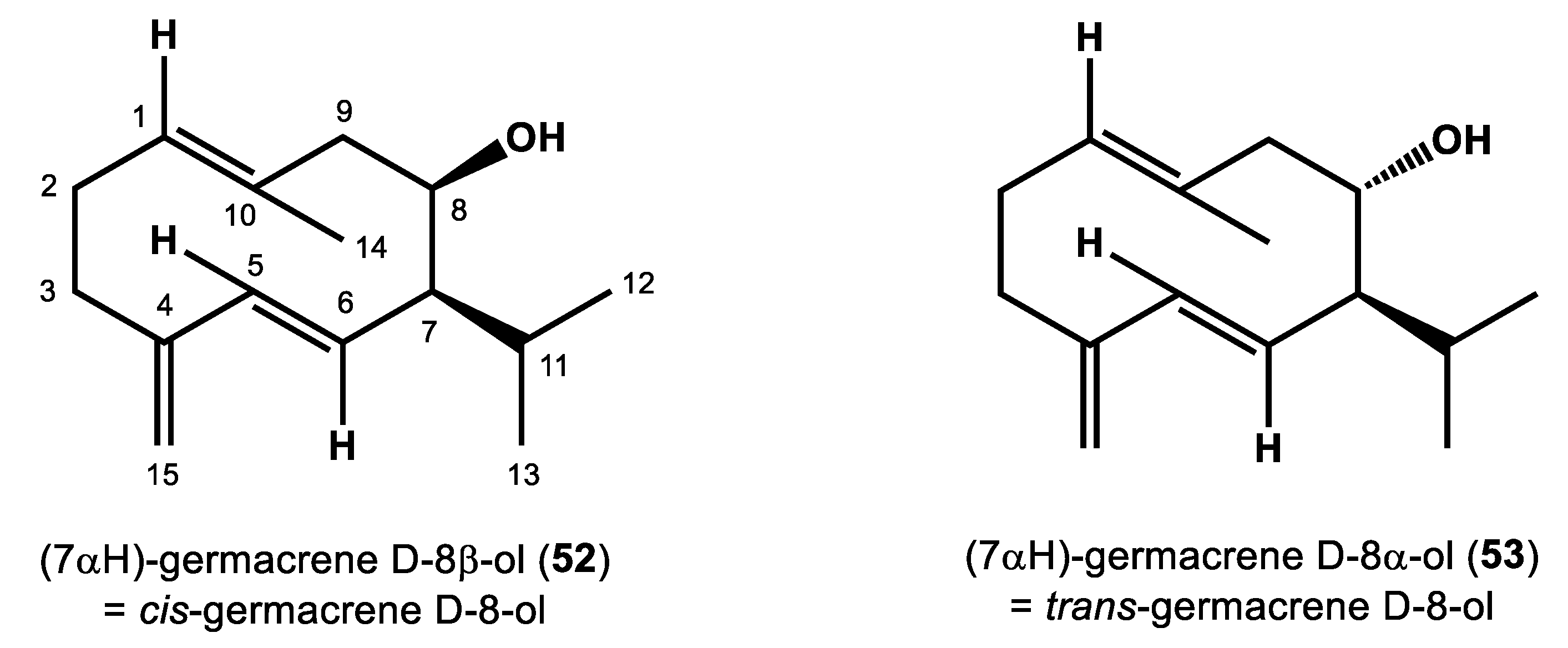
2.1.2. Structure Elucidation of Compounds 52 and 53
2.1.3. Structure Elucidation of Compound 56
2.2. Chemical Composition of Leaf Essential Oil from I. dewevrei
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Isolation and Fractionation
3.3. Gas Chromatography
3.4. Gas Chromatography–Mass Spectrometry in Electron Impact Mode
3.5. Gas Chromatography–High Resolution Mass Spectrometry
3.6. Nuclear Magnetic Resonance
3.7. Identification of Individual Components
3.8. Spectral Data
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Couvreur, T.L.P. Revealing the Secrets of African Annonaceae: Systematics, Evolution and Biogeography of the Syncarpous Genera Isolona and Monodora. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2008; p. 294.
