Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis
Abstract
:1. Introduction
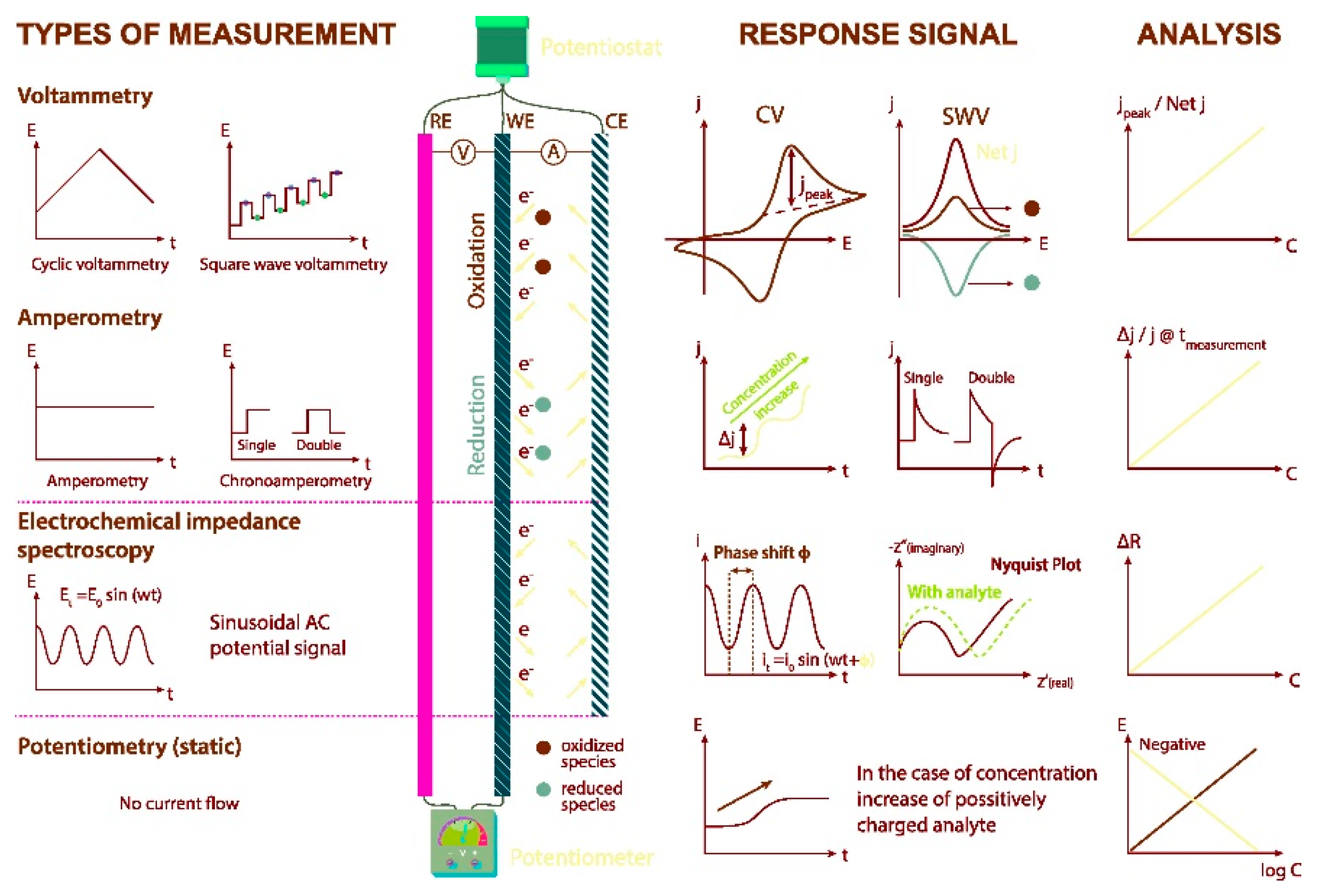
2. Electrochemical Techniques
3. Nanomaterials, Nanotubes, Nanoparticles, and Nanocomposites
3.1. Carbon-Based Nanomaterials
3.2. Gold Based Nanomaterials
3.3. Hybrid Nanocomposites
4. Electrochemical Sensors for Food Analysis: Some Examples
4.1. Phenolic Antioxidants
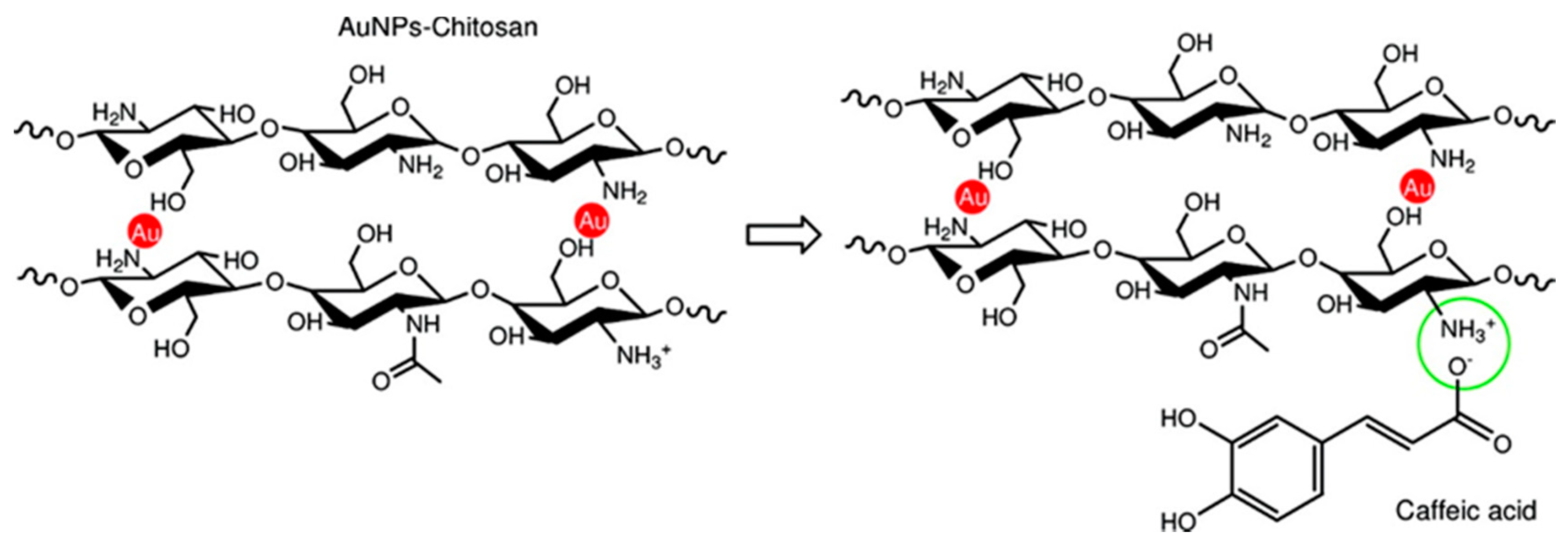
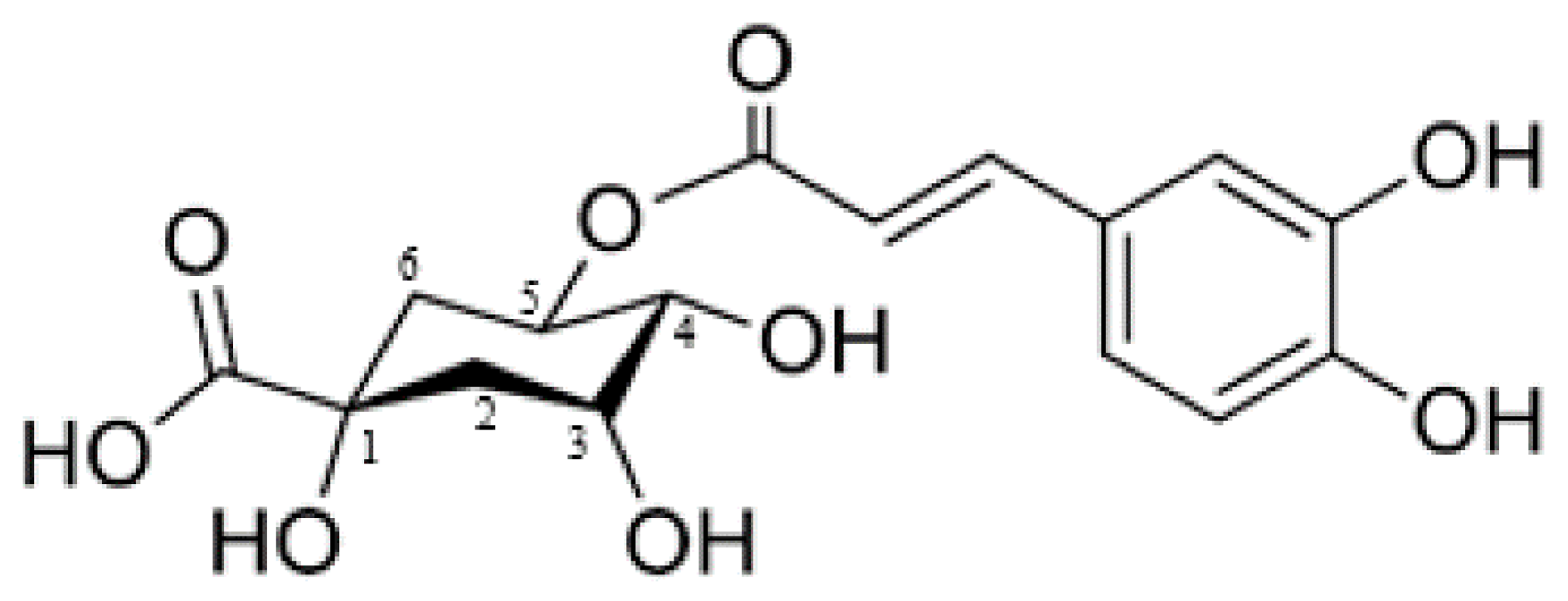
4.1.1. Caffeic Acid
4.1.2. Chlorogenic Acid
4.1.3. Rosmarinic Acid
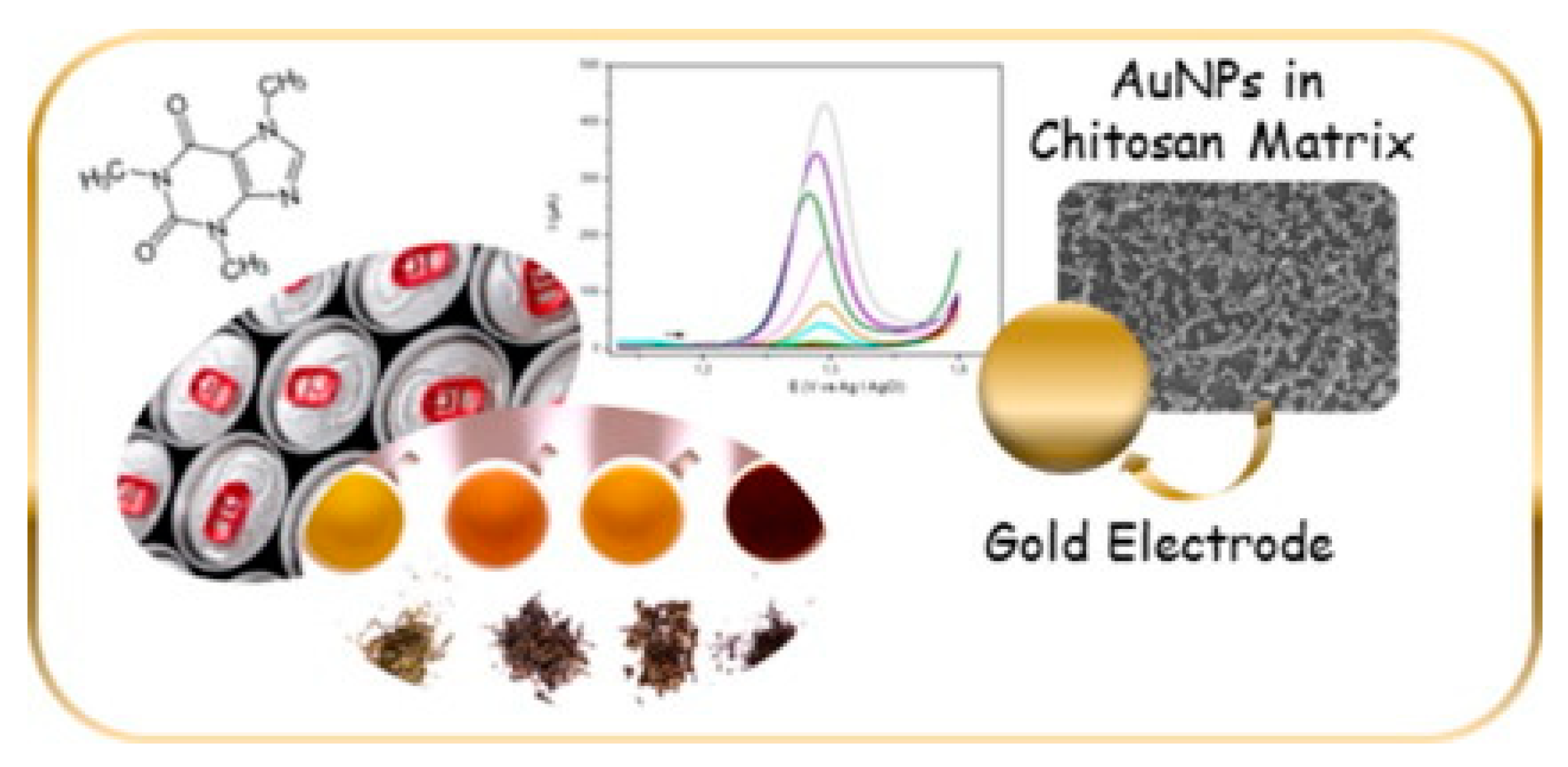
4.2. Caffeine
4.3. Ascorbic Acid
4.4. Nitrite
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Logothetidis, S. Nanostructured Materials and Their Applications; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–22. [Google Scholar]
- Roco, M.C.; Mirkin, C.A.; Dincer Hersam, M.C. Nanotechnology Research Directions for Societal Needs in 2020: Retrospective and Outlook; Springer Science Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Sattler, K.D. Handbook of Nanophysics: Principles and Methods; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Umasankar, Y.; Adhikari, B.-R.; Chen, A. Effective immobilization of alcohol dehydrogenase on carbon nanoscaffolds for ethanol biofuel cell. Bioelectrochemistry 2017, 118, 83–90. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Foster, W.C.; Down, P.M.; Zhang, Y.; Ji, X.; Lorenzo, E.; Kononovs, D.; Saprykin, I.A.; Yakovlev, N.V.; Pozdnyakov, A.G.; et al. Graphene encapsulated silicon carbide nanocomposites for high and low power energy storage applications. J. Carbon Res. 2017, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Duran, N.; Marcato, P.D. Nanobiotechnology perspectives. Role of nanotechnology in the food industry: A review. Int. J. Food Sci. Technol. 2013, 48, 1127–1134. [Google Scholar] [CrossRef]
- Srilatha, B. Nanotechnology in Agriculture. J. Nanomed. Nanotechnol. 2011, 2, 5. [Google Scholar]
- Manikandan, V.S.; Adhikarib, B.R.; Chen, A. Nanomaterial based electrochemical sensors for the safety and quality control of food and beverages. Analyst 2018, 143, 4537–4554. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, P.N. Bioelectrochemistry 45: Fundamentals, Experimental Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Durst, R.A. Chemically modified electrodes: Recommended terminology and definitions. Pure Appl. Chem. 1997, 69, 1317–1324. [Google Scholar] [CrossRef]
- Hierlemann, A.; Gutierrez-Osuna, R. Higher-Order Chemical Sensing. Chem. Rev. 2008, 108, 563–613. [Google Scholar] [CrossRef]
- Bonizzoni, M.; Anslyn, E.V. Combinatorial Methods for Chemical and Biological Sensors. J. Am. Chem. Soc. 2009, 131, 14597–14598. [Google Scholar] [CrossRef]
- Suni, I.I. Impedance methods for electrochemical sensors using nanomaterials. Trends Anal. Chem. 2008, 27, 604–611. [Google Scholar] [CrossRef]
- Katz, E.; Willner, I. Probing Biomolecular Interactions at Conductive and Semiconductive Surfaces by Impedance Spectroscopy: Routes to Impedimetric Immunosensors, DNA-Sensors, and Enzyme Biosensors. Electroanalysis 2003, 15, 913–947. [Google Scholar] [CrossRef]
- Wang, J. Analytical Electrochemistry, 2nd ed.; Wiley/VCH: New York, NY, USA, 2000. [Google Scholar]
- Bockris, J.O.M.; Reddy, A.K.N.; Gamboa-Aldeco, M. Modern Electrochemistry 2A: Fundamentals of Electrodics, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; Volume 2. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2001. [Google Scholar]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Singha, N.; Tomar, V.; Chandra, R. A review on electrochemical detection of serotonin based on surface modified electrodes. Biosens. Bioelectron. 2018, 107, 76–93. [Google Scholar] [CrossRef] [PubMed]
- Brownson, D.A.; Banks, C.E. Graphene electrochemistry: An overview of potential applications. Analyst 2010, 135, 2768–2778. [Google Scholar] [CrossRef] [PubMed]
- Teradal, N.L.; Jelinek, R. Carbon Nanomaterials: Carbon Nanomaterials in Biological Studies and Biomedicine. Adv. Healthc. Mater. 2017, 6, 1700574. [Google Scholar] [CrossRef]
- Porto, L.S.; Silva, N.; de Oliveira, A.E.F.; Pereira, A.C.; Borges, K.B. Carbon nanomaterials: Synthesis and applications to development of electrochemical sensors in determination of drugs and compounds of clinical interest. Rev. Anal. Chem. 2019, 38, 20190017. [Google Scholar] [CrossRef]
- Bobrinetskiy, I.I.; Knezevic, N.Z. Graphene-based biosensors for on-site detection of contaminants in food. Anal. Methods 2018, 10, 5061–5071. [Google Scholar] [CrossRef]
