Optimization of Nazarov Cyclization of 2,4-Dimethyl-1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach
Abstract
:1. Introduction
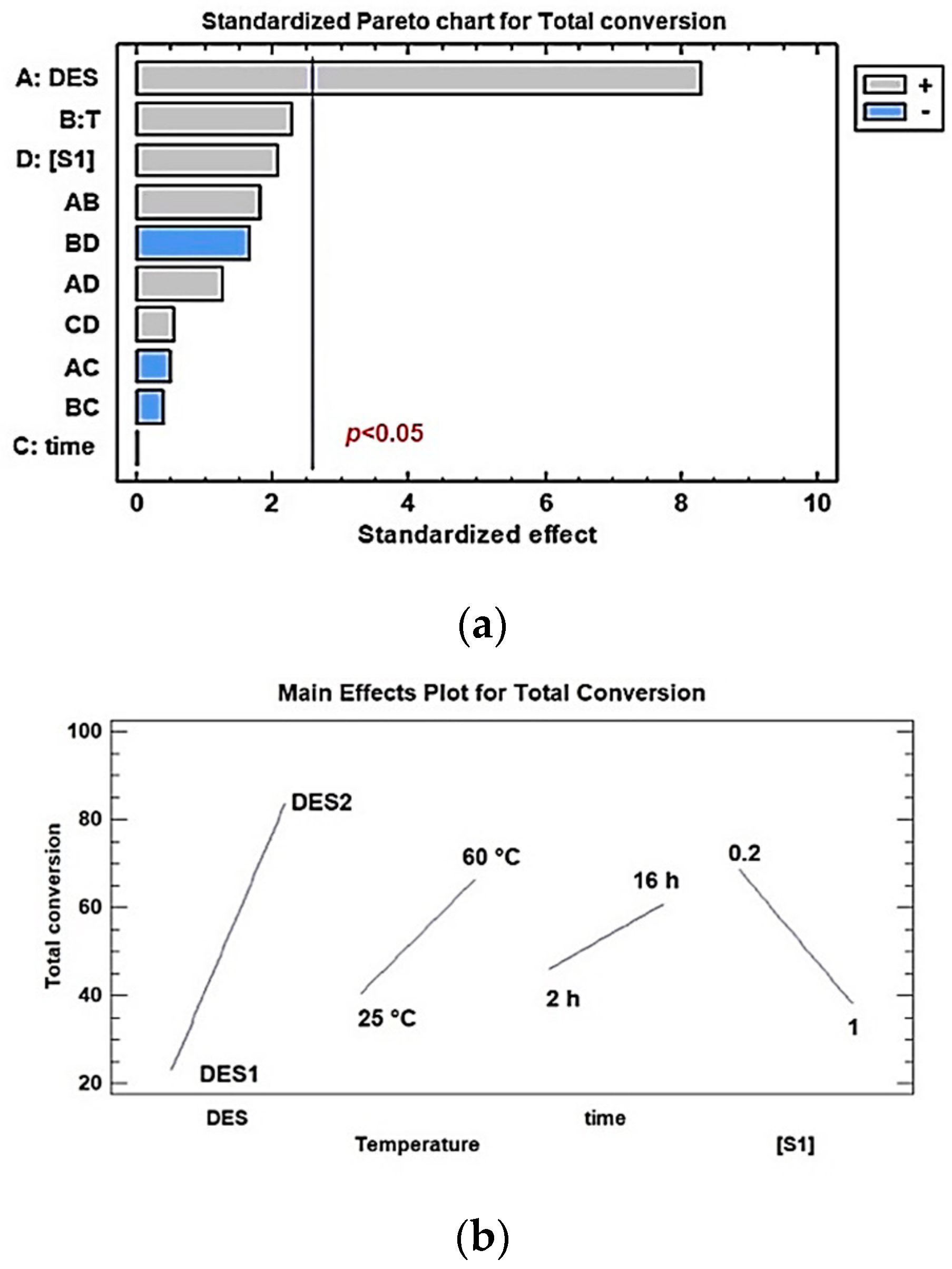
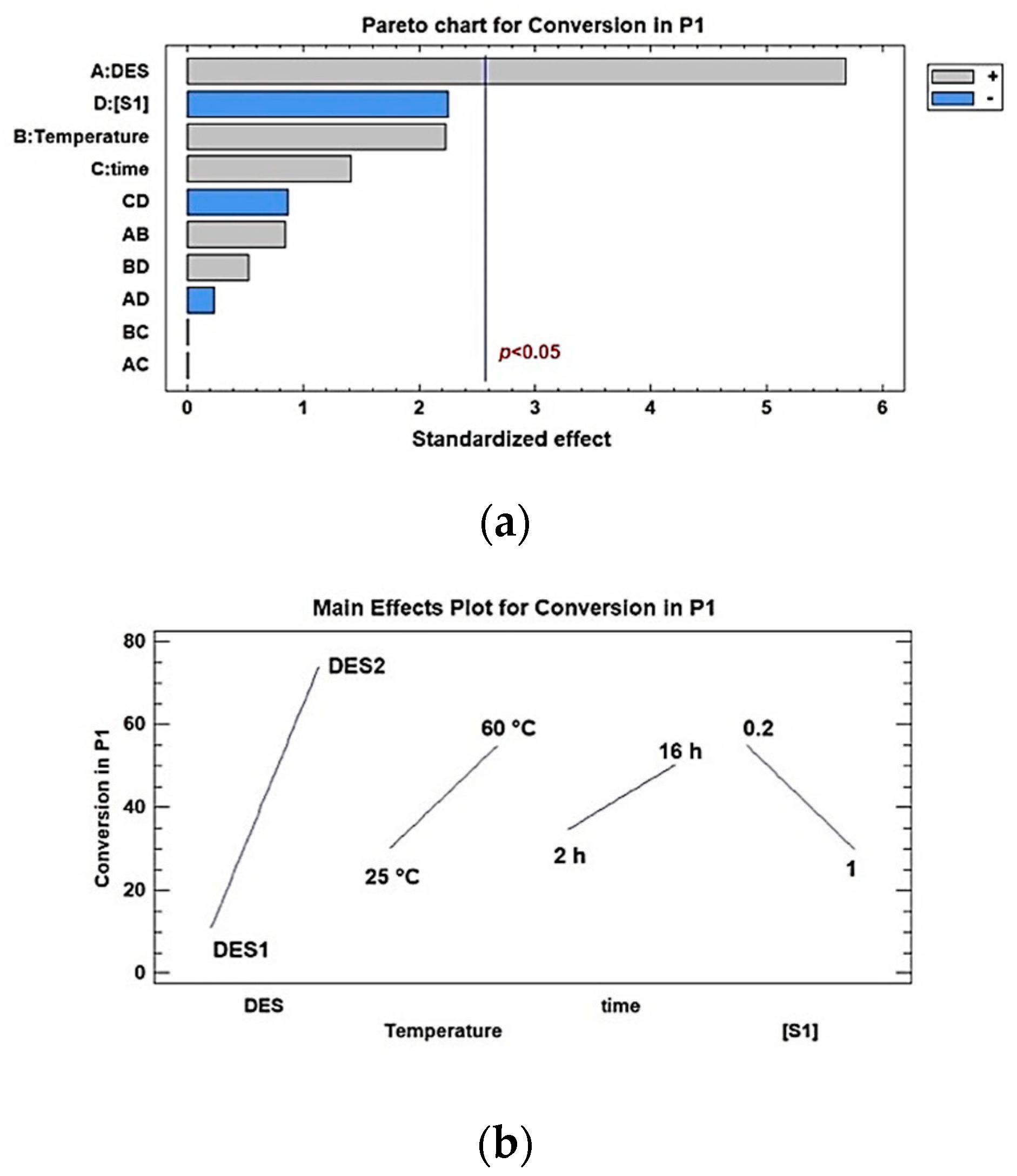
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Preparation of DES1
3.3. Preparation of DES2
3.4. General Procedure for the Nazarov Cyclization Reaction.
3.5. Statistical analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AA | Acetic Acid |
| ANOVA | ANalysis Of Variance |
| BA | Bronsted Acid |
| BBD | Box–Behnken Design |
| CPME | CycloPentyl Methyl Ether |
| DCE | 1,2-DiChloroEthane |
| DES | Deep Eutectic Solvent |
| DoE | Design of Experiment |
| DW | Durbin-Watson |
| EG | Ethylene Glycol |
| HBA | Hydrogen Bond Acceptor |
| HBD | Hydrogen Bond Donor |
| LA | Lewis Acid |
| MAE | Mean Absolute Error |
| NaDES | Natural Deep Eutectic Solvent |
| SRA | Surface Responding Analysis |
| TPMPBr | TriPhenylMethyl Phosphonium Bromide |
| VOC | Volatile Organic Compound |
References and Note
- Nazarov, I.N.; Kuznetzova, A.I. Acetylene derivatives; transformation of cyclopentanone; isomerization of 1,3-dimethyl-delta-1-cyclopentane-5 into 1,3-dimethyl-delta 3-cyclopentane-5. Izv. Akad. Nauk. SSSR 1951, 3, 295–310. [Google Scholar]
- Guo, L.-D.; Hu, J.; Zhang, Y.; Tu, W.; Zhang, Y.; Pu, F.; Xu, J. Enantioselective total synthesis of (−)-caldaphnidine o via a radical cyclization cascade. J. Am. Chem. Soc. 2019, 141, 13043–13048. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, B.; Cai, S.; Gao, S. Total synthesis of gracilamine. Angew. Chem. Int. Ed. 2014, 53, 9539–9543. [Google Scholar] [CrossRef] [PubMed]
- Kakde, B.N.; Kumari, P.; Bisai, A. Total synthesis of (±)-taiwaniaquinol F and related taiwaniaquinoids. J. Org. Chem. 2015, 80, 9889–9899. [Google Scholar] [CrossRef]
- Willoughby, D.A.; Moore, A.R.; Colville-Nash, P.R. Cyclopentenone prostaglandins-new allies in the war on inflammation. Nat. Med. 2000, 6, 137–138. [Google Scholar] [CrossRef]
- Vinogradov, M.G.; Turova, O.V.; Zlotin, S.G. Nazarov reaction: Current trends and recent advances in the synthesis of natural compounds and their analogs. Org. Biomol. Chem. 2017, 15, 8245–8269. [Google Scholar] [CrossRef]
