Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications
Abstract
:1. Introduction
2. Bioaccessibility and Bioavailability of Lignans during Gastrointestinal Digestion and Fermentation Process
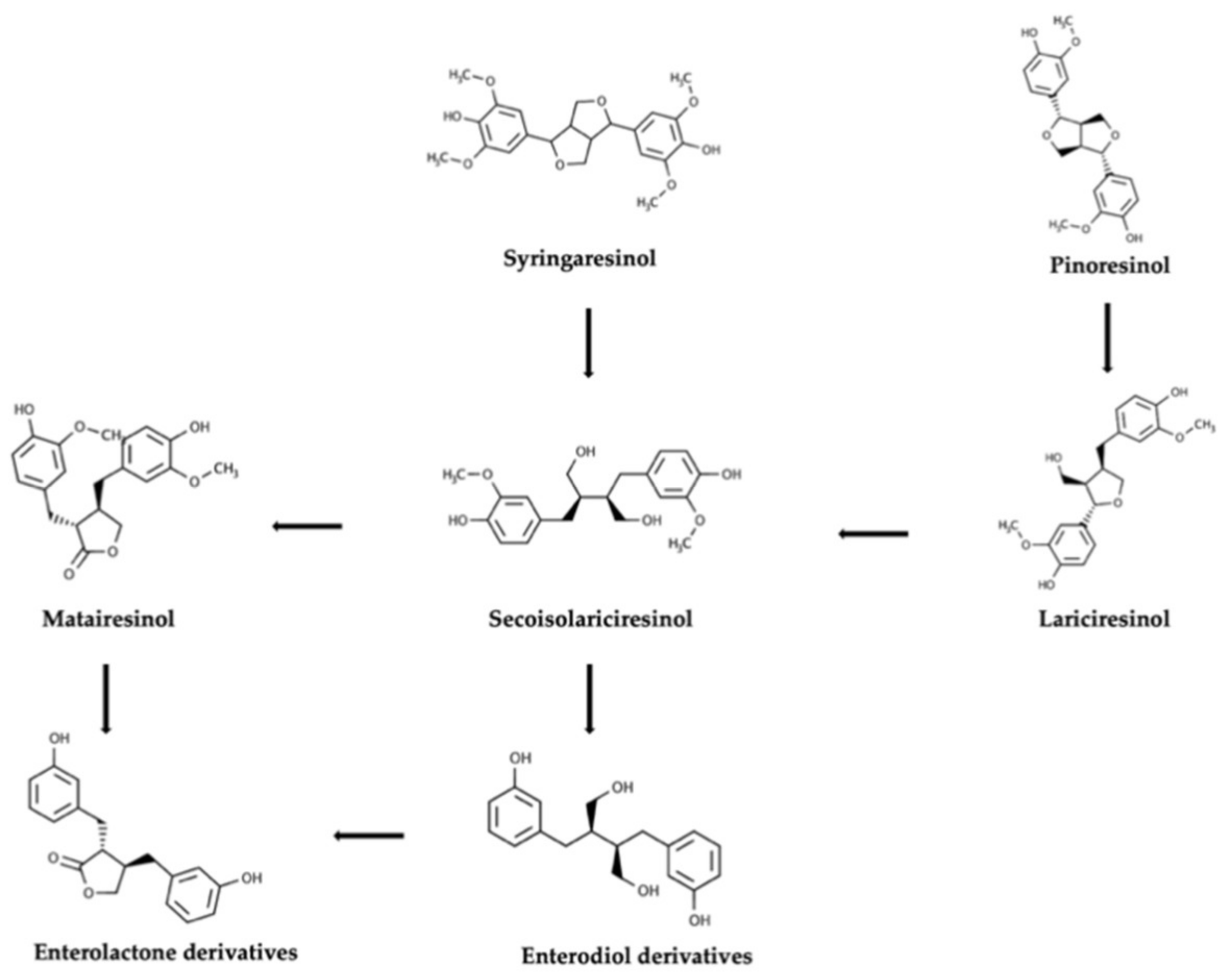
3. Intraindividual and Interindividual Variability in the Conversion of Plant Lignans
4. Modulation of Gut Microbiota by Enterolignans
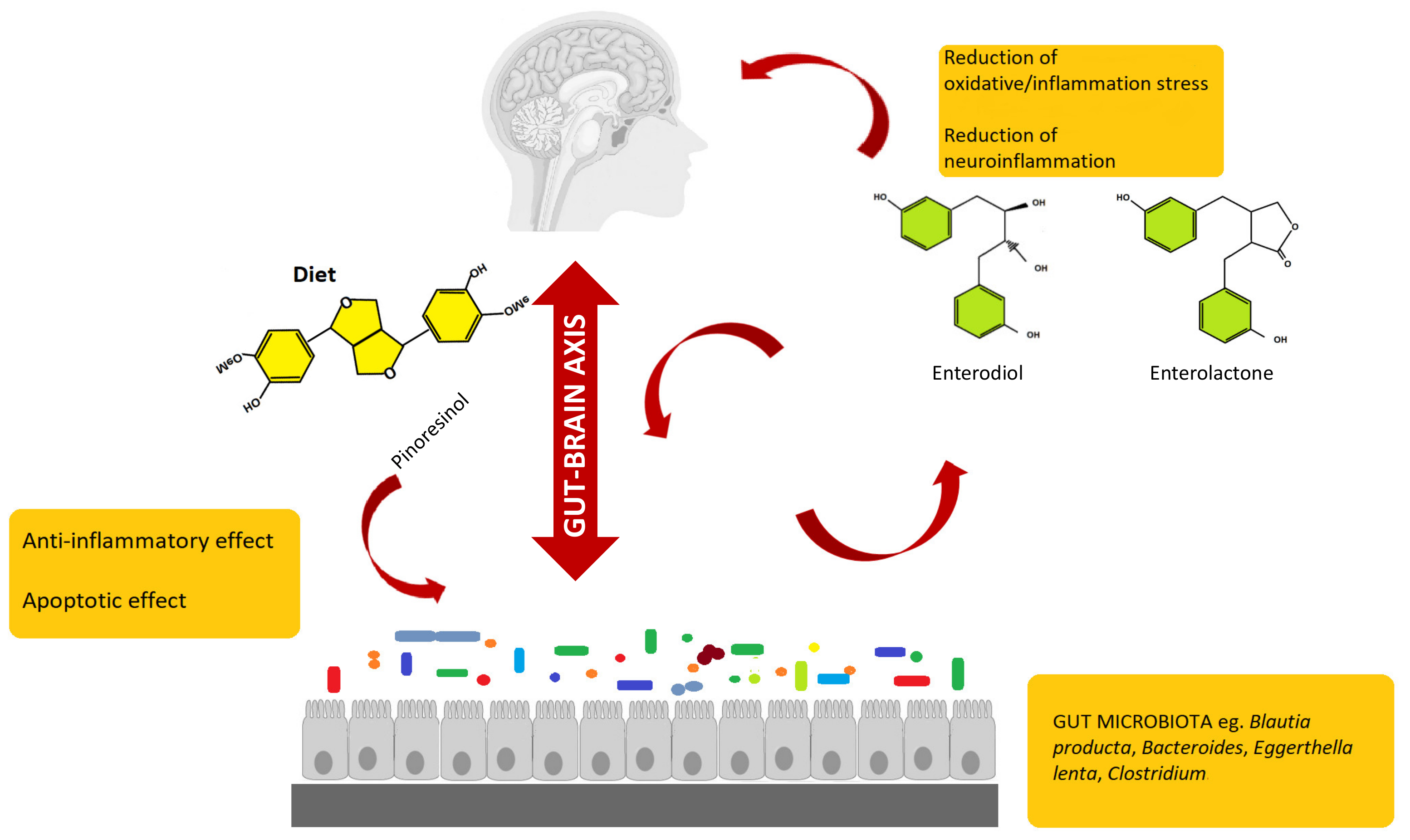
5. Potential of Enterolignans as Health-Promoters and Modulators of the Gut–Brain Axis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordoñez, M.; Llorach, R.; Farran-Codina, A.; Barupal, D.K.; Neveu, V.; Manach, C.; Andres-Lacueva, C.; Scalbert, A. Systematic analysis of the polyphenol metabolome using the Phenol-Explorer database. Mol. Nutr. Food Res. 2015, 60, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally lignan-rich foods: A dietary tool for health promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef] [Green Version]
- Zaineddin, A.K.; Buck, K.; Vrieling, A.; Heinz, J.; Flesch-Janys, D.; Linseisen, J.; Chang-Claude, J. The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: A German case-control study. Nutr. Cancer 2012, 64, 652–665. [Google Scholar] [CrossRef]
- Peterson, J.; Dwyer, J.; Adlercreutz, H.; Scalbert, A.; Jacques, P.; McCullough, M.L. Dietary lignans: Physiology and potential for cardiovascular disease risk reduction. Nutr. Rev. 2010, 68, 571–603. [Google Scholar] [CrossRef] [Green Version]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remón, A.; M’Hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- Landete, J.M. Plant and mammalian lignans: A review of source, intake, metabolism, intestinal bacteria and health. Food Res. Int. 2012, 46, 410–424. [Google Scholar] [CrossRef]
- Miles, F.L.; Navarro, S.L.; Schwarz, Y.; Gu, H.; Djukovic, D.; Randolph, T.W.; Shojaie, A.; Kratz, M.; Hullar, M.A.J.; Lampe, P.D.; et al. Plasma metabolite abundances are associated with urinary enterolactone excretion in healthy participants on controlled diets. Food Funct. 2017, 8, 3209–3218. [Google Scholar] [CrossRef]
- Fuentealba, C.; Figuerola, F.; Estévez, A.M.; Basatías, J.M.; Munoz, O. Bioaccessibility of lignans from flaxseed (Linum usitatissimum L.) determined by single-batch in vitro simulation of the digestive process. J. Sci. Food Agric. 2014, 94, 1729–1738. [Google Scholar] [CrossRef]
- Setchell, K.D.R.; Brown, N.M.; Zimmer-Nechemias, L.; Wolfe, B.; Jha, P.; Heubi, J.E. Metabolism of secoisolariciresinol-diglycoside the dietary precursor to the intestinally derived lignan enterolactone in humans. Food Funct. 2014, 5, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Teponno, R.B.; Kusari, S.; Spiteller, M. Recent advances in research on lignans and neolignans. Nat. Prod. Rep. 2016, 33, 1044–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, Y.; Jones, J.; Mansell, K.; Whiting, S.; Fowler, S.; Thorpe, L.; Billinsky, J.; Viveky, N.; Cheng, P.C.; Almousa, A.; et al. Influence of flaxseed lignan supplementation to older adults on biochemical and functional outcome measures of inflammation. J. Am. Coll. Nutr. 2017, 36, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Bolvig, A.K.; Adlercreutz, H.; Theil, P.K.; Jørgensen, H.; Knudsen, K.E.B. Absorption of plant lignans from cereals in an experimental pig model. Br. J. Nutr. 2016, 115, 1711–1720. [Google Scholar] [CrossRef] [Green Version]
- Quartieri, A.; García-Villalba, R.; Amaretti, A.; Raimondi, S.; Leonardi, A.; Rossi, M.; Tomàs-Barberàn, F. Detection of novel metabolites of flaxseed lignans in vitro and in vivo. Mol. Nutr. Food Res. 2016, 60, 1590–1601. [Google Scholar] [CrossRef]