- Aké, A.L. Flore de Côte d’Ivoire: Étude descriptive et biogéographique avec quelques notes ethno-botaniques. Thèse de Doctorat ès-Sciences Naturelles, Université d’Abidjan, Abidjan, Côte d’Ivoire, 1984; p. 1206. [Google Scholar]
- Hocquemiller, R.; Cabalion, P.; Bruneton, J.; Cavé, A. Alcaloïdes des Annonacées XXIII. Alcaloïdes des écorces d’Isolona campanulata Engler et Diels. Plantes Méd. Phytothérapie 1978, 12, 230–234. [Google Scholar]
- Okpekon, A.T. Étude chimique et biologique de plantes ivoiriennes utilisées en médecine traditionnelle en tant qu’antiparasitaires: Afromomum sceptrum K. Schum. (Zingiberaceae), Bridelia ferruginea Benth. (Euphorbiaceae), Isolona cooperi Hutch. & Dalz. et Uvaria afzelii Sc. Elliot (Annonaceae). Ph.D. Thèse, Université Paris-Sud, France–Université de Cocody-Abidjan, Côte d’Ivoire, Paris, France, 2006; p. 283. [Google Scholar]
- Kabran, A.F. Étude phytochimique de plantes ivoiriennes à activité antiparasitaire. Ph.D. Thèse, Université Paris-Sud, France–Université de Cocody-Abidjan, Côte d’Ivoire, Paris, France, 2013; p. 290. [Google Scholar]
- Boti, J.B.; Koukoua, G.; N’Guessan, Y.T.; Muselli, A.; Bernardini, A.F.; Casanova, J. Composition of the leaf, stem bark and root bark oils of Isolona cooperi investigated by GC (retention index), GC-MS and 13C-NMR spectroscopy. Phytochem. Anal. 2005, 16, 357–363. [Google Scholar] [CrossRef]
- Boti, J.B.; Yao, A.P.; Koukoua, G.; N’Guessan, Y.T.; Casanova, J. Components and chemical variability of Isolona campanulata Engler & Diels leaf oil. Flavour Fragr. J. 2006, 21, 166–170. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Ouattara, Z.A.; Yapi, A.T.; Bighelli, A.; Tomi, F.; Casanova, J. Leaf essential oil from Ivoirian Isolona dewevrei (Annonaceae): Chemical composition and structure elucidation of four new natural sesquiterpenes. Flavour Fragr. J. 2020. [Google Scholar] [CrossRef]
- Kambiré, D.A.; Boti, J.B.; Ouattara, Z.A.; Yapi, A.T.; Nelly, B.; Bighelli, A.; Tomi, F. Chemical composition of root and stem bark essential oils from Ivorian Isolona dewevrei: Structural elucidation of a new natural germacrone. Nat. Prod. Res. 2020, accepted. [Google Scholar] [CrossRef]
- Nea, F.; Kambiré, D.A.; Genva, M.; Tanoh, E.A.; Wognin, E.L.; Martin, H.; Brostaux, Y.; Tomi, F.; Lognay, G.C.; Tonzibo, Z.F.; et al. Composition, Seasonal Variation, and Biological Activities of Lantana camara Essential Oils from Côte d’Ivoire. Molecules 2020, 25, 2400. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Boti, J.B.; Yapi, A.T.; Ouattara, Z.A.; Paoli, M.; Bighelli, A.; Tomi, F.; Casanova, J. Composition and intraspecific chemical variability of leaf essential oil of Laggera pterodonta (DC.) Sch. Bip. ex Oliv. from Côte d’Ivoire. Chem. Biodivers. 