- Sanz, R.; Miguel, D.; Rodriguez, F. Gold(I)-catalyzed tandem reactions initiated by 1,2-indole migrations. Angew. Chem. Int. Ed. 2008, 47, 7354–7357. [Google Scholar] [CrossRef]
- Shimada, N.; Stewart, C.; Bow, W.F.; Jolit, A.; Wong, K.; Zhou, Z.; Tius, M.A. Neutral nazarov-type cyclization catalyzed by palladium(0). Angew. Chem. Int. Ed. 2012, 51, 5727–5729. [Google Scholar] [CrossRef]
- Vaidya, T.; Cheng, R.; Carlsen, P.N.; Frontier, A.J.; Eisenberg, R. Cationic cyclizations and rearrangements promoted by a heterogeneous gold catalyst. Org. Lett. 2014, 16, 800–803. [Google Scholar] [CrossRef]
- Zhu, D.; Cao, X.; Yu, B. Au(I) π-bis(tert-butyldimethylsilyl)acetylene triphenylphosphine complex, an effective pre-catalyst for Au(I)-catalyzed reactions. Org. Chem. Front. 2015, 2, 360–365. [Google Scholar] [CrossRef]
- Petrović, M.; Scarpi, D.; Fiser, B.; Gómez-Bengoa, E.; Occhiato, E.G. Annulated N-heterocycles by tandem gold(I)-catalyzed [3,3]-rearrangement/nazarov reaction of propargylic ester derivatives: An experimental and computational study. Eur. J. Org. Chem. 2015, 2015, 3943–3956. [Google Scholar] [CrossRef]
- Rosocha, G.; Batey, R.A. Synthesis of 2-bromo-1-aryl-1H-indenes via a Ag(I) promoted domino 2π-electrocyclic ring-opening/4π-electrocyclization reaction of 1,2-diaryl substituted gem-dibromocyclopropanes. Tetrahedron 2013, 69, 8758–8768. [Google Scholar] [CrossRef]
- Ouyang, J.; Kennemur, J.L.; De, C.K.; Farès, C.; List, B. Strong and confined acids enable a catalytic asymmetric nazarov cyclization of simple divinyl ketones. J. Am. Chem. Soc. 2019, 141, 3414–3418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogler, M.; Süsse, L.; LaFortune, J.H.; Stephan, D.W.; Oestreich, M. Electrophilic phosphonium cations as lewis acid catalysts in diels—Alder reactions and nazarov cyclizations. Organometallics 2018, 37, 3303–3313. [Google Scholar] [CrossRef]
- Jolit, A.; Walleser, P.M.; Yap, G.P.; Tius, M.A. Catalytic enantioselective nazarov cyclization: Construction of vicinal all-carbon-atom quaternary stereocenters. Angew. Chem. Int. Ed. 2014, 53, 6180–6183. [Google Scholar] [CrossRef]