- Gaya, P.; Peirotén, Á.; Medina, M.; Landete, J.M. Bifidobacterium adolescentis INIA P784: The first probiotic bacterium capable of producing enterodiol from lignan extracts. J. Funct. Foods 2017, 29, 269–274. [Google Scholar] [CrossRef]
- Peirotén, Á.; Gaya, P.; Álvarez, I.; Bravo, D.; Landete, J.M. Influence of different lignan compounds on enterolignan production by Bifidobacterium and Lactobacillus strains. Int. J. Food Microbiol. 2019, 289, 17–23. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Motilva, M.-J. Metabolic and microbial modulation of the large intestine ecosystem by non-absorbed diet phenolic compounds: A review. Molecules 2015, 20, 17429–17468. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Saarinen, N.M.; Thompson, L.U. Sesamin is one of the major precursors of mammalian lignans in sesame seed (Sesamum indicum) as observed in vitro and in rats. J. Nutr. 2006, 136, 906–912. [Google Scholar] [CrossRef] [Green Version]
- Corona, G.; Kreimes, A.; Barone, M.; Turroni, S.; Brigidi, P.; Keleszade, E.; Costabile, A. Impact of lignans in oilseed mix on gut microbiome composition and enterolignan production in younger healthy and premenopausal women: An in vitro pilot study. Microb. Cell Factories 2020, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Struijs, K.; Vincken, J.-P.; Gruppen, H. Bacterial conversion of secoisolariciresinol and anhydrosecoisolariciresinol. J. Appl. Microbiol. 2009, 107, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Kezimana, P.; Dmitriev, A.A.; Kudryavtseva, A.V.; Romanova, E.V.; Melnikova, N.V. Secoisolariciresinol diglucoside of flaxseed and its metabolites: Biosynthesis and potential for nutraceuticals. Front. Genet. 2018, 9, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, H.-J.; Wei, Z.-Y.; Lu, P.-C.; Huang, P.-L.; Lee, K.-T. Bioconversion of pinoresinol into matairesinol by use of recombinant Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 2687–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeds, A.; Saarinen, N.M.; Hurmerinta, T.T.; Penttinen, P.E.; Sjöholm, R.E.; Mäkelä, S.I. Urinary excretion of lignans after administration of isolated plant lignans to rats: The effect of single dose and ten-day exposures. J. Chromatogr. B 2004, 813, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Lærke, H.N.; Mortensen, M.A.; Hedemann, M.S.; Knudsen, K.E.B.; Penalvo, J.L.; Adlercreutz, H. Quantitative aspects of the metabolism of lignans in pigs fed fibre-enriched rye and wheat bread. Br. J. Nutr. 2009, 102, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Lagkouvardos, I.; Kläring, K.; Heinzmann, S.S.; Platz, S.; Scholz, B.; Engel, K.-H.; Schmitt-Kopplin, P.; Haller, D.; Rohn, S.; Skurk, T.; et al. Gut metabolites and bacterial community networks during a pilot intervention study with flaxseeds in healthy adult men. Mol. Nutr. Food Res. 2015, 59, 1614–1628. [Google Scholar] [CrossRef]
- Nurmi, T.; Mursu, J.; Peñalvo, J.L.; Poulsen, H.E.; Voutilainen, S. Dietary intake and urinary excretion of lignans in Finnish men. Br. J. Nutr. 2010, 103, 677–685. [Google Scholar] [CrossRef] [Green Version]
- Milder, I.E.J.; Kuijsten, A.; Arts, I.C.W.; Feskens, E.J.M.; Kampman, E.; Hollman, P.C.H.; Veer, P.V. Relation between plasma enterodiol and enterolactone and dietary intake of lignans in a Dutch endoscopy-based population. J. Nutr. 2007, 137, 1266–1271. [Google Scholar] [CrossRef] [Green Version]