- Bounegru, A.V.; Apetrei, C. Carbonaceous Nanomaterials Employed in the Development of Electrochemical Sensors Based on Screen-Printing Technique-A Review. Catalysts 2020, 10, 680. [Google Scholar] [CrossRef]
- Kirchner, E.-M.; Hirsch, T. Recent developments in carbon-based two-dimensional materials: Synthesis and modification aspects for electrochemical sensors. Microchim. Acta 2020, 187, 441–462. [Google Scholar] [CrossRef]
- Kour, R.; Arya, S.; Young, S.-J.; Gupta, V.; Bandhoria, P.; Khosla, A. Review-Recent Advances in Carbon Nanomaterials as Electrochemical Biosensors. J. Electrochem. Soc. 2020, 16, 037555. [Google Scholar] [CrossRef]
- Pandey, H.; Khare, P.; Singh, S.; Pratap Singh, S. Carbon nanomaterials integrated molecularly imprinted polymers for biological sample analysis: A critical review. Mater. Chem. Phys. 2020, 239, 121966. [Google Scholar] [CrossRef]
- Yang, C.; Jacobs, C.B.; Nguyen, M.D.; Ganesana, M.; Zestos, A.G.; Ivanov, I.N.; Puretzky, A.A.; Rouleau, C.M.; Geohegan, D.B.; Venton, B.J. Carbon Nanotubes Grown on Metal Microelectrodes for the Detection of Dopamine. Anal. Chem. 2016, 88, 645–652. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, F.; Sun, X.; Wei, G.F.; Tian, Y.; Liu, Z.P.; Huang, R.; Yu, Y.; Peng, H. Engineering Carbon Nanotube Fiber for Real-Time Quantification of Ascorbic Acid Levels in a Live Rat Model of Alzheimer’s Disease. Anal. Chem. 2017, 89, 1831–1837. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guo, S.; Zhai, Y.; Wang, E. Nafion–graphene nanocomposite film as enhanced sensing platform for ultrasensitive determination of cadmium. Electrochem. Commun. 2009, 11, 1085–1088. [Google Scholar] [CrossRef]
- Li, X.-R.; Liu, J.; Kong, F.-Y.; Liu, X.-C.; Xu, J.-J.; Chen, H.-Y. Potassium-doped graphene for simultaneous determination of nitrite and sulfite in polluted water. Electrochem. Commun. 2012, 20, 109–112. [Google Scholar] [CrossRef]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef]
- Mustafa, F.; Andreescu, S. Nanotechnology-based approaches for food sensing and packaging applications. RSC Adv. 2020, 10, 19309. [Google Scholar] [CrossRef]
- Xiaoa, T.; Huang, J.; Wang, D.; Meng, T.; Yang, X. Au and Au-Based nanomaterials: Synthesis and recent progress in electrochemical sensor applications. Talanta 2020, 206, 120210. [Google Scholar] [CrossRef]
- Liu, Z.; Nemec-Bakk, A.; Khaper, N.; Chen, A. Sensitive Electrochemical Detection of Nitric Oxide Release from Cardiac and Cancer Cells via a Hierarchical Nanoporous Gold Microelectrode. Anal. Chem. 2017, 89, 8036–8043. [Google Scholar] [CrossRef]
- Salaün, P.; van den Berg, C.M. Voltammetric Detection of Mercury, and Copper in Seawater Using a Gold Microwire Electrode. Anal. Chem. 2006, 78, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Alves, G.M.; Magalhães, J.M.; Salaün, P.; Van den Berg, C.M.; Soares, H.M. Simultaneous electrochemical determination of arsenic, copper, lead, and mercury in unpolluted fresh waters using a vibrating gold microwire electrode. Anal. Chim. Acta 2011, 703, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xing, G.; Liqin, W.; Zhaon, L.; Ding, Y. Nanoporous Gold Leaf for Amperometric Determination of Nitrite. Electroanalysis 2011, 23, 381–386. [Google Scholar]
- Jia, F.; Yu, C.; Ai, Z.; Zhang, L. Fabrication of Nanoporous Gold Film Electrodes with Ultrahigh Surface Area and Electrochemical Activity. Chem. Mater. 2007, 19, 3648–3653. [Google Scholar] [CrossRef]
- Li, J.; Lin, X. Electrocatalytic oxidation of hydrazine and hydroxylamine at gold nanoparticle-polypyrrole nanowire modified glassy carbon electrode. Sens. Actuators B 2007, 126, 527–535. [Google Scholar] [CrossRef]
- Manikandan, V.S.; Liu, Z.; Chen, A. Simultaneous detection of hydrazine, sulfite, and nitrite based on a nanoporous gold microelectrode. J. Electroanal. Chem. 2018, 819, 524–532. [Google Scholar] [CrossRef]
- Yoon, H. Current Trends in Sensors Based on Conducting Polymer Nanomaterials. Nanomaterials 2013, 3, 524–549. [Google Scholar] [CrossRef] [Green Version]
- Al-Mokaram, A.; Amir, M.A.; Yahya, R.; Abdi, M.M.; Mahmud, N.M.E. The Development of Non-Enzymatic Glucose Biosensors Based on Electrochemically Prepared Polypyrrole–Chitosan–Titanium Dioxide Nanocomposite Films. Nanomaterials 2017, 7, 129. [Google Scholar] [CrossRef] [Green Version]
- Govindhan, M.; Amiri, M.; Chen, A. Au nanoparticle/graphene nanocomposite as a platform for the sensitive detection of NADH in human urine. Biosens. Bioelectron. 2015, 66, 474–480. [Google Scholar] [CrossRef]