- Jolit, A.; Dickinson, C.F.; Kitamura, K.; Walleser, P.M.; Yap, G.P.; Tius, M.A. Catalytic enantioselective nazarov cyclization. Eur. J. Org. Chem. 2017, 2017, 6067–6076. [Google Scholar] [CrossRef]
- Vaidya, T.; Atesin, A.C.; Herrick, I.R.; Frontier, A.J.; Eisenberg, R. Cationic cyclizations and rearrangements promoted by a heterogeneous gold catalyst. Angew. Chem. Int. Ed. 2010, 49, 3363–3366. [Google Scholar] [CrossRef]
- Zhang, Q.; Vigier, K.O.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [Green Version]
- Di Pietro, M.E.; Colombo Dugoni, G.; Ferro, M.; Mannu, A.; Castiglione, F.; Costa Gomes, M.; Fourmentin, S.; Mele, A. Do cyclodextrins encapsulate volatiles in deep eutectic systems? ACS Sustain. Chem. Eng. 2019, 7, 17397–17405. [Google Scholar] [CrossRef]
- Peng, L.; Hu, Z.; Lu, Q.; Tang, Z.; Jiao, Y.; Xu, X. DESs: Green solvents for transition metal catalyzed organic reactions. Chin. Chem. Lett. 2019, 30, 2151–2156. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Li, X.; Row, K.H. Development of deep eutectic solvents applied in extraction and separation. J. Sep. Sci. 2016, 39, 3505–3520. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Hao, J.W.; Mo, L.P.; Zhang, Z.H. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015, 5, 48675–48704. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, S.; Lu, X.; Zhang, X. Electrodeposition in Ionic Liquids. ChemPhysChem 2016, 17, 335–351. [Google Scholar] [CrossRef]
- Wagle, D.V.; Zhao, H.; Baker, G.A. Deep eutectic solvents: Sustainable media for nanoscale and functional materials. Acc. Chem. Res. 2014, 47, 2299–2308. [Google Scholar] [CrossRef]
- Olivares, B.; Martinez, F.; Rivas, L.; Calderon, C.; Munita, J.M.; Campodonico, P.R. A natural deep eutectic solvent formulated to stabilize β-lactam antibiotics. Sci. Rep. 2018, 8, 14900. [Google Scholar] [CrossRef] [Green Version]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Di Pietro, M.E.; Garroni, S.; Mele, A. From deep eutectic solvents to deep band gap systems. J. Mol. Liq. 2020, 301, 112441–112449. [Google Scholar] [CrossRef]