- Eeckhaut, E.; Struijs, K.; Possemiers, S.; Vincken, J.-P.; De Keukeleire, D.; Verstraete, W. Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J. Agric. Food Chem. 2008, 56, 4806–4812. [Google Scholar] [CrossRef]
- Chang, H.; Yao, S.; Tritchler, D.; Hullar, M.A.; Lampe, J.W.; Thompson, L.U.; McCann, S.E. Genetic variation in steroid and xenobiotic metabolizing pathways and enterolactone excretion before and after flaxseed intervention in African American and European American women. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 265–274. [Google Scholar] [CrossRef] [Green Version]
- Kuijsten, A.; Arts, I.C.W.; Veer, P.V.; Hollman, P.C.H. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J. Nutr. 2005, 135, 2812–2816. [Google Scholar] [CrossRef] [PubMed]
- Hålldin, E.; Eriksen, A.K.; Brunius, C.; Da Silva, A.B.; Bronze, M.; Hanhineva, K.; Aura, A.-M.; Landberg, R. Factors explaining interpersonal variation in plasma enterolactone concentrations in humans. Mol. Nutr. Food Res. 2019, 63, e1801159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, A.F.; Zang, Y. A review of lignan metabolism, milk enterolactone concentration, and antioxidant status of dairy cows fed flaxseed. Molecules 2019, 24, 41. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.L.; Kirk, R.D.; DaSilva, N.A.; Ma, H.; Seeram, N.P.; Bertin, M.J. Polyphenol microbial metabolites exhibit gut and blood–brain barrier permeability and protect murine microglia against LPS-induced inflammation. Metabolites 2019, 9, 78. [Google Scholar] [CrossRef] [Green Version]
- Possemiers, S.; Bolca, S.; Eeckhaut, E.; Depypere, H.; Verstraete, W. Metabolism of isoflavones, lignans and prenylflavonoids by intestinal bacteria: Producer phenotyping and relation with intestinal community. FEMS Microbiol. Ecol. 2007, 61, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B.; Serena, A.; Kjaer, A.K.B.; Tetens, I.; Heinonen, S.-M.; Nurmi, T.; Adlercreutz, H. Rye bread in the diet of pigs enhances the formation of enterolactone and increases its levels in plasma, urine and feces. J. Nutr. 2003, 133, 1368–1375. [Google Scholar] [CrossRef] [PubMed]
- Heinonen, S.; Nurmi, T.; Liukkonen, K.; Poutanen, K.; Wähälä, K.; Deyama, T.; Nishibe, S.; Adlercreutz, H. In vitro metabolism of plant lignans: New precursors of mammalian lignans enterolactone and enterodiol. J. Agric. Food Chem. 2001, 49, 3178–3186. [Google Scholar] [CrossRef] [PubMed]
- Hullar, M.A.J.; Lancaster, S.M.; Li, F.; Tseng, E.; Beer, K.; Atkinson, C.; Wähälä, K.; Copeland, W.K.; Randolph, T.W.; Newton, K.M.; et al. Enterolignan-producing phenotypes are associated with increased gut microbial diversity and altered composition in premenopausal women in the united states. Cancer Epidemiol. Biomarkers Prev. 2004, 24, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Knust, U.; Spiegelhalder, B.; Strowitzki, T.; Owen, R.W. Contribution of linseed intake to urine and serum enterolignan levels in German females: A randomised controlled intervention trial. Food Chem. Toxicol. 2006, 44, 1057–1064. [Google Scholar] [CrossRef]
- Peñalvo, J.L.; Heinonen, S.-M.; Aura, A.-M.; Adlercreutz, H. Dietary sesamin is converted to enterolactone in humans. J. Nutr. 2005, 135, 1056–1062. [Google Scholar] [CrossRef]
- Saarinen, N.M.; Smeds, A.; Mäkelä, S.I.; Ämmälä, J.; Hakala, K.; Pihlava, J.M.; Ryhänen, E.L.; Sjöholm, R.; Santti, R. Structural determinants of plant lignans for the formation of enterolactone in vivo. J. Chromatogr. B 2002, 777, 311–319. [Google Scholar] [CrossRef]