2019, 17, e1900504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Ouattara, Z.A.; Tonzibo, Z.F.; Filippi, J.J.; Bighelli, A.; Tomi, F. Two new eudesman-4-ol epoxides from the stem essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Boti, J.B.; Filippi, J.J.; Tonzibo, Z.F.; Tomi, F. Characterization of a new epoxy-hydroxycarvotanacetone derivative from the leaf essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2019, 33, 2109–2112. [Google Scholar] [CrossRef] [PubMed]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Garcia, G.; Tomi, P.; Bighelli, A.; Tomi, F. Chemical composition of leaf essential oil of Piper umbellatum and aerial part essential oil of Piper guineense from Côte d’Ivoire. Nat. Prod. Commun. 2019, 14, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Tomi, F.; Bradesi, P.; Bighelli, A.; Casanova, J. Computer-aided identification of individual components of essential oils using carbon-13 NMR spectroscopy. J. Magn. Reson. Anal. 1995, 1, 25–34. [Google Scholar]
- Ouattara, Z.A.; Boti, J.B.; Ahibo, A.C.; Sutour, S.; Casanova, J.; Tomi, F.; Bighelli, A. The key role of 13C NMR analysis in the identification of individual components of Polyalthia longifolia leaf oil. Flavour Fragr. J. 2014, 29, 371–379. [Google Scholar] [CrossRef]
- Gerhard, U.; Thomas, S.; Mortishire-Smith, R. Accelerated metabolite identification by ‘extraction NMR’. J. Pharm. Biomed. Anal. 2003, 32, 531–538. [Google Scholar] [CrossRef]
- Yapi, T.A.; Boti, J.B.; Attioua, B.K.; Ahibo, A.C.; Bighelli, A.; Casanova, J.; Tomi, F. Three new natural compounds from the root bark essential oil from Xylopia aethiopica. Phytochem. Anal. 2012, 23, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Wahlburg, H.C.; Marschall, H.; Rustaiyan, A. New cadinene and bisabolene derivatives from the essential oil of Pulicaria gnaphalodes. Liebigs Ann. Chem. 1993, 1117–1123. [Google Scholar] [CrossRef]
- Rustaiyan, A.; Monfared, A.; Masoudi, S. The Essential Oil of Ferula flabelliloba Rech. F. et Aell. J. Essent. Oil Res. 2001, 13, 403–404. [Google Scholar] [CrossRef]
- Weyerstahl, P.; Marschall, H.; Wahlburg, H.C.; Cristiansen, C.; Rustaiyan, A.; Mirdjalili, F. Constituents of the essential oil of Pulicaria gnaphalodes (Vent.) Boiss. from Iran. Flavour Fragr. J. 1999, 14, 121–130. [Google Scholar] [CrossRef]
- Lis, A.; Nazaruk, J.; Mielczarek, J.; Kalemba, D. Comparative Study of Chemical Composition of Essential Oils from Different Organs of Erigeron annuus (L.) Pers. J. Essent. Oil-Bear. Plants 2008, 11, 17–21. [Google Scholar] [CrossRef]
- Cachet, T.; Brevard, H.; Chaintreau, A.; Demyttenaere, J.; French, L.; Gassenmeier, K.; Joulain, D.; Koenig, T.; Leijs, H.; Liddle, P.; et al. IOFI recommended practice for the use of predicted relative-response factors for the rapid quantification of volatile flavouring compounds by GC-FID. Flavour Fragr. J. 2016, 31, 191–194. [Google Scholar] [CrossRef] [Green Version]
- König, W.A.; Hochmuth, D.H.; Joulain, D. Terpenoids and Related Constituents of Essential Oils. Library of MassFinder 2.1; Institute of Organic Chemistry: Hamburg, Germany, 2001. [Google Scholar]
- Terpenoids Library Website. Available online: https://massfinder.com/wiki/Terpenoids_Library_List (accessed on 23 June 2020).
- National Institute of Standards and Technology. PC Version of the Mass Spectral Library; National Institute of Standards and Technology: Norwalk, CT, USA, 2014.