- Mannu, A.; Di Pietro, M.E.; Mele, A. Band-Gap energies of choline chloride and triphenylmethylphosphoniumbromide-based systems. Molecules 2020, 25, 1495. [Google Scholar] [CrossRef] [Green Version]
- Abbott, A.P. Application of hole theory to the viscosity of ionic and molecular liquids. ChemPhysChem 2004, 5, 1242–1246. [Google Scholar] [CrossRef] [PubMed]
- Nejrotti, S.; Iannicelli, M.; Jamil, S.S.; Arnodo, D.; Blangetti, M.; Prandi, C. Natural deep eutectic solvents as an efficient and reusable active system for the nazarov cyclization. Green Chem. 2020, 22, 110–117. [Google Scholar] [CrossRef]
- Azizi, N.; Dezfooli, S.; Hashemi, M.M. Greener synthesis of spirooxindole in deep eutectic solvent. J. Mol. Liq. 2014, 194, 62–67. [Google Scholar] [CrossRef]
- Pednekar, S.; Bhalerao, R.; Ghadge, N. One-pot multi-component synthesis of 1,4-dihydropyridine derivatives in biocompatible deep eutectic solvents. J. Chem. Sci. 2013, 125, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Malhotra, S.V. Applications of ionic liquids in organic synthesis. Aldrichimica Acta 2002, 36, 75–83. [Google Scholar] [CrossRef]
- El-Sayed, T.H.; Aboelnaga, A.; El-Atawy, M.A.; Hagar, M. Ball milling promoted N-heterocycles synthesis. Molecules 2018, 23, 1348. [Google Scholar] [CrossRef] [Green Version]
- Nuvoli, L.; Conte, P.; Fadda, C.; Reglero Ruiz, J.A.; García, J.M.; Baldino, S.; Mannu, A. Structural, thermal, and mechanical properties of gelatin-based films integrated with tara gum. Polymer 2020. [Google Scholar] [CrossRef]
- Banerjee, B. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017, 35, 1–14. [Google Scholar] [CrossRef]
- El Ashry, E.S.H.; Kassem, A.A.; Ramadan, E. Microwave irradiation for accelerating organic reactions-Part II: Six-, seven-membered, spiro, and fused heterocycles. Adv. Heterocycl. Chem. 2006, 90, 1. [Google Scholar]
- Jiju, A. Introduction to industrial experimentation. In Design of Experiments for Engineers and Scientists, 2nd ed.; Jiju, A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; pp. 1–6. [Google Scholar]
