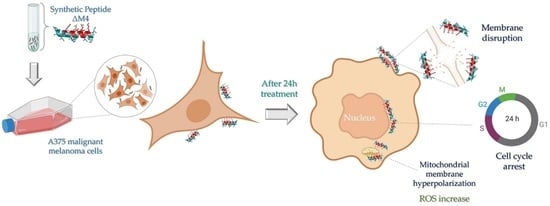
Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells
Abstract
1. Introduction
2. Results
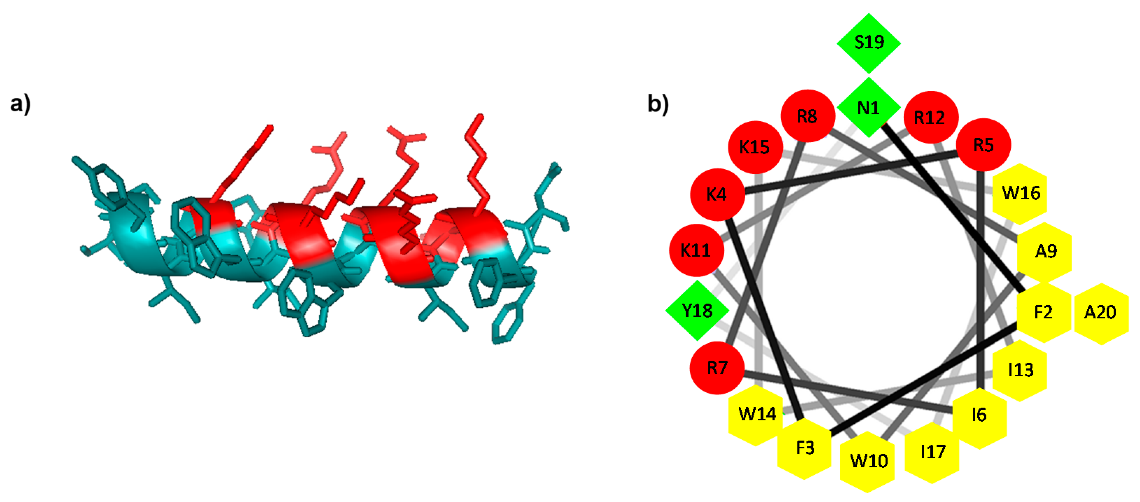
2.1. Design and Prediction of the 3D Structure of ΔM4
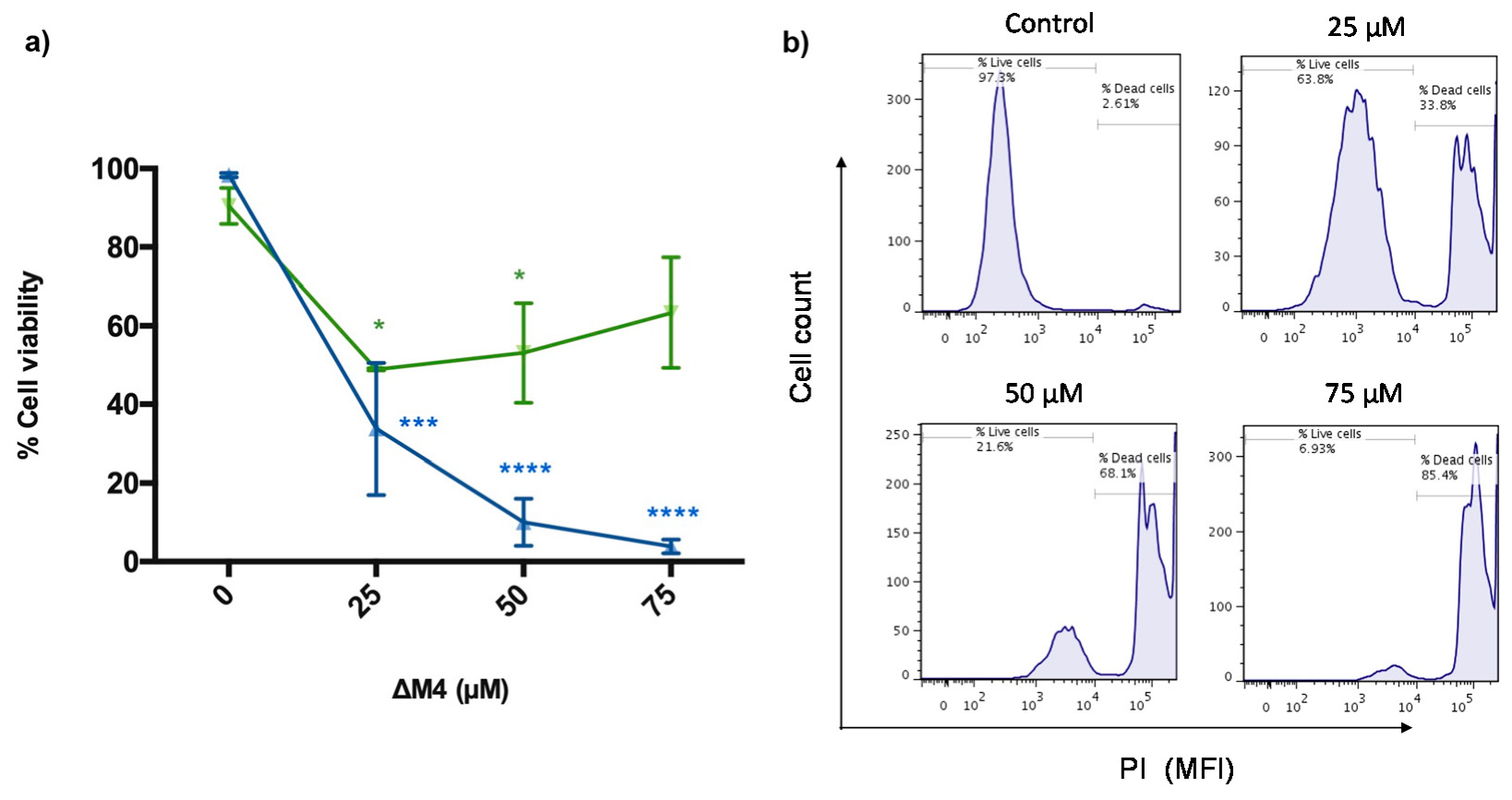
2.2. ΔM4 Disrupts the Plasma Membrane and Selectively Reduces Melanoma Skin Cancer Cell Viability in a Dose-Response Relationship
2.3. ΔM4-Induced Morphological Changes in Melanoma Skin Cancer Cells
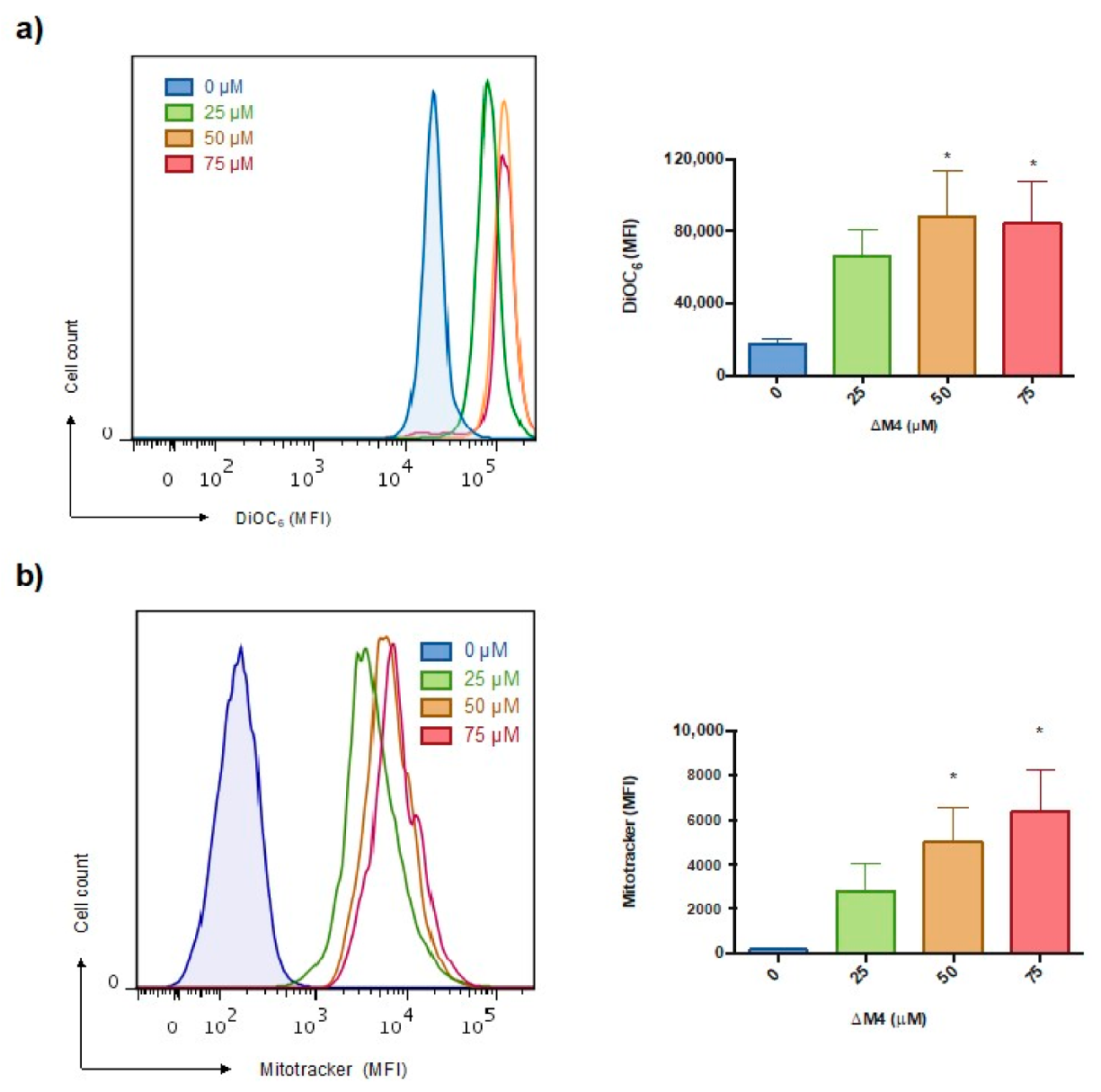
2.4. Mitochondrial Membrane Hyperpolarization and ROS Production Are Stimulated in A375 Cells by ΔM4 Treatment
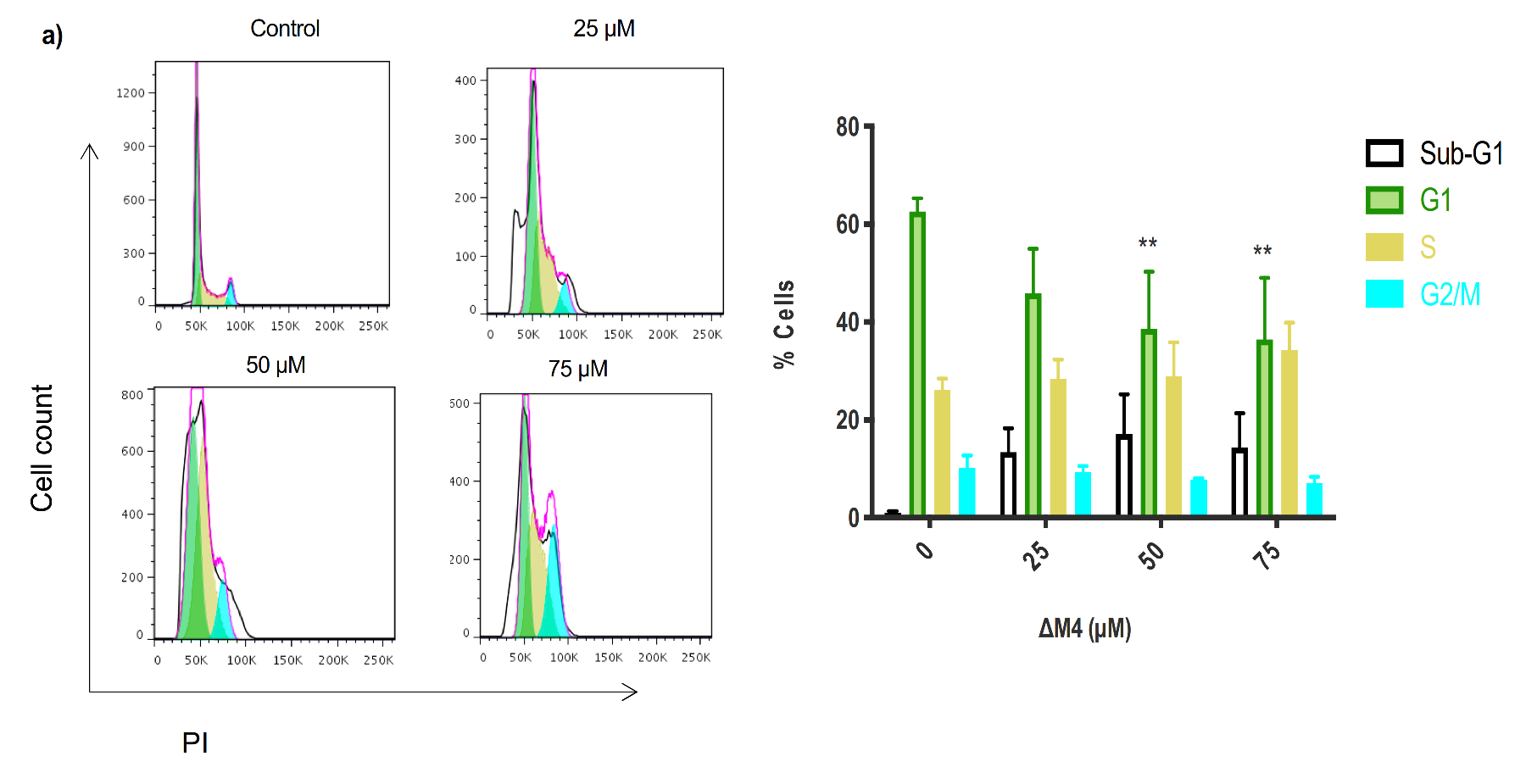
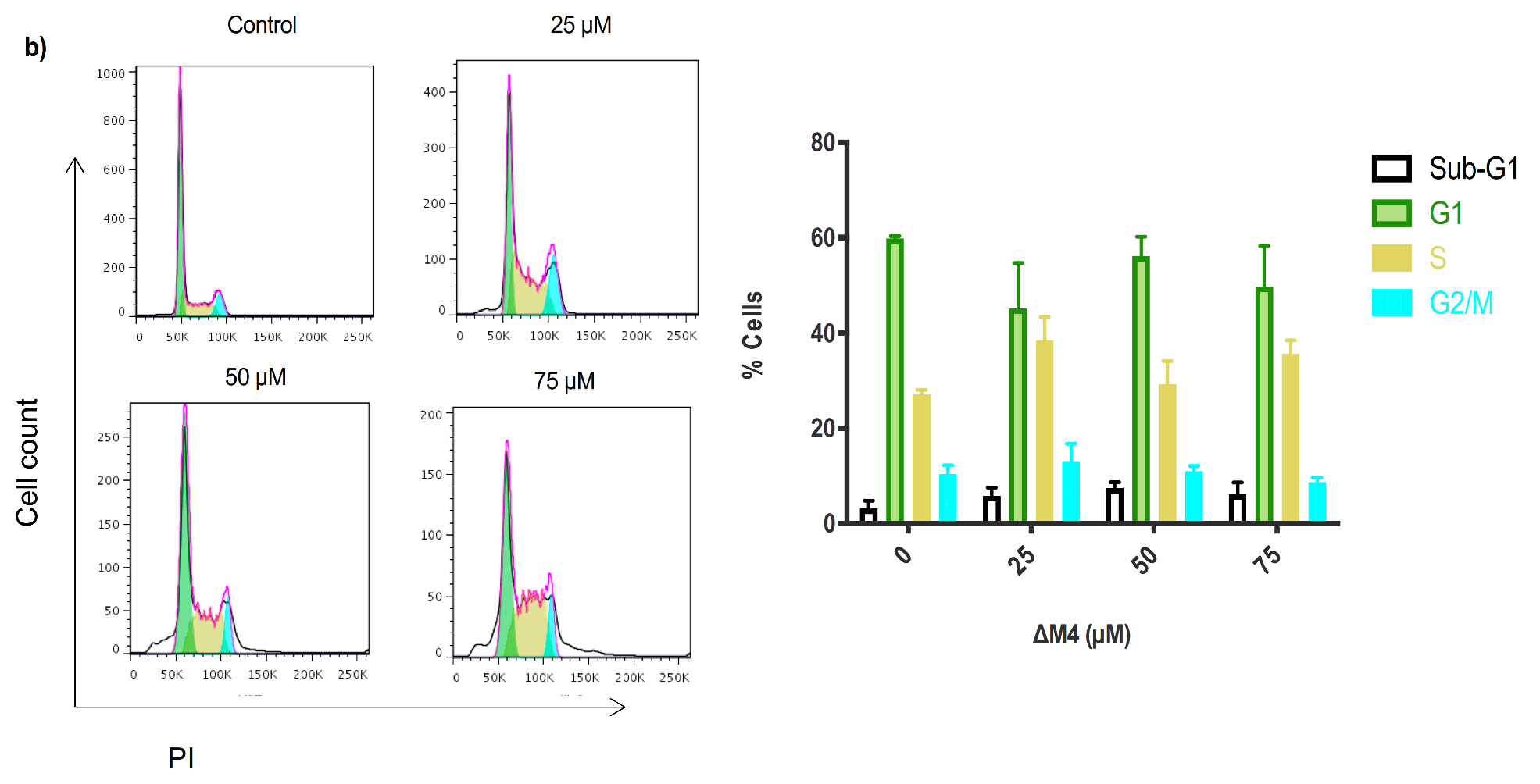
2.5. Exposure to ∆M4 Induces Cell Cycle Arrest at the S-Phase in A375 Cells
3. Discussion
4. Materials and Methods
4.1. Prediction of the Peptide Structure
4.2. Peptide Synthesis
4.3. Cell Culture
4.4. Treatment Conditions
4.5. Evaluation of the Cytoplasmic Membrane Integrity as a Measure of the Cell Viability
4.6. Morphological Analysis
4.7. Cell Size and Granularity, and Cell Cycle Analysis by Flow Cytometry
4.8. Evaluation of the Mitochondrial Membrane Potential
4.9. Mitochondrial ROS Detection
4.10. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cooper, G.M.; Hausman, R.E. The Development and Causes of Cancer. In The Cell: A Molecular Approach, 4th ed.; Sinauer Associates: Sunderland, MA, USA, 2007; pp. 719–723. [Google Scholar]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016. [Google Scholar]
- Steward, B.; Wild, C.P. World Cancer Report 2014; WHO Press: Geneva, Switzerland, 2014; pp. 16–69. [Google Scholar]