- Adams, R.P. Identification of Essential Oils Components by Gas. In Chromatography/Quadrupole Mass Spectroscopy; Allured: Carol Stream, IL, USA, 2001; p. 455. [Google Scholar]



| Compound 38 | |||||||
|---|---|---|---|---|---|---|---|
| C | δ 13C (ppm) | DEPT | δ 1H (ppm) | Multiplicity (J (Hz)) | COSY 1H–1H | HMBC H → C | NOESY 1H–1H |
| 1 | 86.63 | C | - | - | - | - | - |
| 2 | 30.71 | CH2 | a 2.15 | m | 2b, 3a, 3b | 1, 3, 4, 6, 10 | 2b, 3a |
| b 2.29 | m | 2a, 3a, 3b | 1, 3, 4, 6, 10 | 2a, 3b | |||
| 3 | 30.13 | CH2 | a 2.00 | m | 2a, 2b, 3b | 1, 2, 4, 5, 15 | 2a, 3b |
| b 2.17 | m | 2b, 3a, 3b | 1, 2, 4, 5, 15 | 2b, 3a, 15 | |||
| 4 | 133.78 | C | - | - | - | - | - |
| 5 | 122.89 | CH | 5.57 | quint (1.5) | 6 | 1, 4, 6, 7, 15 | 6, 13, 15 |
| 6 | 51.98 | CH | 2.25 | m | 5, 7 | 1, 2, 4, 5, 7, 10 | 5, 11, 13 |
| 7 | 54.01 | CH | 1.16 | t (9.3) | 6, 8, 11 | 5, 6, 8, 11, 12, 13 | 2b, 8, 9a, 14 |
| 8 | 81.75 | CH | 4.27 | d (5.2) | 7, 9a, 9b | 6, 7, 9, 10, 11 | 7, 9a, 14 |
| 9 | 43.42 | CH2 | a 1.02 | dd (11.0, 3.8) | 8, 9b, 10 | 7, 8, 10, 14 | 9b, 8, 7, 14 |
| b 2.21 | dd (11.0, 5.2) | 8, 9a, 10 | 1, 7, 8, 10, 14 | 9a, 10 | |||
| 10 | 41.18 | CH | 2.02 | m | 14, 9a, 9b | 1, 2, 6, 8, 9, 14 | 9b, 14 |
| 11 | 33.27 | CH | 1.45 | dsept (9.3, 6.7) | 7, 12, 13 | 6, 7, 8, 12, 13 | 6, 12, 13 |
| 12 | 21.81 | CH3 | 0.94 | d (6.7) | 11 | 7, 11, 13 | 11, 13 |
| 13 | 19.82 | CH3 | 0.87 | d (6.7) | 11 | 7, 11, 12 | 5, 6, 11, 12 |
| 14 | 19.71 | CH3 | 1.07 | d (7.4) | 10 | 1, 9, 10 | 7, 8, 9a, 10 |
| 15 | 22.65 | CH3 | 1.59 | br s | - | 3, 4, 5 | 3b, 5 |
| Compound 52 | Compound 53 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | δ 13C (ppm) | DEPT | δ 1H (ppm) | Multiplicity (J (Hz)) | COSY 1H–1H | HMBC H → C | NOESY 1H–1H | δ 13C (ppm) | δ 1H (ppm) | Multiplicity (J (Hz)) | NOESY 1H–1H |
| 1 | 132.27 | CH | 5.14 | br dd (10.7, 4.8) | 2a, 2b | 2, 9, 10, 14 | 2b, 6, 9b | 130.27 | 5.63 | br dd (10.6, 5.0) | 2b, 6, 9b |
| 2 | 29.36 | CH2 | a 1.99 | m | 1, 2b, 3a, 3b | 1, 3, 4, 5, 10, 15 | 3b, 14 | 30.58 | a 2.16 | m | 3b, 14 |
| b 2.46 | m | 1, 2a, 3a, 3b | 1, 3, 4, 5, 10, 15 | 1, 3a, 15b | b 2.19 | m | 1, 3a, 15b | ||||