- Vlahopoulou, G.; Petretto, G.L.; Garroni, S.; Piga, C.; Mannu, A. Variation of density and flash point in acid degummed waste cooking oil. J. Food Process. Preserv. 2018, 42, e13533. [Google Scholar] [CrossRef]
- Omer, E.; Hendawy, S.; ElGendy, A.N.; Mannu, A.; Petretto, G.L.; Pintore, G. Effect of irrigation systems and soil conditioners on the growth and essential oil composition of rosmarinus officinalis L. cultivated in Egypt. Sustainability 2020, 12, 6611. [Google Scholar] [CrossRef]
- Ghinato, S.; Dilauro, G.; Perna, F.M.; Capriati, V.; Blangetti, M.; Prandi, C. Directed ortho-metalation–nucleophilic acyl substitution strategies in deep eutectic solvents: The organolithium base dictates the chemoselectivity. Chem. Commun. 2019, 55, 7741–7744. [Google Scholar] [CrossRef] [PubMed]
- Arnodo, D.; Ghinato, S.; Nejrotti, S.; Blangetti, M.; Prandi, C. Lateral lithiation in deep eutectic solvents: Regioselective functionalization of substituted toluene derivatives. Chem. Commun. 2020, 56, 2391–2394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahbaz, K.; Baroutian, S.; Mjalli, F.S.; Hashim, M.A.; AlNashef, I.M. Densities of ammonium and phosphonium based deep eutectic solvents: Prediction using artificial intelligence and group contribution techniques. Thermochim. Acta 2012, 527, 59–66. [Google Scholar] [CrossRef]
- Su, H.Z.; Yin, J.M.; Liu, Q.S.; Li, C.P. Properties of four deep eutectic solvents: Density, electrical conductivity, dynamic viscosity, and refractive index. Acta Phys. Chim. Sin. 2015, 31, 1468–1473. [Google Scholar] [CrossRef]
- William, R.; Wang, S.; Mallick, A.; Liu, X.-W. Interrupting nazarov reaction with different trapping modality: Utilizing potassium alkynyltrifluoroborate as a σ-nucleophile. Org. Lett. 2016, 18, 4458–4461. [Google Scholar] [CrossRef]
- Weissman, S.A.; Anderson, N.G. Design of experiments (DoE) and process optimization. A review of recent publications. Org. Process. Res. Dev. 2015, 19, 1605–1633. [Google Scholar] [CrossRef]
- Peng, X.; Yang, G.; Shi, Y.; Zhou, Y.; Zhang, M.; Li, S. Box–behnken design based statistical modeling for the extraction and physicochemical properties of pectin from sunflower heads and the comparison with commercial low-methoxyl pectin. Sci. Rep. 2020, 10, 3595. [Google Scholar] [CrossRef]