- Narayanan, D.L.; Saladi, R.N.; Fox, J.L. Ultraviolet radiation and skin cancer. Int. J. Dermatol. 2010, 49, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Garnier-Suillerot, A.; Marbeuf-Gueye, C.; Salerno, M.; Loetchutinat, C.; Fokt, I.; Krawczyk, M.; Kowalczyk, T.; Priebe, W. Analysis of drug transport kinetics in multidrug-resistant cells: Implications for drug action. Curr. Med. Chem. 2001, 8, 51–64. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Zunino, F. Overview of Tumor Cell Chemoresistance Mechanisms. In Chemosensitivity; Humana Press: Totowa, NJ, USA, 2005; Volume II, pp. 127–148. [Google Scholar]
- Brogden, K.A.; Ackermann, M.; McCray, P.B.; Tack, B.F. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents 2003, 22, 465–478. [Google Scholar] [CrossRef]
- Reddy, K.; Yedery, R.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Hancock, R.E.; Haney, E.F.; Gill, E.E. The immunology of host defence peptides: Beyond antimicrobial activity. Nat. Rev. Immunol. 2016, 16, 321–334. [Google Scholar] [CrossRef]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Genet. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Lee, A.C.-L.; Harris, J.L.; Khanna, K.K.; Hong, J.-H. A Comprehensive Review on Current Advances in Peptide Drug Development and Design. Int. J. Mol. Sci. 2019, 20, 2383. [Google Scholar] [CrossRef]
- Lazarev, V.N.; Govorun, V.M. Antimicrobial peptides and their use in medicine. Appl. Biochem. Microbiol. 2010, 46, 803–814. [Google Scholar] [CrossRef]
- Marqus, S.; Pirogova, E.; Piva, T.J. Evaluation of the use of therapeutic peptides for cancer treatment. J. Biomed. Sci. 2017, 24, 1–15. [Google Scholar] [CrossRef]
