Zeaxanthin, a Molecule for Photoprotection in Many Different Environments
Abstract
1. Overview of Sources and Roles of Carotenoids
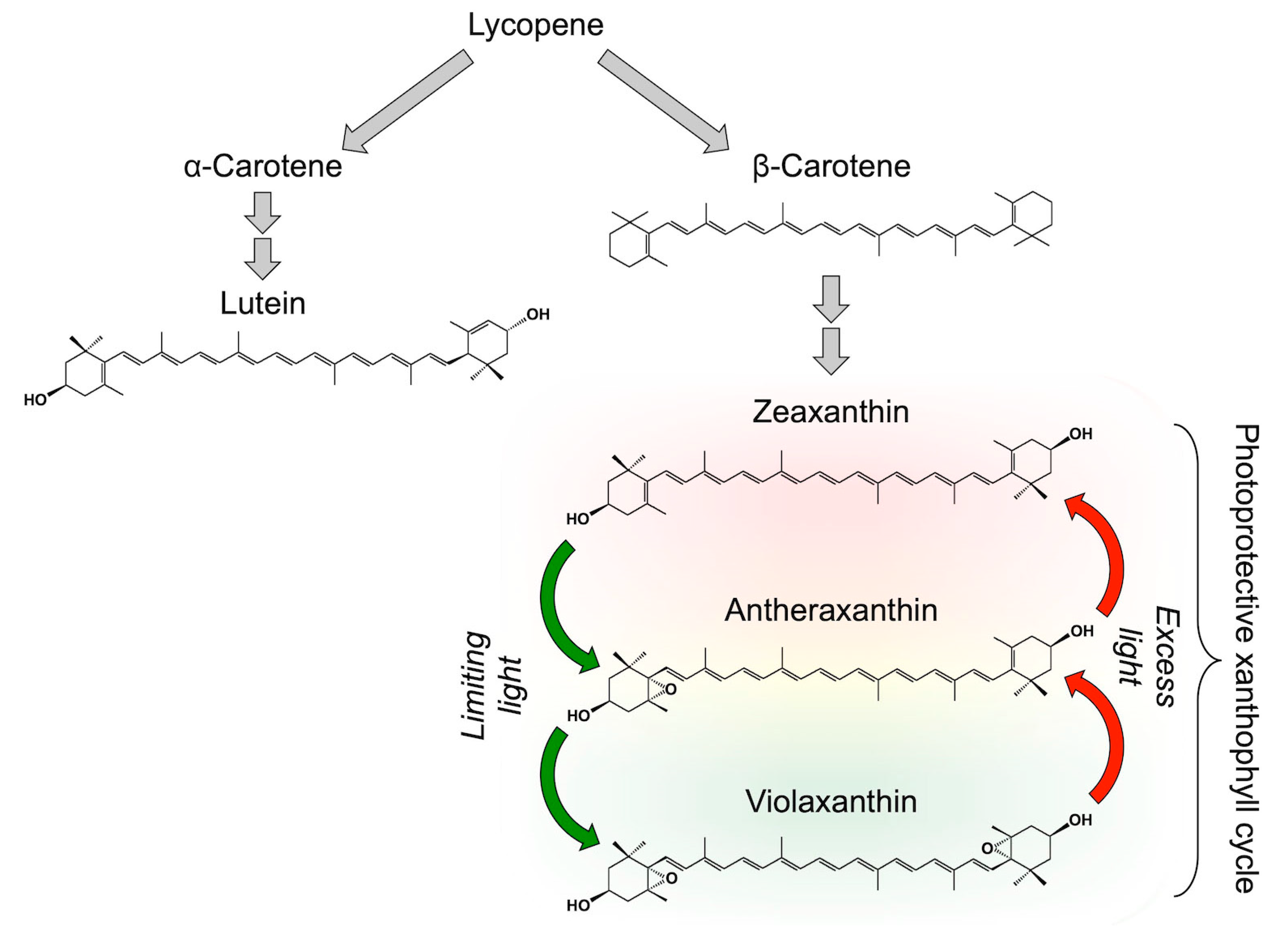
2. Carotenoid Biosynthesis and Biochemical Control of Xanthophyll Cycle Conversions