- Klingbeil, E.; De La Serre, C.B. Microbiota modulation by eating patterns and diet composition: Impact on food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1254–R1260. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-Q.; Zhang, A.-H.; Miao, J.-H.; Sun, H.; Yan, G.-L.; Wu, F.-F.; Wang, X.-J. Gut microbiota as important modulator of metabolism in health and disease. RSC Adv. 2018, 8, 42380–42389. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hjorth, M.F.; Roager, H.M.; Larsen, T.M.; Poulsen, S.K.; Licht, T.R.; Bahl, M.I.; Zohar, Y.; Astrup, A. Pre-treatment microbial Prevotella-to-Bacteroides ratio, determines body fat loss success during a 6-month randomized controlled diet intervention. Int. J. Obes. 2018, 42, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Christensen, L.; Roager, H.M.; Astrup, A.; Hjorth, M.F. Microbial enterotypes in personalized nutrition and obesity management. Am. J. Clin. Nutr. 2018, 108, 645–651. [Google Scholar] [CrossRef]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut microbiome of the Hadza hunter-gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [Green Version]
- Bess, E.N.; Bisanz, J.E.; Yarza, F.; Bustion, A.E.; Rich, B.E.; Li, X.; Kitamura, S.; Waligurski, E.; Ang, Q.Y.; Alba, D.L.; et al. Genetic basis for the cooperative bioactivation of plant lignans by Eggerthella lenta and other human gut bacteria. Nat. Microbiol. 2020, 5, 56–66. [Google Scholar] [CrossRef]
- Clavel, T.; Doré, J.; Blaut, M. Bioavailability of lignans in human subjects. Nutr. Res. Rev. 2006, 19, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Nose, M.; Fujimoto, T.; Takeda, T.; Nishibe, S.; Ogihara, Y. Structural transformation of lignan compounds in rat gastrointestinal tract. Planta Med. 1992, 58, 520–523. [Google Scholar] [CrossRef]
- Patel, D.; Vaghasiya, J.; Pancholi, S.S.; Paul, A. Therapeutic potential of secoisolariciresinol diglucoside: A plant lignan. Int. J. Pharm. Sci. Drug Res. 2012, 4, 15–18. [Google Scholar]
- Wang, L.-Q.; Meselhy, M.R.; Li, Y.; Qin, G.-W.; Hattori, M. Human intestinal bacteria capable of transforming secoisolariciresinol diglucoside to mammalian lignans, enterodiol and enterolactone. Chem. Pharm. Bull. 2000, 48, 1606–1610. [Google Scholar] [CrossRef] [Green Version]
- Studenik, S.; Vogel, M.; Diekert, G. Characterization of an O-demethylase of Desulfitobacterium hafniense DCB-2. J. Bacteriol. 2012, 194, 3317–3326. [Google Scholar] [CrossRef] [Green Version]
- Engelmann, T.; Kaufmann, F.; Diekert, G. Isolation and characterization of a veratrol:corrinoid protein methyl transferase from Acetobacterium dehalogenans. Arch. Microbiol. 2001, 175, 376–383. [Google Scholar] [CrossRef]
- Kaufmann, F.; Wohlfarth, G.; Diekert, G. Isolation of O-demethylase, an ether-cleaving enzyme system of the homoacetogenic strain MC. Arch. Microbiol. 1997, 168, 136–142. [Google Scholar] [CrossRef]
- Naidu, D.; Ragsdale, S.W. Characterization of a three-component vanillate O-demethylase from Moorella thermoacetica. J. Bacteriol. 2001, 183, 3276–3281. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-X.; Deng, C.-Y.; Zhang, Y.-T.; Liu, Z.-M.; Wang, P.-Z.; Liu, S.-L.; Qian, W.; Yang, D.-H. Cloning, expression, and characterization of a four-component O-demethylase from human intestinal bacterium Eubacterium limosum ZL-II. Appl. Microbiol. Biotechnol. 2016, 100, 9111–9124. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J.M. Phytoestrogen metabolism by adult human gut microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef] [Green Version]