- Chen, C.H.; Lu, T.K. Development and challenges of antimicrobial peptides for therapeutic applications. Antibiotics 2020, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, A.; Pirri, G.; Nicoletto, S. Antimicrobial peptides: An overview of a promising class of therapeutics. Open Life Sci. 2007, 2, 1–33. [Google Scholar] [CrossRef]
- Gordon, Y.J.; Romanowski, E.G.; McDermott, A.M. A Review of Antimicrobial Peptides and Their Therapeutic Potential as Anti-Infective Drugs. Curr. Eye Res. 2005, 30, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2016, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Veiga, A.S.; Castanho, M.A.R.B. From antimicrobial to anticancer peptides. A review. Front. Microbiol. 2013, 4, 294. [Google Scholar] [CrossRef] [PubMed]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The Use of Therapeutic Peptides to Target and to Kill Cancer Cells. Curr. Med. Chem. 2012, 19, 3794–3804. [Google Scholar] [CrossRef]
- Alves, A.C.; Ribeiro, D.; Nunes, C.; Reis, S. Biophysics in cancer: The relevance of drug-membrane interaction studies. Biochim. Biophys. Acta Biomembr. 2016, 1858, 2231–2244. [Google Scholar] [CrossRef]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-Mediated Delivery of Chemical Probes and Therapeutics to Mitochondria. Accounts Chem. Res. 2016, 49, 1893–1902. [Google Scholar] [CrossRef]
- Eike, L.-M.; Yang, N.; Rekdal, Ø.; Sveinbjørnsson, B. The oncolytic peptide LTX-315 induces cell death and DAMP release by mitochondria distortion in human melanoma cells. Oncotarget 2015, 6, 34910. [Google Scholar] [CrossRef] [PubMed]