| 3 | 34.61 | CH2 | a 2.13 | m | 1, 2a, 2b | 1, 2, 4, 5, 15 | 2b, 6, 15b | 32.26 | a 2.22 | m | 2b, 6, 15b |
| b 2.48 | m | 1, 2a, 2b | 1, 2, 4, 5, 15 | 2a, 5, 8, 14 | b 2.46 | m | 2a, 5, 14 | ||||
| 4 | 148.76 | C | - | - | - | - | - | 148.79 | - | - | - |
| 5 | 137.36 | CH | 5.79 | d (16.1) | 6 | 3, 4, 6, 7, 15 | 3b, 7, 8, 14 | 127.64 | 6.06 | d (16.3) | 3b, 7, 14 |
| 6 | 130.18 | CH | 5.56 | dd (16.1, 9.8) | 5, 7 | 4, 5, 7, 8, 11 | 1, 2b, 9b, 11, 13 | 134.81 | 5.93 | dd (16.3, 9.8) | 1, 2b, 9b, 11, 13 |
| 7 | 57.56 | CH | 1.93 | dt (9.8, 2.5) | 6, 8, 11 | 5, 6, 8,11, 12, 13 | 5, 8, 12, 13, 14 | 53.96 | 2.12 | m | 5, 12, 13, 14 |
| 8 | 69.57 | CH | 4.12 | m | 7, 9a, 9b | 6, 7, 9, 10, 11 | 3b, 5, 7, 14 | 73.13 | 4.09 | m | 9a, 13 |
| 9 | 47.28 | CH2 | a 2.39 | dd (14.0, 2.3) | 8 | 1, 7, 8, 10, 14 | 9b, 14 | 45.68 | a 2.02 | dd (14.2, 4.0) | 8, 9b, 14 |
| b 2.56 | dd (14.0, 5.3) | 8 | 1, 7, 8, 10, 14 | 1, 6, 9a, 11 | b 2.70 | dd (14.2, 6.8) | 1, 6, 9a, 11 | ||||
| 10 | 132.55 | C | - | - | - | - | - | 134.16 | - | - | - |
| 11 | 28.47 | CH | 1.69 | m | 7, 12, 13 | 6, 7, 8, 12, 13 | 6, 9b, 12, 13 | 27.44 | 1.97 | m | 6, 9b, 12, 13 |
| 12 | 20.52 | CH3 | 0.97 | d (6.7) | 11 | 7, 11, 13 | 7, 11, 13 | 20.64 | 0.99 | d (6.8) | 7, 11, 13 |
| 13 | 21.58 | CH3 | 0.87 | d (6.7) | 11 | 7, 11, 12 | 6, 7, 11, 12 | 21.86 | 0.94 | d (6.8) | 6, 8, 11, 12 |
| 14 | 19.34 | CH3 | 1.71 | br s | - | 1, 2, 8, 9, 10 | 2a, 3b, 5, 7, 8, 9a | 19.53 | 1.44 | br s | 2a, 3b, 5, 7, 9a |
| 15 | 109.34 | CH2 | a 4.78 | br d (2.3) | 15b | 2, 3, 4, 5 | 3b, 5, 15b | 112.25 | 4.71 | br d (2.2) | 3b, 5, 15b |
| b 4.82 | br d (2.3) | 15a | 2, 3, 4, 5 | 2b, 3a, 15a | 4.88 | br d (2.2) | 2b, 3a, 15a | ||||
| C | δ 13C (ppm) | DEPT | δ 1H (ppm) | Multiplicity (J (Hz)) | COSY 1H–1H | HMBC H → C | NOESY 1H–1H a |
|---|---|---|---|---|---|---|---|
| 1 | 130.31 | C | - | - | - | - | - |
| 2 | 26.70 | CH2 | a 1.99 | m | 2b, 3 | 1, 3, 4, 6, 10 | 2b, 3 |
| b 2.74 | ddd (12.2, 3.6, 3.1) | 2a, 3 | 1, 3, 4, 6, 10 | 2a, 3, 14 | |||
| 3 | 32.05 | CH2 | a 2.04 | m | 2a, 2b | 1, 2, 4, 5, 15 | 2a, 2b, 15 |
| 4 | 134.83 | C | - | - | - | - | - |
| 5 | 123.99 | CH | 5.45 | m (1.5) | 6 | 1, 4, 6, 7, 15 | 6, 15 |