- Woting, A.; Clavel, T.; Loh, G.; Blaut, M. Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbiol. Ecol. 2010, 72, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.P.; Costa, I.; Almeida, A.F.; Tavares, L.; Pais, T.F.; Pinto, P.; Ventura, M.R.; et al. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Liu, K.; Luo, M.; Wei, S. The bioprotective effects of polyphenols on metabolic syndrome against oxidative stress: Evidences and perspectives. Oxidative Med. Cell. Longev. 2019, 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Reddy, V.P.; Aryal, P.; Robinson, S.; Rafiu, R.; Obrenovich, M.; Perry, G. Polyphenols in Alzheimer’s disease and in the gut-brain axis. Microorganisms 2020, 8, 199. [Google Scholar] [CrossRef] [Green Version]
- Crispi, S.; Filosa, S.; Di Meo, F. Polyphenols-gut microbiota interplay and brain neuromodulation. Neural Regen. Res. 2018, 13, 2055–2059. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Waśkiewicz, A.; Zujko, M.E.; Szcześniewska, D.; Stepaniak, U.; Pająk, A.; Drygas, W. Are total and individual dietary lignans related to cardiovascular disease and its risk factors in postmenopausal women? A nationwide study. Nutrients 2018, 10, 865. [Google Scholar] [CrossRef] [Green Version]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Creus-Cuadros, A.; Tresserra-Rimbau, A.; Quifer-Rada, P.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Fitó, M.; Estruch, R.; Gómez-Gracia, E.; Lapetra, J.; et al. Associations between both lignan and yogurt consumption and cardiovascular risk parameters in an elderly population: Observations from a cross-sectional approach in the PREDIMED study. J. Acad. Nutr. Diet. 2017, 117, 609–622. [Google Scholar] [CrossRef]
- Song, Y.; Shan, B.; Zeng, S.; Zhang, J.; Jin, C.; Liao, Z.; Wang, T.; Zeng, Q.; He, H.; Wei, F.; et al. Raw and wine processed Schisandra chinensis attenuate anxiety like behavior via modulating gut microbiota and lipid metabolism pathway. J. Ethnopharmacol. 2020, 266, 113426. [Google Scholar] [CrossRef]
- Soleymani, S.; Habtemariam, S.; Rahimi, R.; Nabavi, S.M. The what and who of dietary lignans in human health: Special focus on prooxidant and antioxidant effects. Trends Food Sci. Technol. 2020, 106, 382–390. [Google Scholar] [CrossRef]
- Serra, D.; Almeida, L.M.; Dinis, T.C.P. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Das, M.; Devi, K.P. A mini review on the protective effect of lignans for the treatment of neurodegenerative disorders. J. Nutr. Food Lipid Sci. 2019, 2019, 40–53. [Google Scholar] [CrossRef] [Green Version]
- Bondonno, C.P.; Yang, X.; Croft, K.D.; Considine, M.J.; Ward, N.C.; Rich, L.; Puddey, I.B.; Swinny, E.; Mubarak, A.; Hodgson, J.M. Flavonoid-rich apples and nitrate-rich spinach augment nitric oxide status and improve endothelial function in healthy men and women: A randomized controlled trial. Free Radic. Biol. Med. 2012, 52, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Laranjinha, J.; Santos, R.M.; Lourenço, C.F.; Ledo, A.; Barbosa, R.M. Nitric oxide signaling in the brain: Translation of dynamics into respiration control and neurovascular coupling. Ann. N. Y. Acad. Sci. 2012, 1259, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.-F. Impact of microbiota on central nervous system and neurological diseases: The gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.A. The tantalizing links between gut microbes and the brain. Nature 2015, 526, 312–314. [Google Scholar] [CrossRef] [Green Version]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The central nervous system and the gut microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [Green Version]