- Li, S.-S.; Hu, Y.-Y.; Wang, A.-J.; Weng, X.; Chen, J.-R.; Feng, J.-J. Simple synthesis of worm-like Au–Pd nanostructures supported on reduced graphene oxide for highly sensitive detection of nitrite. Sens. Actuators B 2015, 208, 468–474. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakian, T.; Ghaedi, H. Surface decoration of multi-walled carbon nanotubes modified carbon paste electrode with gold nanoparticles for electro-oxidation and sensitive determination of nitrite. Biosens. Bioelectron. 2014, 51, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.-L.; Lee, H.-H.; Yang, J.-M.; Wu, C.-C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 2011, 26, 3450–3455. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Chen, A. Voltammetric detection of the α-dicarbonyl compound: Methylglyoxal as a flavoring agent in wine and beer. Anal. Chim. Acta 2012, 751, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Mathiyarasu, J.; Senthilkumar, S.; Phani, K.L.N.; Yegnaraman, V. PEDOT-Au nanocomposite film for electrochemical sensing. Mater. Lett. 2008, 62, 571–573. [Google Scholar] [CrossRef]
- Bianchini, C.; Zane, D.; Curulli, A. Gold microtubes assembling architecture for an impedimetric glucose biosensing system. Sens. Actuators B 2015, 220, 734–742. [Google Scholar] [CrossRef]
- Wang, J.; Musameh, M. Carbon-nanotubes doped polypyrrole glucose biosensor. Anal. Chim. Acta 2005, 539, 209–2013. [Google Scholar] [CrossRef]
- Valentini, F.; Galache Fernandez, L.; Tamburri, E.; Palleschi, G. Single Walled Carbon Nanotubes/polypyrrole–GOx composite films to modify gold microelectrodes for glucose biosensors: Study of the extended linearity. Biosens. Bioelectron. 2013, 43, 75–78. [Google Scholar] [CrossRef]
- Adamson, T.L.; Eusebio, F.A.; Cook, C.B.; La Belle, J.T. The promise of electrochemical impedance spectroscopy as novel technology for the management of patients with diabetes mellitus. Analyst 2012, 137, 4179–4187. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biochemistry; CRC Press: Andover, MA, USA, 2005. [Google Scholar]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Gordon, M.H. Significance of Dietary Antioxidants for Health. Int. J. Mol. Sci. 2012, 13, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Bors, W.; Heller, W.; Michel, C.; Stettmaier, K. Flavonoids, and polyphenols: Chemistry and biology. In Handbook of Antioxidants; Cadenas, E., Packer, L., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 409–466. [Google Scholar]
- Guo, Q.; Yue, Q.; Zhao, J.; Wang, L.; Wang, H.; Wei, X.; Liu, J.; Jia, J. How far can hydroxyl radicals travel? An electrochemical study based on a DNA mediated electron transfer process. Chem. Commun. 2011, 47, 11906–11908. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Apak, R.; Demirci Çekiç, S.; Üzer, A.; Çelik, S.E.; Bener, M.; Bekdeşer, B.; Can, Z.; Sağlam, Ş.; Önem, A.N.; Erçağ, E. Novel Spectroscopic and Electrochemical Sensors and Nanoprobes for the Characterization of Food and Biological Antioxidants. Sensors 2018, 18, 186. [Google Scholar] [CrossRef] [Green Version]
- Bounegru, A.V.; Apetrei, C. Voltammetric Sensors Based on Nanomaterials for Detection of Caffeic Acid in Food Supplements. Chemosensors 2020, 8, 41. [Google Scholar] [CrossRef]
- Di Carlo, G.; Curulli, A.; Trani, A.; Zane, D.; Ingo, G.M. Enhanced electrochemical response of structurally related antioxidant at nanostructured hybrid films. Sens. Actuators B 2014, 191, 703–710. [Google Scholar] [CrossRef]
- Di Carlo, G.; Curulli, A.; Toro, R.G.; Bianchini, C.; De Caro, T.; Padeletti, G.; Zane, D.; Ingo, G.M. Green Synthesis of Gold–Chitosan Nanocomposites for Caffeic Acid Sensing. Langmuir 2012, 28, 5471–5479. [Google Scholar] [CrossRef]
- Curulli, A.; Di Carlo, G.; Ingo, G.M.; Riccucci, C.; Zane, D.; Bianchini, C. Chitosan Stabilized Gold Nanoparticle-Modified Au Electrodes for the Determination of Polyphenol Index in Wines: A Preliminary Study. Electroanalysis 2012, 24, 897–904. [Google Scholar] [CrossRef]
- Kang, N.J.; Lee, K.W.; Shin, B.J.; Jung, S.K.; Hwang, M.K.; Bode, A.M.; Heo, Y.-S.; Lee, H.J.; Dong, Z. Caffeic acid, a phenolic phytochemical in coffee, directly inhibits Fyn kinase activity and UVB-induced COX-2 expression. Carcinogenesis 2008, 30, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Filik, H.; Çetintaş, G.; Aslıhan Avan, A.; Aydar, S.; Naci Koç, S.; Boz, İ. Square-wave stripping voltammetric determination of caffeic acid on electrochemically reduced graphene oxide–Nafion composite film. Talanta 2013, 116, 245–250. [Google Scholar] [CrossRef]