| 6 | 34.93 | CH | 2.86 | br d (11.0) | 5, 7 | 1, 2, 4, 5, 7, 10 | 5, 9b, 11, 13 |
| 7 | 48.03 | CH | 1.15 | br dd (11.0, 4.3) | 6, 8, 11 | 5, 6, 8, 11, 12, 13 | 8, 9a, 14 |
| 8 | 65.54 | CH | 4.17 | m | 7, 9a, 9b | 6, 7, 9, 10, 11 | 7, 9a, 14 |
| 9 | 41.81 | CH2 | a 2.04 | m | 8, 9b | 1, 7, 8, 10, 14 | 9b, 8, 7, 14 |
| b 2.30 | dd (17.3, 4.1) | 8, 9a | 1, 7, 8, 10, 14 | 9a, 6, 11, 12 | |||
| 10 | 119.79 | C | - | - | - | - | - |
| 11 | 27.14 | CH | 2.10 | dsept (7.0, 4.1) | 7, 12, 13 | 6, 7, 8, 12, 13 | 6, 12, 13 |
| 12 | 18.50 | CH3 | 1.04 | d (7.0) | 11 | 7, 11, 13 | 9b, 11, 13 |
| 13 | 21.79 | CH3 | 1.05 | d (7.0) | 11 | 7, 11, 12 | 6, 11, 12 |
| 14 | 18.74 | CH3 | 1.67 | br s | - | 1, 9, 10 | 2b, 8, 7 |
| 15 | 23.62 | CH3 | 1.69 | br s | - | 3, 4, 5 | 3, 5 |
| N° | Compounds | RIa | RIp | RFF | S1 (%) | S2 (%) | Identification |
|---|---|---|---|---|---|---|---|
| 1 | α-Thujene | 923 | 1016 | 0.765 | tr | 0.1 | RI, MS |
| 2 | α-Pinene | 931 | 1013 | 0.765 | 0.1 | 0.1 | RI, MS |
| 3 | Sabinene | 965 | 1120 | 0.765 | 0.1 | 0.4 | RI, MS, 13C-NMR |
| 4 | β-Pinene | 970 | 1109 | 0.765 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 5 | Myrcene | 981 | 1158 | 0.765 | 0.3 | 0.3 | RI, MS, 13C-NMR |
| 6 | α-Terpinene | 1009 | 1178 | 0.765 | 0.1 | 0.1 | RI, MS |
| 7 | p-Cymene | 1012 | 1268 | 0.698 | tr | 0.1 | RI, MS, 13C-NMR |
| β | Limonene | 1021 | 1199 | 0.765 | 1.1 | 1.1 | RI, MS, 13C-NMR |
| 9 | (Z)-β-Ocimene | 1025 | 1230 | 0.765 | 3.4 | 4.5 | RI, MS, 13C-NMR |
| 10 | (E)-β-Ocimene | 1036 | 1247 | 0.765 | 4.5 | 4.2 | RI, MS, 13C-NMR |
| 11 | γ-Terpinene | 1048 | 1242 | 0.765 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 12 | Linalool | 1083 | 1543 | 0.869 | tr | 0.1 | RI, MS |
| 13 | allo-Ocimene | 1117 | 1370 | 0.765 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 14 | Terpinen-4-ol | 1161 | 1597 | 0.869 | - | 0.1 | RI, MS |
| 15 | Geraniol | 1233 | 1843 | 0.869 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 16 | Geranial | 1244 | 1740 | 0.887 | 0.1 | 0.1 | RI, MS, 13C-NMR |
| 17 | δ-Elemene | 1334 | 1464 | 0.751 | tr | 0.5 | RI, MS, 13C-NMR |
| 18 | α-Cubebene | 1347 | 1452 | 0.751 | 0.1 | 0.1 | RI, MS |
| 19 | α-Copaene | 1374 | 1485 | 0.751 | 0.9 | 0.7 | RI, MS, 13C-NMR |