- Cussotto, S.; Sandhu, K.V.; Dinan, T.G.; Cryan, J.F. The neuroendocrinology of the microbiota-gut-brain axis: A behavioral perspective. Front. Neuroendocrinol. 2018, 51, 80–101. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. The vagus nerve in the neuro-immune axis: Implications in the pathology of the gastrointestinal tract. Front. Immunol. 2017, 8, 1452. [Google Scholar] [CrossRef]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Vagus nerve stimulation: A new promising therapeutic tool in inflammatory bowel disease. J. Intern. Med. 2017, 282, 46–63. [Google Scholar] [CrossRef] [Green Version]
- Travagli, R.A.; Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, R.; Wisniewski, P.J.; Alderman, B.L.; Campbell, S.C. Microbes and mental health: A review. Brain Behav. Immun. 2017, 66, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut microbiota and the neuroendocrine system. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohajeri, M.H.; La Fata, G.; Steinert, R.E.; Weber, P. Relationship between the gut microbiome and brain function. Nutr. Rev. 2018, 76, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Interactions between gut microbes and host cells control gut barrier and metabolism. Int. J. Obes. Suppl. 2016, 6, S28–S31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, J.A.; Rinaman, L.; Cryan, J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress 2017, 7, 124–136. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.R.; Osadchiy, V.; Kalani, A.; Mayer, E.A. The brain-gut-microbiome axis. Cell. Mol. Gastroenterol. Hepatol. 2018, 6, 133–148. [Google Scholar] [CrossRef] [Green Version]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Saulnier, D.M.; Ringel, Y.; Heyman, M.B.; Foster, J.A.; Bercik, P.; Shulman, R.J.; Versalovic, J.; Verdu, E.F.; Dinan, T.G.; Hecht, G.; et al. The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes. 2013, 4, 17–27. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Holzer, P.; Farzi, A. Neuropeptides and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014, 817, 195–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cryan, J.F.; Dinan, T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012, 13, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Mittal, R.; Debs, L.H.; Patel, A.P.; Nguyen, D.; Patel, K.; O’Connor, G.; Grati, M.; Mittal, J.; Yan, D.; Eshraghi, A.A.; et al. Neurotransmitters: The critical modulators regulating gut-brain axis. J. Cell. Physiol. 2017, 232, 2359–2372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Kawaguchi, K.; Kiyama, R. Differential and directional estrogenic signaling pathways induced by enterolignans and their precursors. PLoS ONE 2017, 12, e0171390. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.S.; Hattori, M. Further studies on a human intestinal bacterium Ruminococcus sp. END-1 for transformation of plant lignans to mammalian lignans. J. Agric. Food Chem. 2009, 57, 7537–7542. [Google Scholar] [CrossRef]
- Parikh, M.; Maddaford, T.G.; Austria, J.A.; Aliani, M.; Netticadan, T.; Pierce, G.N. Dietary flaxseed as a strategy for improving human health. Nutrients 2019, 11, 1171. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Liu, J.; Wang, S.; Zeng, Z.; Li, T.; Liu, Y.; Mastriani, E.; Li, Q.-H.; Bao, H.-X.; Zhou, Y.-J.; et al. Enterolactone has stronger effects than enterodiol on ovarian cancer. J. Ovarian Res. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Ezzat, S.M.; Shouman, S.A.; ElKhoely, A.; Attia, Y.M.; Elsesy, M.E.; El Senousy, A.S.; Choucry, M.A.; El Gayed, S.H.; El Sayed, A.A.; Sattar, E.A.; et al. Anticancer potentiality of lignan rich fraction of six Flaxseed cultivars. Sci. Rep. 2018, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-H.; Fang, J.; Li, H.; Demark-Wahnefried, W.; Lin, X. Enterolactone induces apoptosis in human prostate carcinoma LNCaP cells via a mitochondrial-mediated, caspase-dependent pathway. Mol. Cancer Ther. 2007, 6, 2581–2590. [Google Scholar] [CrossRef] [Green Version]