- Constance, J.E.; Lim, C.S. Targeting malignant mitochondria with therapeutic peptides. Ther. Deliv. 2012, 3, 961–979. [Google Scholar] [CrossRef]
- Do, N.; Weindl, G.; Grohmann, L.; Salwiczek, M.; Koksch, B.; Korting, H.C.; Schäfer-Korting, M. Cationic membrane-active peptides-anticancer and antifungal activity as well as penetration into human skin. Exp. Dermatol. 2014, 23, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Duong, D.T.; Singh, S.; Bagheri, M.; Verma, N.K.; Schmidtchen, A.; Malmsten, M. Pronounced peptide selectivity for melanoma through tryptophan end-tagging. Sci. Rep. 2016, 6, 24952. [Google Scholar] [CrossRef] [PubMed]
- Riedl, S.; Leber, R.; Rinner, B.; Schaider, H.; Lohner, K.; Zweytick, D. Human lactoferricin derived di-peptides deploying loop structures induce apoptosis specifically in cancer cells through targeting membranous phosphatidylserine. Biochim. Biophys. Acta Biomembr. 2015, 1848, 2918–2931. [Google Scholar] [CrossRef] [PubMed]
- Lewies, A.; Wentzel, J.F.; Miller, H.C.; Du Plessis, L.H. The antimicrobial peptide nisin Z induces selective toxicity and apoptotic cell death in cultured melanoma cells. Biochimie 2018, 144, 28–40. [Google Scholar] [CrossRef]
- Camilio, K.A.; Berge, G.; Ravuri, C.S.; Rekdal, Ø.; Sveinbjørnsson, B. Complete regression and systemic protective immune responses obtained in B16 melanomas after treatment with LTX-315. Cancer Immunol. Immunother. 2014, 63, 601–613. [Google Scholar] [CrossRef]
- Ma, R.; Kwok, H.F. New opportunities and challenges of venom-based and bacteria-derived molecules for anticancer targeted therapy. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef]