| 20 | β-Elemene | 1385 | 1583 | 0.751 | 1.6 | 1.7 | RI, MS, 13C-NMR |
| 21 | (E)-β-Caryophyllene * | 1416 | 1589 | 0.751 | 5.3 | 5.7 | RI, MS, 13C-NMR |
| 22 | α-Santalene * | 1416 | 1565 | 0.751 | 0.1 | 0.3 | RI, MS, 13C-NMR |
| 23 | γ-Elemene # | 1426 | 1630 | 0.751 | tr | 0.5 | RI, MS, 13C-NMR |
| 24 | (E)-β-Farnesene | 1446 | 1660 | 0.751 | 0.1 | 0.1 | RI, MS |
| 25 | α-Humulene | 1448 | 1662 | 0.751 | 1.7 | 1.3 | RI, MS, 13C-NMR |
| 26 | α-Curcumene | 1469 | 1766 | 0.707 | tr | tr | RI, MS, 13C-NMR |
| 27 | γ-Muurolene | 1471 | 1683 | 0.751 | 0.3 | 0.3 | RI, MS, 13C-NMR |
| 28 | Germacrene D | 1474 | 1700 | 0.751 | 23.6 | 20.5 | RI, MS, 13C-NMR |
| 29 | trans-β-Bergamotene | 1478 | 1676 | 0.751 | tr | 0.2 | RI, MS, 13C-NMR |
| 30 | β-Selinene | 1484 | 1710 | 0.751 | 0.1 | tr | RI, MS |
| 31 | Bicyclogermacrene | 1489 | 1721 | 0.751 | 1.8 | 1.6 | RI, MS, 13C-NMR |
| 32 | α-Selinene | 1493 | 1717 | 0.751 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 33 | β-Bisabolene | 1500 | 1719 | 0.751 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 34 | δ-Cadinene | 1512 | 1753 | 0.751 | 2.5 | 2.4 | RI, MS, 13C-NMR |
| 35 | cis-Lanceol | 1517 | 2087 | 0.819 | 0.9 | 0.7 | RI, MS, 13C-NMR |
| 36 | (Z)-γ-Bisabolene | 1521 | 1721 | 0.751 | 1.4 | 1.5 | RI, MS, 13C-NMR |
| 37 | trans-Sesquisabinene hydrate | 1530 | 1984 | 0.819 | tr | 0.1 | RI, MS |
| 38 | (10βH)-1β,8β-Oxido-cadin-4-ene | 1534 | 1853 | 0.830 | 7.3 | 8.7 | QTOF-MS, 1D, 2D-NMR |
| 39 | β-Elemol | 1536 | 2077 | 0.819 | tr | 0.2 | RI, MS, 13C-NMR |
| 40 | (E)-Nerolidol | 1547 | 2034 | 0.819 | 0.5 | 1.1 | RI, MS, 13C-NMR |
| 41 | Germacrene B # | 1549 | 1818 | 0.751 | 1.3 | 2.4 | RI, MS, 13C-NMR |
| 42 | cis-Sesquisabinene hydrate | 1562 | 2079 | 0.819 | 0.3 | 0.2 | RI, MS, 13C-NMR |
| 43 | Caryophyllene oxide | 1567 | 1973 | 0.830 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 44 | Germacrene D-8-one | 1584 | 2066 | 0.841 | 8.9 | 8.7 | RI, MS, 13C-NMR |
| 45 | Humulene oxide II | 1597 | 2042 | 0.830 | 0.4 | 0.2 | RI, MS, 13C-NMR |
| 46 | Alismol | 1609 | 2245 | 0.830 | 0.1 | 0.3 | RI, MS, 13C-NMR |
| 47 | γ-Eudesmol | 1620 | 2172 | 0.819 | 1.2 | 1.2 | RI, MS, 13C-NMR |