- Ferro, M.; Mannu, A.; Panzeri, W.; Theeuwen, C.H.J.; Mele, A. An integrated approach to optimizing cellulose mercerization. Polymers 2020, 12, 1559. [Google Scholar] [CrossRef]
- Mannu, A.; Ferro, M.; Colombo Dugoni, G.; Garroni, S.; Taras, A.; Mele, A. Response surface analysis of density and flash point in recycled waste cooking oils. Chem. Data Collect. 2020, 25, 100329. [Google Scholar] [CrossRef]
- Dopar, M.; Kusic, H.; Koprivanac, N. Treatment of simulated industrial wastewater by photo-fenton process. Part I: The optimization of process parameters using design of experiments (DOE). Chem. Eng. J. 2011, 173, 267–279. [Google Scholar] [CrossRef]
- Alagumurthi, N.; Palaniradja, K.; Soundararajan, V. Optimization of grinding process through design of experiment (DOE)—A comparative study. Mater. Manuf. Process. 2006, 21, 19–21. [Google Scholar] [CrossRef]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The effect of water upon deep eutectic solvent nanostructure: An unusual transition from ionic mixture to aqueous solution. Angew. Chem. Int. Ed. 2017, 56, 9782–9785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Considering a DES as a molecular compound with stoichiometry determined by the original molar ratio of the two components
- Daneshfar, Z.; Rostami, A. Cellulose sulfonic acid as a green, efficient, and reusable catalyst for nazarov cyclization of unactivated dienones and pyrazoline synthesis. RSC Adv. 2015, 5, 104695–104707. [Google Scholar] [CrossRef]
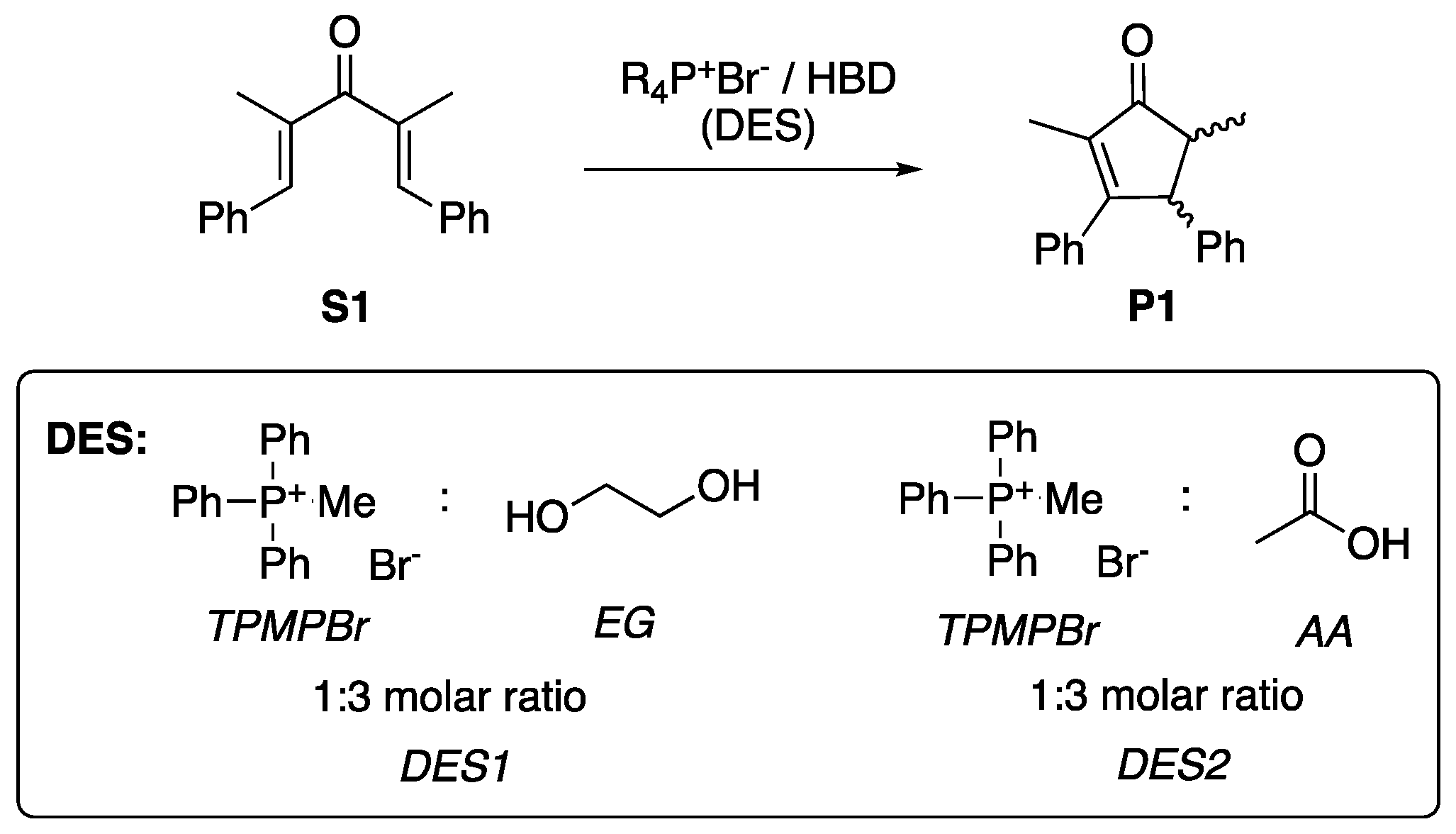


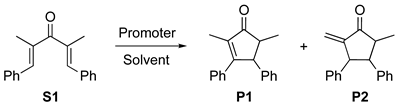
| Entry | Promoter | Solvent | S1 (%) 2 | P1 (%) 2 | cis-P1 (%) 2 | P2 (%) 2 |
|---|---|---|---|---|---|---|
| 1 | TPMPBr | CPME | 91 | - | - | - |
| 2 | AA | CPME | 41 | 10 | 60 | 22 |
| 3 | TPMPBr | DCE | 2 | 78 | 9 | - |
| 4 | AA | DCE | 5 | 17 | 59 | 30 |
| 5 | TPMPBr | EG | 82 | 62 | 94 | - |
| 6 | AA | EG | 0 | 30 | 57 | 22 |
| Factor | Level − 1 | Level + 1 | Unit |
|---|---|---|---|
| Temperature | 25 | 60 | °C |