- Oñate-Garzón, J.; Ausili, A.; Manrique-Moreno, M.; Torrecillas, A.; Aranda, F.J.; Patiño, E.; Gomez-Fernández, J.C. The increase in positively charged residues in cecropin D-like Galleria mellonella favors its interaction with membrane models that imitate bacterial membranes. Arch. Biochem. Biophys. 2017, 629, 54–62. [Google Scholar] [CrossRef]
- Popiołkiewicz, J.; Polkowski, K.; Skierski, J.S.; Mazurek, A.P. In vitro toxicity evaluation in the development of new anticancer drugs—Genistein glycosides. Cancer Lett. 2005, 229, 67–75. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Sica, V.; Izzo, V.; Durand, S.; Müller, K.; Liu, P.; Zitvogel, L.; Rekdal, Ø.; et al. The oncolytic peptide LTX-315 kills cancer cells through Bax/Bak-regulated mitochondrial membrane permeabilization. Oncotarget 2015, 6, 26599–26614. [Google Scholar] [CrossRef]
- Zhou, X.-R.; Zhang, Q.; Tian, X.-B.; Cao, Y.-M.; Liu, Z.-Q.; Fan, R.; Ding, X.-F.; Zhu, Z.; Chen, L.; Luo, S.-Z. From a pro-apoptotic peptide to a lytic peptide: One single residue mutation. Biochim. Biophys. Acta Biomembr. 2016, 1858, 1914–1925. [Google Scholar] [CrossRef]
- De Planque, M.R.R.; Bonev, B.B.; Demmers, J.A.A.; Greathouse, D.V.; Koeppe, R.E.; Separovic, F.; Watts, A.A.; Killian, J.A. Interfacial Anchor Properties of Tryptophan Residues in Transmembrane Peptides Can Dominate over Hydrophobic Matching Effects in Peptide-Lipid Interactions&dagger. Biochemie 2003, 42, 5341–5348. [Google Scholar] [CrossRef]