- Köse, L.; Gülçin, İ. Inhibition effects of some lignans on carbonic anhydrase, acetylcholinesterase and butyrylcholinesterase enzymes. Rec. Nat. Prod. 2017, 11, 558–561. [Google Scholar] [CrossRef]
- Cuong, T.D.; Hung, T.M.; Han, H.-Y.; Roh, H.S.; Seok, J.-H.; Lee, J.K.; Jeong, J.Y.; Choi, J.S.; Kim, J.A.; Min, B.S. Potent acetylcholinesterase inhibitory compounds from Myristica fragrans. Nat. Prod. Commun. 2014, 9, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, M.; Liu, Y.; Pi, Z.; Li, S.; Hu, M.; He, Y.; Yue, K.; Liu, T.; Liu, Z.; Song, F.; et al. Systematically characterize the anti-alzheimer’s disease mechanism of lignans from S. chinensis based on in-vivo ingredient analysis and target-network pharmacology strategy by UHPLC⁻Q-TOF-MS. Molecules 2019, 24, 1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giuliano, C.; Siani, F.; Mus, L.; Ghezzi, C.; Cerri, S.; Pacchetti, B.; Bigogno, C.; Blandini, F. Neuroprotective effects of lignan 7-hydroxymatairesinol (HMR/lignan) in a rodent model of Parkinson’s disease. Nutrition 2020, 69, 110494. [Google Scholar] [CrossRef]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The gut microbiota: A key factor in the therapeutic effects of (poly)phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
| Compound | Structure | Class | Major Food Sources |
|---|---|---|---|
| Secoisolariciresinol |  | Dibenzylbutane lignan | Flaxseed (257.6 mg/100 g FW) Cashew nut (6.7 mg/100 g FW) |
| Matairesinol |  | Dibenzylbutyrolactone lignan | Sesame seed (29.8 mg/100 FW) Flaxseed (6.7 mg/100 g FW) |
| Lariciresinol |  | Furan lignan | Broccoli (97.2 mg/100 g FW) Kale (59.9 mg/100 g FW) |
| Medioresinol |  | Furofuran lignan | Sesame seed (4.1 mg/100 g FW) Cloudberry (0.48 mg/100 g FW) |
| Pinoresinol |  | Furofuran lignan | Olive oil (2.4 mg/100 g FW) EVOO (0.42 mg/100 g FW) |
| Syringaresinol |  | Furofuran lignan | Rye, whole grain flour (0.9 mg/100 g FW) Avocado (0.4 mg/100 g FW) |
| Sesamin |  | Furofuran lignan | Sesame seed, oil (644.5 mg/100 g FW) Sesame seed (538.1 mg/100 g FW) |
| Sesamolin |  | Furofuran lignan | Sesame seed, oil (287.3 mg/100 g FW) Sesame seed (133.9 mg/100 g FW) |
| Plant Lignan Source | Experiment Type | Main Findings | Reference |
|---|---|---|---|
| Wheat bread; Rye bread | Crossover intervention using pigs. | Conversion of parent lignans in EL. MAT and SECO showed the higher conversion rate. | [36] |
| Oilseeds mix | In vitro fermentation using women feces. | Lower effectiveness in the conversion ED in EL in postmenopausal period. | [20] |
| BeneFlax® | In vivo study based on healthy adults. | Plasma concentration of flaxseed lignans SECO, ED, and EL correlated with daily oral supplementation of flaxseed lignan–enriched complex. | [13] |
| LinumLife Extra | SHIME (considering low and high enterolignan producers) | Marked differences in EL/ED ratio over the experimental period. | [29] |
| Whole flaxseed and flaxseed flour | In vitro fermentation using pooled human feces. | Major ED production as resulting by flaxseed flour.Similar production of EL for both food matrices. | [10] |
| Flaxseed extract | In vitro fermentation using children feces. | Dihydroxy-ED detected as the major metabolites. | [32] |