- Leite, F.; de Jesus Rodrigues Santos, F.R.W.; Tatsuo Kubota, L. Selective determination of caffeic acid in wines with electrochemical sensor based on molecularly imprinted siloxanes. Sens. Actuators B 2014, 193, 238–246. [Google Scholar] [CrossRef]
- Thangavelu, K.; Palanisamy, S.; Chen, S.M.; Velusamy, V.; Chen, T.W.; Kannan Ramarajc, S. Electrochemical Determination of Caffeic Acid in Wine Samples Using Reduced Graphene Oxide/Polydopamine Composite. J. Electrochem. Soc. 2016, 163, B726–B731. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, J.; Yue, R.; Yang, T.; Gao, L. Facile one-pot synthesis of Au–PEDOT/rGO nanocomposite for highly sensitive detection of caffeic acid in red wine sample. Electrochim. Acta 2016, 196, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, B.; Gao, Y.; Yang, T.; Yue, R.; Xu, J.; Gao, L. Facile one-pot preparation of Pd–Au/PEDOT/graphene nanocomposites and their high electrochemical sensing performance for caffeic acid detection. Rsc Adv. 2016, 6, 89157–89166. [Google Scholar] [CrossRef]
- Karthik, R.; Vinoth Kumar, J.; Chen, S.-M.; Senthil Kumar, P.; Selvam, V.; Muthuraj, V. A selective electrochemical sensor for caffeic acid and photocatalyst for metronidazole drug pollutant-A dual role by rod-like SrV2O6. Sci. Rep. 2017, 7, 7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thangavelu, K.; Raja, N.; Chen, S.-M.; Liao, W.-C. Nanomolar electrochemical detection of caffeic acid in fortified wine samples based on gold/palladium nanoparticles decorated graphene flakes. J. Colloids Interface Sci. 2017, 501, 77–85. [Google Scholar] [CrossRef]
- Bharath, G.; Alhseinat, E.; Madhu, R.; SM Mugo Alwasel, S.; Harrath, A.H. Facile synthesis of Au@α-Fe2O3@RGO ternary nanocomposites for enhanced electrochemical sensing of caffeic acid toward biomedical applications. J. Alloys Compd. 2018, 750, 819–827. [Google Scholar] [CrossRef]
- Gao, L.; Yue, R.; Xu, J.; Liu, Z.; Chai, J. Pt-PEDOT/rGO nanocomposites: One-pot preparation and superior electrochemical sensing performance for caffeic acid in tea. J. Electroanal. Chem. 2018, 816, 14–20. [Google Scholar] [CrossRef]
- Shi, Y.; Xu, H.; Gu, Z.; Wang, C.; Du, Y. Sensitive detection of caffeic acid with trifurcate PtCu nanocrystals modified glassy carbon electrode. Colloids Surf. A 2019, 567, 27–31. [Google Scholar] [CrossRef]
- Chen, T.-W.; Rajaji, U.; Chen, S.-M.; Govindasamy, M.; Selvin, S.S.P.; Manavalan, S.; Arumugam, R. Sonochemical synthesis of graphene oxide sheets supported Cu2S nanodots for high sensitive electrochemical determination of caffeic acid in red wine and soft drinks. Compos. Part B 2019, 158, 419–427. [Google Scholar] [CrossRef]
- Karabozhikova, V.; Tsa, V. Electroanalytical determination of caffeic acid–Factors controlling the oxidation reaction in the case of PEDOT-modified electrodes. Electrochim. Acta 2019, 293, 439–446. [Google Scholar] [CrossRef]
- Erady, V.; Mascarenhas, R.J.; Satpati, A.K.; Bhakta, A.K.; Mekhalif, Z.; Delhalle, J.; Dhason, A. Carbon paste modified with Bi decorated multi-walled carbon nanotubes and CTAB as a sensitive voltammetric sensor for the detection of Caffeic acid. Microchem. J. 2019, 146, 73–82. [Google Scholar] [CrossRef]
- Manikandantr, V.S.; Sidhureddy, B.; Thiruppathi, A.R.; Chen, A. Sensitive Electrochemical Detection of Caffeic Acid in Wine Based on Fluorine-Doped Graphene Oxide. Sensors 2019, 19, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchini, C.; Curulli, A.; Pasquali, M.; Zane, D. Determination of caffeic acid in wine using PEDOT film modified electrode. Food Chem. 2014, 156, 81–86. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, N.; Najafi, M.; Adeh, N.B. Highly defective mesoporous carbon–Ionic liquid paste electrode as sensitive voltammetric sensor for determination of chlorogenic acid in herbal extracts. Sens. Actuators B 2017, 243, 838–846. [Google Scholar] [CrossRef]
- Ma, X.; Yang, H.; Xiong, H.; Li, X.; Gao, J.; Gao, Y. Electrochemical Behavior and Determination of Chlorogenic Acid Based on Multi-Walled Carbon Nanotubes Modified Screen-Printed Electrode. Sensors 2016, 16, 1797. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Chen, Y.; Huang, W.; Wang, Y.; Hu, X. A novel AuNPs-doped COFs composite as electrochemical probe for chlorogenic acid detection with enhanced sensitivity and stability. Sens. Actuators B 2018, 276, 362–369. [Google Scholar] [CrossRef]
- Chokkareddy, R.; Redhi, G.G.; Karthick, T. A lignin polymer nanocomposite based electrochemical sensor for the sensitive detection of chlorogenic acid in coffee samples. Heliyon 2019, 5, e01457. [Google Scholar] [CrossRef] [Green Version]