- Ladokhin, A.S. Evaluation of Lipid Exposure of Tryptophan Residues in Membrane Peptides and Proteins. Anal. Biochem. 1999, 276, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.M.; Whelan, E.J. Characterisation of the interactions of aromatic amino acids with diacetyl phosphatidylcholine. Phys. Chem. Chem. Phys. 2004, 6, 1012–1017. [Google Scholar] [CrossRef]
- Ulmschneider, M.B.; Sansom, M.S. Amino acid distributions in integral membrane protein structures. Biochim. Biophys. Acta Biomembr. 2001, 1512, 1–14. [Google Scholar] [CrossRef]
- Kelly, G.J.; Kia, A.F.-A.; Hassan, F.; O’Grady, S.; Morgan, M.P.; Creaven, B.S.; McClean, S.; Harmey, J.H.; Devocelle, M. Polymeric prodrug combination to exploit the therapeutic potential of antimicrobial peptides against cancer cells. Org. Biomol. Chem. 2016, 14, 9278–9286. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Wong, S.W.; Ge, L.; Shaw, C.; Siu, S.W.; Kwok, H.F. In Vitro and MD Simulation Study to Explore Physicochemical Parameters for Antibacterial Peptide to Become Potent Anticancer Peptide. Mol. Ther. Oncolytics 2020, 16, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vitale, I.; Abrams, J.M.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; Dawson, T.M.; Dawson, V.L.; Eldeiry, W.S.; Fulda, S.; et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012, 19, 107–120. [Google Scholar] [CrossRef]
- Shubin, A.V.; Demidyuk, I.V.; Komissarov, A.A.; Rafieva, L.M.; Kostrov, S.V. Cytoplasmic vacuolization in cell death and survival. Oncotarget 2016, 7, 55863–55889. [Google Scholar] [CrossRef]
- Zurgil, N.; Shafran, Y.; Fixler, D.; Deutsch, M. Analysis of Early Apoptotic Events in Individual Cells by Fluorescence Intensity and Polarization Measurements. Biochem. Biophys. Res. Commun. 2002, 290, 1573–1582. [Google Scholar] [CrossRef]