| Time | 2 | 16 | h |
| Substrate concentration | 1.0 | 0.2 | mmol S1/g DES |
| DES | DES11 | DES22 | |
| Responses | |||
| Total conversion | % of consumed S1 | ||
| Conversion to P1 | % of P1 formed | ||
| Factor | Level − 1 1 | Level + 1 1 | Unit |
|---|---|---|---|
| Temperature | 25 | 60 | °C |
| Time | 1 | 5 | h |
| Amount of DES | 0.2 | 1.0 | g DES for 0.2 mmol S1 |
| Responses | |||
| Total conversion | % of consumed S1 | ||
| Conversion to P1 | % of P1 formed | ||
| Selectivity | % of cis isomer | ||
| Response Variables | |||||
| Name | Unit | Analyse | Goal | Impact | Sensitivity |
| Conv. to P1 | % | Mean | Maximize | 3.0 | Medium |
| Cis selectivity | % | Mean | Maximize | 3.0 | Medium |
| Total Conv. | % | Mean | Maximize | 3.0 | Medium |
| Experimental variable factors | |||||
| Name | Unit | Type | Role | Low | High |
| A: temperature | °C | Continuous | Controllable | 25.0 | 60.0 |
| B: time | h | Continuous | Controllable | 1.0 | 5.0 |
| C: DES amount | g | Continuous | Controllable | 0.2 | 1.0 |
| Theoretical | Experimental | |
|---|---|---|
| Total conversion | 96.55% | 96% |
| Conversion to P1 | 85.28% | 82% |
Sample Availability: Samples of the compounds are not available from the authors.
| |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nejrotti, S.; Mannu, A.; Blangetti, M.; Baldino, S.; Fin, A.; Prandi, C. Optimization of Nazarov Cyclization of 2,4-Dimethyl-1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules 2020, 25, 5726. https://doi.org/10.3390/molecules25235726
Nejrotti S, Mannu A, Blangetti M, Baldino S, Fin A, Prandi C. Optimization of Nazarov Cyclization of 2,4-Dimethyl-1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach. Molecules. 2020; 25(23):5726. https://doi.org/10.3390/molecules25235726
Chicago/Turabian StyleNejrotti, Stefano, Alberto Mannu, Marco Blangetti, Salvatore Baldino, Andrea Fin, and Cristina Prandi. 2020. "Optimization of Nazarov Cyclization of 2,4-Dimethyl-1,5-diphenylpenta-1,4-dien-3-one in Deep Eutectic Solvents by a Design of Experiments Approach" Molecules 25, no. 23: 5726. https://doi.org/10.3390/molecules25235726