- Manivannan, K.; Sivakumar, M.; Cheng, C.-C.; Luc, C.-H.; Chen, J.-K. An effective electrochemical detection of chlorogenic acid in real samples: Flower-like ZnO surface covered on PEDOT: PSS composites modified glassy carbon electrode. Sens. Actuators B 2019, 301, 127002. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Sun, J.; Chen, S.; Chen, Y.; Fang, Y. Nanomolar detection of chlorogenic acid at the cross-section surface of the pencil lead electrode. Sens. Actuators B 2020, 321, 128550. [Google Scholar] [CrossRef]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Shahbaz, A.I.M.; Amir, R.M.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; Salehi, B.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef] [Green Version]
- Mohamadi, M.; Mostafavi, A.; Torkzadeh-Mahanic, M. Voltammetric Determination of Rosmarinic Acid on Chitosan/Carbon Nanotube Composite-Modified Carbon Paste Electrode Covered with DNA. J. Electrochem. Soc. 2015, 162, B344–B349. [Google Scholar] [CrossRef]
- Alipour, S.; Azar, A.; Husain, S.W.; Rajabi, H.R. Determination of Rosmarinic acid in plant extracts using a modified sensor based on magnetic imprinted polymeric nanostructures. Sens. Actuators B 2020, 323, 128668. [Google Scholar] [CrossRef]
- Trani, A.; Petrucci, R.; Marrosu, G.; Zane, D.; Curulli, A. Selective electrochemical determination of caffeine at a gold-chitosan nanocomposite sensor: May little change on nanocomposites synthesis affect selectivity? J. Electroanal. Chem. 2017, 788, 99–106. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, F.; Zhao, W.; Zhou, J.; Liu, Y.; Zou, L.; Ye, B. Voltammetric sensor for caffeine based on a glassy carbon electrode modified with Nafion and graphene oxide. Microchim. Acta 2011, 174, 383–390. [Google Scholar] [CrossRef]
- de Jesus Rodrigues Santos, W.; Santhiago, M.; Pagotto Yoshida, I.V.; Kubota, L.T. Electrochemical sensor based on imprinted sol–gel and nanomaterial for determination of caffeine. Sens. Actuators B 2012, 166–167, 739–745. [Google Scholar] [CrossRef]
- Yu Heng Khoo, W.; Pumera, M.; Bonanni, A. Graphene platforms for the detection of caffeine in real samples. Anal. Chim. Acta 2013, 804, 92–97. [Google Scholar] [CrossRef]
- Yang, G.; Zhao, F.; Zeng, B. Facile fabrication of a novel anisotropic gold nanoparticle–chitosan–ionic liquid/graphene modified electrode for the determination of theophylline and caffeine. Talanta 2014, 127, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Khalili Boroujeni, M.; Ensafi, A.A. Caffeine electrochemical sensor using imprinted film as recognition element based on polypyrrole, sol-gel, and gold nanoparticles hybrid nanocomposite modified pencil graphite electrode. Biosens. Bioelectron. 2014, 60, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Shehata, M.; Azab, S.M.; Fekry, A.M. May glutathione and graphene oxide enhance the electrochemical detection of caffeine on carbon paste sensor in aqueous and surfactant media for beverages analysis? Synth. Met. 2019, 256, 116122. [Google Scholar] [CrossRef]
- Fekry, A.M.; Shehata, M.; Azab, S.M.; Walcarius, A. Voltammetric detection of caffeine in pharmacological and beverages samples based on simple nano- Co (II, III) oxide modified carbon paste electrode in aqueous and micellar media. Sens. Actuators B 2020, 302, 127172. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra Debiprosad, R. Review on nanomaterials-enabled electrochemical sensors for ascorbic acid detection. Anal. Biochem. 2019, 586, 113415. [Google Scholar] [CrossRef] [PubMed]
- Siddeeg, S.M.; Salem Alsaiari, N.; Tahoon, M.A.; Ben Rebah, F. The Application of Nanomaterials as Electrode Modifiers for the Electrochemical Detection of Ascorbic Acid: Review. Int. J. Electrochem. Sci. 2020, 15, 3327–3346. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; He, W.; Uyama, H.; Xie, Q.; Zhang, L.; Yang, F. Electrochemical sensor based on carbon supported NiCoO2 nanoparticles for selective detection of ascorbic acid. Biosens. Bioelectron. 2014, 55, 446–451. [Google Scholar] [CrossRef]
- Khalilzadeh, M.A.; Borzoo, M. Green synthesis of silver nanoparticles using onion extract and their application for the preparation of a modified electrode for determination of ascorbic acid. J. Food Drug Anal. 2016, 24, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.M.; Asiri AM Rahman, M.M. Non-enzymatic simultaneous detection of acetylcholine and ascorbic acid using ZnO⋅CuO nanoleaves: Real sample analysis. Microchem. J. 2020, 159, 105534. [Google Scholar] [CrossRef]