- Matarrese, P.; Gambardella, L.; Cassone, A.; Vella, S.; Cauda, R.; Malorni, W. Mitochondrial Membrane Hyperpolarization Hijacks Activated T Lymphocytes Toward the Apoptotic-Prone Phenotype: Homeostatic Mechanisms of HIV Protease Inhibitors. J. Immunol. 2003, 170, 6006–6015. [Google Scholar] [CrossRef] [PubMed]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Pluskota-Karwatka, D. Modifications of nucleosides by endogenous mutagens—DNA adducts arising from cellular processes. Bioorganic Chem. 2008, 36, 198–213. [Google Scholar] [CrossRef] [PubMed]
- Satyanarayana, A.; Kaldis, P. A dual role of Cdk2 in DNA damage response. Cell Div. 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Saldivar, J.C.; Hamperl, S.; Bocek, M.J.; Chung, M.; Bass, T.E.; Cisneros-Soberanis, F.; Samejima, K.; Xie, L.; Paulson, J.R.; Earnshaw, W.C. An intrinsic S/G2 checkpoint enforced by ATR. Science 2018, 361, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Felício, M.R.; Silva, O.N.; Gonçalves, S.; Santos, N.C.; Franco, O.L. Peptides with Dual Antimicrobial and Anticancer Activities. Front. Chem. 2017, 5, 5. [Google Scholar] [CrossRef]
- Brown, M.C.; Staniszewska, I.; Del Valle, L.; Tuszynski, G.P.; Marcinkiewicz, C. Angiostatic activity of obtustatin as α1β1 integrin inhibitor in experimental melanoma growth. Int. J. Cancer 2008, 123, 2195–2203. [Google Scholar] [CrossRef]
- Santa-González, G.A.; Patiño-González, E.; Manrique-Moreno, M. Cell cycle progression data on human skin cancer cells with anticancer synthetic peptide LTX-315 treatment. Data Brief 2020, 30, 105443. [Google Scholar] [CrossRef]