| Single lignan compounds (MAT, SECO, PDG, SYR diglucoside, HMAT). | In vitro fermentation using pooled human feces. | Major conversion rates observed for SECO (72%), MAT (62%), and PDG (55%). | [37] |
| Habitual diet | Food record on urine collected from premenopausal women. | ED was detected over the limit of detection as major metabolite in all urine samples. | [38] |
| Ground linseed | Healthy women increasing the consumption of fruit and vegetables. Collection of serum and urine samples. | Increase in the concentration of ED (as main metabolite) in both serum and urine samples. | [39] |
| Isolate SDG | Plasma and urine samples collected from mean and women. | Extraction of urinary EL was 2-fold higher than enterodiol. Plasma concentration of ED was higher in women. | [31] |
| Wheat and rye diet | Feces and urine collected from pigs. | EL was the predominant circulating lignan found in both biofluids and significantly correlated to the higher plant lignan intake. | [25] |
| Flaxseed | Collection of serum samples from healthy men. | 10-fold increase in serum concentration of ED and EL. | [33] |
| Sesame, flaxseed and sesame seeds | In vitro fermentation and collection of urine from rats. | Higher conversion rate of parent compounds in ED. | [19] |
| Habitual diet | Food record on endoscopy men and women (collection of plasma samples). | Higher levels of EL detected in biofluids. | [28] |
| Whole grains and refined grains | Food record on urine samples collected from health volunteers. | No linear correlation between lignans intake and EL excretion. | [9] |
| Habitual diet | Food record on urine samples collected from healthy men. | EL production was correlated with a higher intake of vegetables and berries consumption. | [27] |
| Pure sesamin and sesame seed | In vitro fermentation using pooled human feces. | EL was the main metabolite of sesamin. | [40] |
| LinumLifeTM | In vitro fermentation using feces collected from women. | ED detected in higher % when compared with EL. High inter-variability detected. | [35] |
| SDG and flaxseed consumption | In vitro fermentation and collection of urine samples. | Great inter- and intra-variability detected when considering the different donors. | [15] |
| Single compounds (SECO, HMAT, and MAT) | Urine samples collected from rats. | Different proportions of excreted EL and ED depending on the lignan precursor. | [41] |
| Flaxseed extracts (high in SDG) | Serum and urine samples collected from postmenopausal women. | Great dose-response effect observed when considering EL and ED production. | [11] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senizza, A.; Rocchetti, G.; Mosele, J.I.; Patrone, V.; Callegari, M.L.; Morelli, L.; Lucini, L. Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules 2020, 25, 5709. https://doi.org/10.3390/molecules25235709
Senizza A, Rocchetti G, Mosele JI, Patrone V, Callegari ML, Morelli L, Lucini L. Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules. 2020; 25(23):5709. https://doi.org/10.3390/molecules25235709
Chicago/Turabian StyleSenizza, Alice, Gabriele Rocchetti, Juana I. Mosele, Vania Patrone, Maria Luisa Callegari, Lorenzo Morelli, and Luigi Lucini. 2020. "Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications" Molecules 25, no. 23: 5709. https://doi.org/10.3390/molecules25235709
APA StyleSenizza, A., Rocchetti, G., Mosele, J. I., Patrone, V., Callegari, M. L., Morelli, L., & Lucini, L. (2020). Lignans and Gut Microbiota: An Interplay Revealing Potential Health Implications. Molecules, 25(23), 5709. https://doi.org/10.3390/molecules25235709