- Mohammadnia, M.S.; Khosrowshahi, E.M.; Naghian, E.; Keihan, A.H.; Sohouli, E.; Plonska-Brzezinska, M.E.; Sobhani-Nasab, A.; Rahimi-Nasrabadi, M.; Ahmadi, F. Application of carbon nanoonion NiMoO4-MnWO4 nanocomposite for modification of glassy carbon electrode: Electrochemical determination of ascorbic acid. Microchem. J. 2020, 159, 105470. [Google Scholar] [CrossRef]
- Brainina Khiena, Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticlesmfor Determination of Ascorbic Acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Zhang, Z.; Nan, F.; Zhao, Y.; Wang, P.; He, F.; Wang, Y. Mesoporous CuCo2O4 rods modified glassy carbon electrode as a novel non-enzymatic amperometric electrochemical sensors with high sensitive ascorbic acid recognition. J. Alloys Compd. 2021, 852, 157045. [Google Scholar] [CrossRef]
- de Faria, L.V.; Pedrosa Lisboa, T.; Marques de Farias, D.; Moreira Araujo, F.; Moura Machado, M.; Arromba de Sousa, R.; Costa Matos, M.A.; Abarza Muñoz, R.A.; Camargo Matos, R. Direct analysis of ascorbic acid in food beverage samples by flow injection analysis using reduced graphene oxide sensor. Food Chem. 2020, 319, 126509. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Liu, T.; Mo, H.; Yuan, Z.; Cui, F.; Jin, Y.; Chen, X. Cu-based metal–organic framework HKUST-1 as effective catalyst for highly sensitive determination of ascorbic acid. Rsc Adv. 2020, 10, 22881. [Google Scholar] [CrossRef]
- Zhuanga, Z.; Chen, W. One-step rapid synthesis of Ni6(C12H25S)12 nanoclusters for electrochemical sensing of ascorbic acid. Analyst 2020, 145, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Liu, Z.; Liu, L.; Shin Low, S.; Lua, Y.; Yu, X.; Zhu, L.; Li, C.; Liu, Q. Smartphone-based integrated voltammetry system for simultaneous detection of ascorbic acid, dopamine, and uric acid with graphene and gold nanoparticles modified screen-printed electrodes. Biosens. Bioelectron. 2018, 119, 55–62. [Google Scholar] [CrossRef]
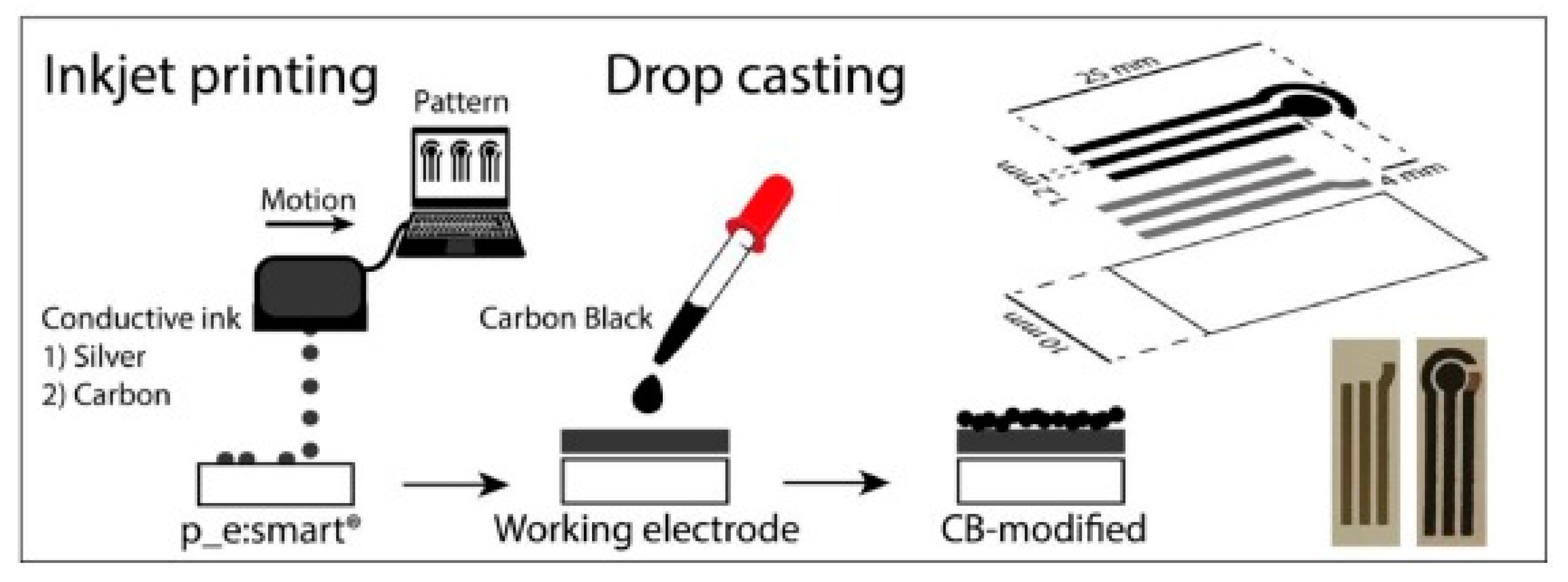
- Cinti, S.; Colozza, N.; Cacciotti, I.; Moscone, D.; Polomoshnov, M.; Sowade, E.; Baumann, R.R.; Arduini, F. Electroanalysis moves towards paper-based printed electronics: Carbon black nanomodified inkjet-printed sensor for ascorbic acid detection as a case study. Sens. Actuators B 2018, 265, 155–160. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2004; p. 417. [Google Scholar]
- Ning, D.; Zhang, H.; Zheng, J. Electrochemical sensor for sensitive determination of nitrite based on the PAMAM dendrimer-stabilized silver nanoparticles. J. Electroanal. Chem. 2014, 717–718, 29–33. [Google Scholar] [CrossRef]
- Yang, B.; Bin, D.; Wang, H.; Zhu, M.; Yang, P.; Du, Y. High quality Pt-graphene nanocomposites for efficient electrocatalytic nitrite sensing. Colloids Surf. A Phys. Eng. Asp. 2015, 481, 43–50. [Google Scholar] [CrossRef]
- Wang, H.; Wen, F.; Chen, Y.; Sun, T.; Meng, Y.; Zhang, Y. Electrocatalytic determination of nitrite based on straw cellulose/Molybdenum sulphide nanocomposite. Biosens. Bioelectron. 2016, 85, 692–697. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.-M.; Zhao, D.-H. Amperometric detection of nitrite in water samples by use of electrodes consisting of palladium-nanoparticle-functionalized multi-walled carbon nanotubes. J. Colloid Interface Sci. 2016, 478, 413–420. [Google Scholar] [CrossRef]
- Mehmeti, E.; Stanković, D.M.; Hajrizi, A.; Kalcher, K. The use of graphene nanoribbons as efficient electrochemical sensing material for nitrite determination. Talanta 2016, 159, 34–39. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Xia, Z.; Liu, C.; Huang, H.; Ye, W. Green synthesis of Pd/Fe3O4 composite based on poly DOPA functionalized reduced graphene oxide for electrochemical detection of nitrite in cured food. Electrochim. Acta 2017, 256, 146–154. [Google Scholar] [CrossRef]
- Aksu, Z.; Alanyalıoglu, M. Fabrication of free-standing reduced graphene oxide composite papers doped with different dyes and comparison of their electrochemical performance for electrocatalytical oxidation of nitrite. Electrochim. Acta 2017, 258, 1376–1386. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tiwari, I.; Foster, C.W.; Banks, C.E. Highly sensitive amperometric sensing of nitrite utilizing bulk modified MnO2 decorated Graphene oxide nanocomposite screen-printed electrodes. Electrochim. Acta 2017, 227, 255–266. [Google Scholar] [CrossRef]
- Losada, J.; García Armada, M.P.; García, E.; Casado, C.M.; Alonso, B. Electrochemical preparation of gold nanoparticles on ferrocenyl-dendrimer film modified electrodes and their application for the electrocatalytic oxidation and amperometric detection of nitrite. J. Electroanal. Chem. 2017, 788, 14–22. [Google Scholar] [CrossRef]
- Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate. J. Hazard. Mater. 2017, 324, 762–772. [Google Scholar] [CrossRef]
- Wang, K.; Wu, C.; Wang, F.; Liu, C.; Yu, C.; Jiang, G. In-situ insertion of carbon nanotubes into metal-organic frameworks-derived-Fe2O3 polyhedrons for highly sensitive electrochemical detection of nitrite. Electrochim. Acta 2018, 285, 128–138. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, Y.; Hong, Z.; Zheng, X.; Lv, R. Magnetic Co@carbon nanocages for facile and binder-free nitrite sensor. J. Electroanal. Chem. 2018, 824, 45–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Nie, J.; Wei, H.; Xu, H.; Wang, Q.; Cong, Y.; Tao, J.; Zhang, Y.; Chu, L.; Zhou, Y.; et al. Electrochemical detection of nitrite ions using Ag/Cu/MWNT nanoclusters electrodeposited on a glassy carbon electrode. Sens. Actuators B 2018, 258, 1107–1116. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Wang, W.; Sun, Y.; Li, P.; Hu, J.; Chen, L.; Gong, W. Synthesis and electrochemical properties of Co3O4-rGO/CNTs composites towards highly sensitive nitrite detection. Appl. Surf. Sci. 2019, 485, 274–282. [Google Scholar] [CrossRef]
- Hameed, R.M.A.; Medany, S.S. Construction of core-shell structured nickel@platinum nanoparticles on graphene sheets for electrochemical determination of nitrite in drinking water samples. Microchem. J. 2019, 145, 354–366. [Google Scholar] [CrossRef]
- Chen, H.; Yang, T.; Liu, F.; Li, W. Electrodeposition of gold nanoparticles on Cu-based metal-organic framework for the electrochemical detection of nitrite. Sens. Actuators B 2019, 286, 401–407. [Google Scholar] [CrossRef]
- Wang, P.; Wang, M.; Zhou, F.; Yang, G.; Qu, L.; Miao, X. Development of a paper-based, inexpensive, and disposable electrochemical sensing platform for nitrite detection. Electrochem. Commun. 2017, 81, 74–78. [Google Scholar] [CrossRef]

| Electrochemical Methods | Electrode Material | Linearity Range (mol·L−1) | LOD (mol·L −1) | Application | Reference |
|---|---|---|---|---|---|
| DPV) | AuNps/Chitosan/AuE | 5.00 × 10−8–2.00 × 10−3 | 2.50 ×10−8 | Red and white wines | [66,67] |
| SWS | Nafion/ER-GO/GCE | 1.0 × 10−7–1.0 × 10−6 | 9.1 × 10−8 | White wines | [69] |
| DPV | MIS/AuE | 5.00 × 10−7–6.00 × 10−5 | 1.50 × 10−7 | White wines | [70] |
| DPV | RGO@PDA/GCE | 5.0 × 10−9–4.55 × 10−4 | 1.20 × 10−9 | Wines | [71] |
| DPV | Au–PEDOT/rGO/GCE | 1.00 × 10−8–4.60 × 10−5 | 4.00 × 10−9 | Red wines | [72] |
| DPV | PdAu/PEDOT/rGO/GCE | 1.90 × 10−9–5.50 × 10−5 | 3.70 × 10−10 | Red wines | [73] |
| Amperometry | SrV2O6/GCE | 1.00 × 10−8–2.07 × 10−4 | 4.00 × 10−9 | No real samples | [74] |
| DPV | Au/PdNPs/GRF/GCE | 3.00 × 10−8–9.40 × 10−4 | 6.00 × 10−9 | Fortified wines | [75] |
| DPV | Au@α-Fe2O3/RGO/GCE | 1.90 × 10−5–1.87 ×10−3 | 9.80 × 10−8 | Coffee samples | [76] |
| DPV | PEDOT/rGO/PtE | 5.0 × 10−9–5.0 × 10−5 | 2.0 × 10−9 | Teas | [77] |
| DPV | PtCu trifurcate nanocrystal/GCE | 1.20 × 10−6–1.90 × 10−3 | 3.50 × 10−7 | Red wines | [78] |
| Amperometry | Cu2S NDs@GOS NC/SPCE | 5.50 × 10−8–2.50 × 10−3 | 2.20 × 10−10 | Soft drinks and red wines | [79] |
| DPV | PEDOT/GCE | thin film 1.50 × 10−7–4.00 × 10−6 thick fim1.50 × 10−6–4.75 × 10−5 | No real samples | [80] | |
| DPV | MWCNTs-Bi/CTABCPE | 6.0 × 10−8–5.0 × 10−4 | 1.91 × 10−9 | Coconut water, teas, and fruit juices | [81] |
| DPV | F-GO/GCE | 5.00 × 10−7–1.00 × 10−4 | 1.80 × 10−8 | Red wines | [82] |
| Electrochemical Methods | Electrode Material | Linearity Range (mol·L−1) | LOD (mol·L−1) | Application | Reference |
|---|---|---|---|---|---|
| DPV | MWCNTs/SPE | 4.8 × 10−4–4.4 × 10−2 | 3.38 × 10−4 | Coffee beans | [86] |
| DPV | DMC/BMIM.PF6/CPE | 2.00 × 10−8–2.50 × 10−6 | 1.00 × 10−8 | Herbal extracts of Calendula officinalis and Echinacea purpurea | [85] |
| DPV | AuNps@TAPB-DMTP-COFs/GCE | 1.00 × 10−8–4.00 × 10−5 | 9.50 × 10−9 | Coffee, fruit juice and herbal extracts | [87] |
| Differential Pulse Voltammetry (DPV) WE | MWCNTs/CuONPs/LGN/GCE | 5.00 × 10−3–5.00 × 10−2 | 1.25 × 10−5 | Coffee | [88] |
| Differential Pulse Voltammetry (DPV) WE) | ZnO@PEDOT:PSS/GCE | 3.00 × 10−8–4.76 × 10−4 | 2.00 × 10−8 | Coffee powder, soft drink | [89] |
| Electrochemical Methods | Electrode Material | Linearity Range (mol·L−1) | LOD (mol·L−1) | Application | Reference |
|---|---|---|---|---|---|
| DPV | Nafion/GO/GCE | 4.00 × 10−7–8.00 × 10−5 | 2.00 × 10−7 | Soft and energy drinks, cola beverage | [95] |