| 48 | δ-Cadinol | 1626 | 2174 | 0.819 | 0.1 | 0.2 | RI, MS, 13C-NMR |
| 49 | Muurola-4,10(14)-dien-8β-ol | 1629 | 2186 | 0.830 | 3.2 | 2.9 | RI, MS, 13C-NMR |
| 50 | α-Cadinol | 1634 | 2231 | 0.819 | 0.6 | 0.6 | RI, MS, 13C-NMR |
| 51 | β-Bisabolol | 1653 | 2144 | 0.819 | 0.2 | 0.2 | RI, MS, 13C-NMR |
| 52 | (7αH)-Germacrene D-8β-ol * | 1657 | 2355 | 0.819 | 7.8 | 7.4 | QTOF-MS, 1D, 2D-NMR |
| 53 | (7αH)-Germacrene D-αβ-ol * | 1657 | 2355 | 0.819 | 2.6 | 2.5 | QTOF-MS, 1D, 2D-NMR |
| 54 | α-Bisabolol | 1664 | 2208 | 0.819 | 1.4 | 1.5 | RI, MS, 13C-NMR |
| 55 | epi-α-Bisabolol | 1667 | 2214 | 0.819 | 0.1 | tr | RI, MS, 13C-NMR |
| 56 | Cadina-1(10),4-dien-8β-ol | 1676 | 2276 | 0.819 | 7.6 | 7.2 | QTOF-MS, 1D, 2D-NMR |
| 57 | Cadina-4,10(14)-dien-8β-ol | 1678 | 2280 | 0.830 | 0.8 | 0.8 | RI, MS, 13C-NMR |
| Hydrocarbon monoterpenes | 10.0 | 11.5 | |||||
| Oxygenated monoterpenes | 0.2 | 0.5 | |||||
| Hydrocarbon sesquiterpenes | 41.2 | 40.2 | |||||
| Oxygenated sesquiterpenes | 44.1 | 44.9 | |||||
| Total | 95.5 | 97.1 |
Sample Availability: Samples of the compounds 38, 52 and 53 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kambiré, D.A.; Boti, J.B.; Yapi, T.A.; Ouattara, Z.A.; Bighelli, A.; Casanova, J.; Tomi, F. New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules 2020, 25, 5613. https://doi.org/10.3390/molecules25235613
Kambiré DA, Boti JB, Yapi TA, Ouattara ZA, Bighelli A, Casanova J, Tomi F. New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules. 2020; 25(23):5613. https://doi.org/10.3390/molecules25235613
Chicago/Turabian StyleKambiré, Didjour Albert, Jean Brice Boti, Thierry Acafou Yapi, Zana Adama Ouattara, Ange Bighelli, Joseph Casanova, and Félix Tomi. 2020. "New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels" Molecules 25, no. 23: 5613. https://doi.org/10.3390/molecules25235613
APA StyleKambiré, D. A., Boti, J. B., Yapi, T. A., Ouattara, Z. A., Bighelli, A., Casanova, J., & Tomi, F. (2020). New Natural Oxygenated Sesquiterpenes and Chemical Composition of Leaf Essential Oil from Ivoirian Isolona dewevrei (De Wild. & T. Durand) Engl. & Diels. Molecules, 25(23), 5613. https://doi.org/10.3390/molecules25235613