- Márquez, C.A.P.; Azevedo, R.B.; Joanitti, G.A.; Júnior, O.R.P.; Fontes, W.; Castro, M.S. Cytotoxic Activity and Antiproliferative Effects of Crude Skin Secretion from Physalaemus nattereri (Anura: Leptodactylidae) on in vitro Melanoma Cells. Toxins 2015, 7, 3989–4005. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System. Available online: http://www.pymol.org (accessed on 27 August 2018).
- Mol, A.R.; Castro, M.S.; Fontes, W. NetWheels: A web application to create high quality peptide helical wheel and net projections. BioRxiv 2018, 416347. [Google Scholar] [CrossRef]

| HaCaT Cells | A375 Cells | Selectivity Index (SX) * | |
|---|---|---|---|
| IC50 values (μM) | 88.56 | 9.31 | 951.23 |
Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa-González, G.A.; Patiño-González, E.; Manrique-Moreno, M. Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells. Molecules 2020, 25, 5684. https://doi.org/10.3390/molecules25235684
Santa-González GA, Patiño-González E, Manrique-Moreno M. Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells. Molecules. 2020; 25(23):5684. https://doi.org/10.3390/molecules25235684
Chicago/Turabian StyleSanta-González, Gloria A., Edwin Patiño-González, and Marcela Manrique-Moreno. 2020. "Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells" Molecules 25, no. 23: 5684. https://doi.org/10.3390/molecules25235684
APA StyleSanta-González, G. A., Patiño-González, E., & Manrique-Moreno, M. (2020). Synthetic Peptide ΔM4-Induced Cell Death Associated with Cytoplasmic Membrane Disruption, Mitochondrial Dysfunction and Cell Cycle Arrest in Human Melanoma Cells. Molecules, 25(23), 5684. https://doi.org/10.3390/molecules25235684