| DPV | MIS/MWCNTs/VTMS/GCE | 7.50 × 10−7–4.00 × 10−5 | 2.20 × 10−7 | Coffees, energy drinks, | [96] |
| DPV | ERGO/GCE | 5.00 × 10−5–3.00 × 10−4 | Not declared | Cola beverage, tea, and soluble coffee | [97] |
| DPV | AuNps/chitosan–ionic liquid/Gr/GCE | 2.50 × 10−8–2.49 × 10−6 | 4.42 × 10−9 | Energy drink, teas, drugs | [98] |
| DPV | AuNps@PPY/PGE | 2.00 × 10−9–5.00 × 10−8 5.00 × 10−8–1.00 × 10−6 | 9.00 × 10−10 | Soft and energy drinks, green tea, human plasma, drugs and urine | [99] |
| DPV | AuNps/chitosan/AuE | 2.00 × 10−6–5.00 × 10−2 | 1.00 × 10−6 | Cola beverages, energy drink, teas | [94] |
| DPV | GO/RG/CPE | 8.00 × 10−6–8.00 × 10−4 | 1.53 × 10−7 | Cola beverages, energy drink, teas, and drugs | [100] |
| DPV | CoON/CPE | 5.00 × 10−6–6.00 × 10−4 | 1.60 × 10−8 | Coffees | [101] |
| Electrochemical Methods | Electrode Material | Linearity Range (mol·L−1) | LOD (mol·L−1) | Application | Reference |
|---|---|---|---|---|---|
| DPV | NiCoO2/C/GCE | 1.00 × 10−5–2.63 × 10−3 | 5.00 × 10−7 | Fetal bovine serum, Vitamin C tableys, Vitamin C drinks | [104] |
| SWV | AgNPs@onion extracts/CPE | 4.00 x 10−7–4.50 × 10−4 | 1.00 × 10−7 | Orange, kiwi and apple juices | [105] |
| DPV | ZnO⋅CuO NLs/GCE | 1.00 × 10−7–1.00 × 10−1 | 1.20 × 10−8 | Human, mouse, and rabbit serum, orange juice, and urine | [106] |
| DPV | CNO-NiMoO4-MnWO4/GCE | 1.00 × 10−6–1.00 × 10−4 | 3.30 × 10−7 | Orange, strawberry, tomato, pineapple juices | [107] |
| Amperometry | Mesoporous CuCo2O4/GCE | 1.00 × 10−4–1. 05 × 10−3 | 2.10 × 10−7 | Vitamin C tablets, Vitamin C effeverscent tablets and urine | [108] |
| LSV | Au-gr/CVE | 1.00 × 10−6–5.75 × 10−3 | 5.00 × 10−8 | Cherry-apple juice, apple juice for children, apple juice and apple nectar clarified | [109] |
| FIA-Amperometry | rGO/GCE | Linearity range not declared | 4.70 × 10−6 | Milk, fermented milk, chocolate milk and multivitamin supplement | [110] |
| DPV | HKUST-1/ITO | 1.00 × 10−5–2. 65 × 10−3 | 3.00 × 10−6 | Vitamin C pills, Vitamin C tablets, Vitamin C effervescent tablets | [111] |
| Amperometry | Ni6 NCs/CB/GCE | 1.00 × 10−6–3.21 × 10−3 | 1.00 × 10−7 | Vitamin C tablets | [112] |
| Electrochemical Methods | Electrode Material | Linearity Range (mol·L−1) | LOD (mol·L−1) | Application | Reference |
|---|---|---|---|---|---|
| DPV | AgNPs@PAMAM/GCE | 4.00 × 10−6–1.44 × 10−3 | 4.00 × 10−7 | Milk and tap water | [116] |
| DPV | PtNPs/rGO/GCE | Linearity range not declared | 1.00 × 10−7 | Beverages | [117] |
| DPV | TOSC-MoS2/GCE | 6.00 × 10−6–4.20 × 10−3 | 2.00 × 10−6 | River and drinking water | [118] |
| Amperometry | f-MWCNT/PdNPs/GCE | 5.00 × 10−8–3.00 × 10−6 | 2.20 × 10−8 | River, pond, and drinking water | [119] |
| DPV | GNs/GCE | 1.00 × 10−6 –1. 05 × 10−4 | 2.20 × 10−7 | Tap water | [120] |
| DPV | Pd/Fe3O4/polyDOPA/RGO/GCE | 2.50 × 10−6–6.47 × 10−3 | 5.00 × 10−7 | River water and sausage | [121] |
| Amperometry | rGO/Acr/GCE | 4.00 × 10−7–3.60 × 10−3 | 1.20 × 10−7 | Milk, mineral and tap water | [122] |
| Amperometry | MnO2/GO-SPE | 1.00 × 10−7–1.00 × 10−3 | 9.00 × 10−8 | Tap and mineral water | [123] |
| DPV | AuNPs/carbosilane-dendrimer/GCE | 1.00 × 10−5–5. 00 × 10−3 | 2.00 × 10−7 | Natural water | [124] |
| SWV | Cu/MWCNT/RGO/GCE | 1.00 × 10−7–7.50 × 10−5 | 3.00 × 10−8 | Tap and mineral waters, sausages, salami, and cheese | [125] |
| DPV | MOFs-derived α-Fe2O3/CNTs/GCE | 1.00 × 10−7–7.50 × 10−5 | 3.00 × 10−8 | Tap and mineral waters, sausages, salami, and cheese | [126] |
| DPV | CoCNM/GCE | 5.00 × 10−6–7.05 × 10−6 | 1.80 × 10−7 | Tap water | [127] |
| DPV | Ag/CuNCs/MWCNTs/GCE | 1.00 × 10−6–1.00 × 1036 | 2.00 × 10−7 | Lake water, drinking water and seawater. | [128] |
| DPV | Co3O4@rGO/CNTs/GCE | 8.00 × 10−6–5.60 × 10−2 | 1.60 × 10−8 | Tap water | [129] |
| DPV | Ni@Pt/Gr/GCE | 1.00 × 10−5–1.50 × 10−2 | Not declared | Tap water | [130] |
| DPV | AuNPs@Cu-MOF/GCE | 1.00 × 10−7–1.00 × 10−2 | 8.20 × 10−8 | River water | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curulli, A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules 2020, 25, 5759. https://doi.org/10.3390/molecules25235759
Curulli A. Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules. 2020; 25(23):5759. https://doi.org/10.3390/molecules25235759
Chicago/Turabian StyleCurulli, Antonella. 2020. "Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis" Molecules 25, no. 23: 5759. https://doi.org/10.3390/molecules25235759
APA StyleCurulli, A. (2020). Nanomaterials in Electrochemical Sensing Area: Applications and Challenges in Food Analysis. Molecules, 25(23), 5759. https://doi.org/10.3390/molecules25235759