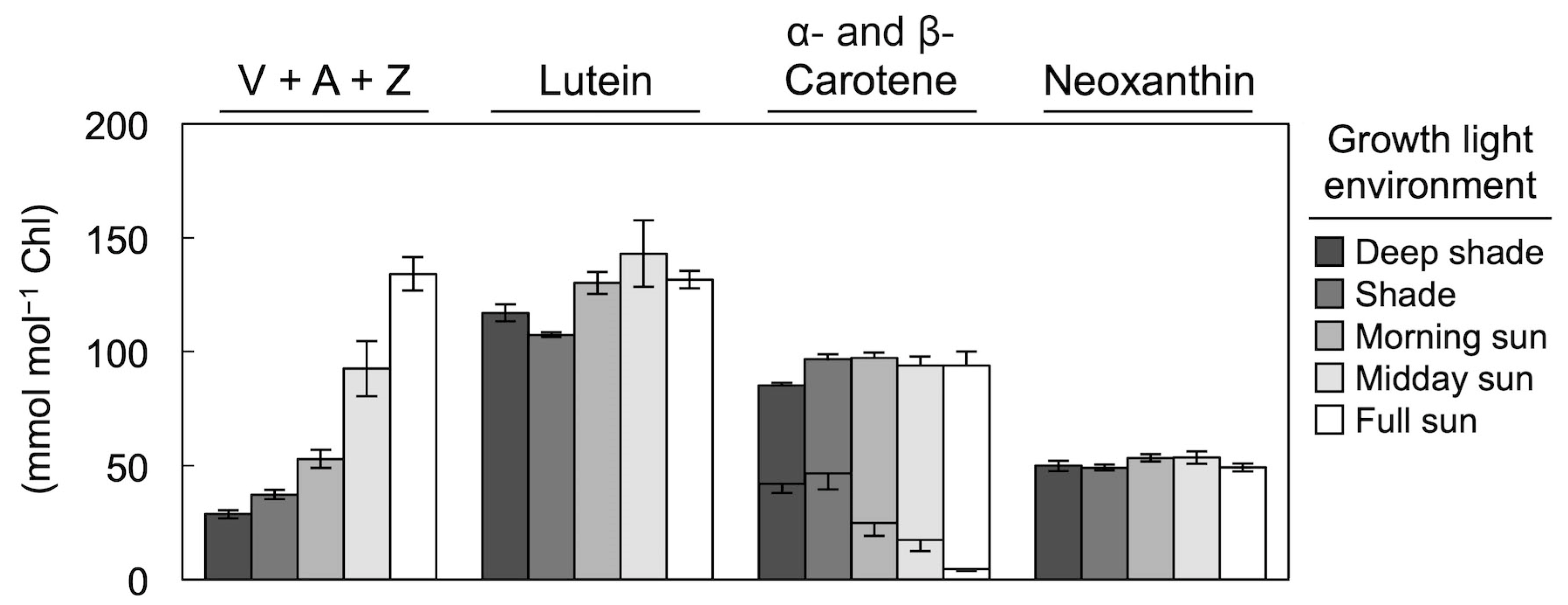
3. Foliar Carotenoid Levels across Multiple Layers of a Rainforest Canopy
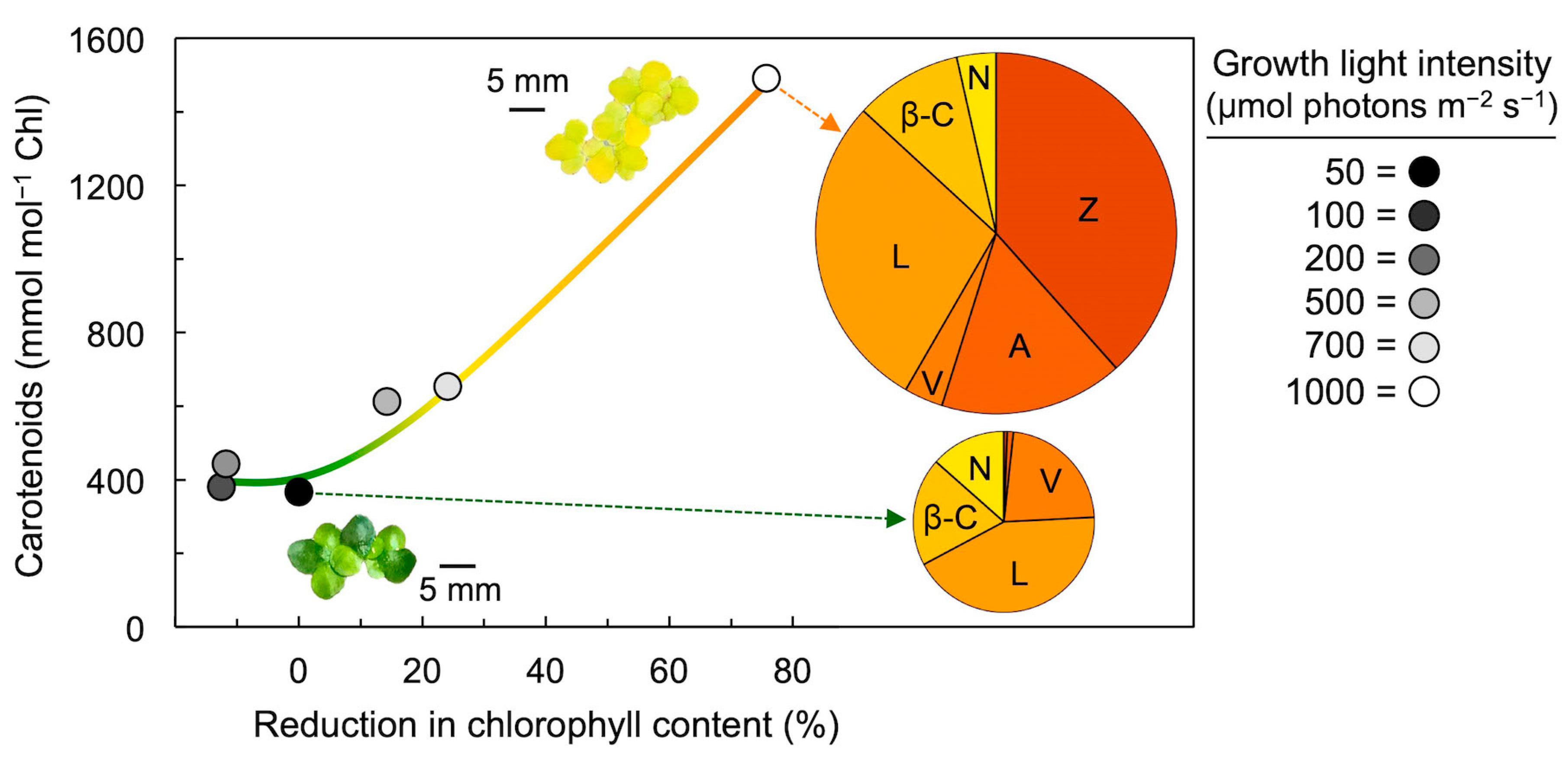
4. Xanthophyll Cycle Conversion State in Response to Growth Light Intensity
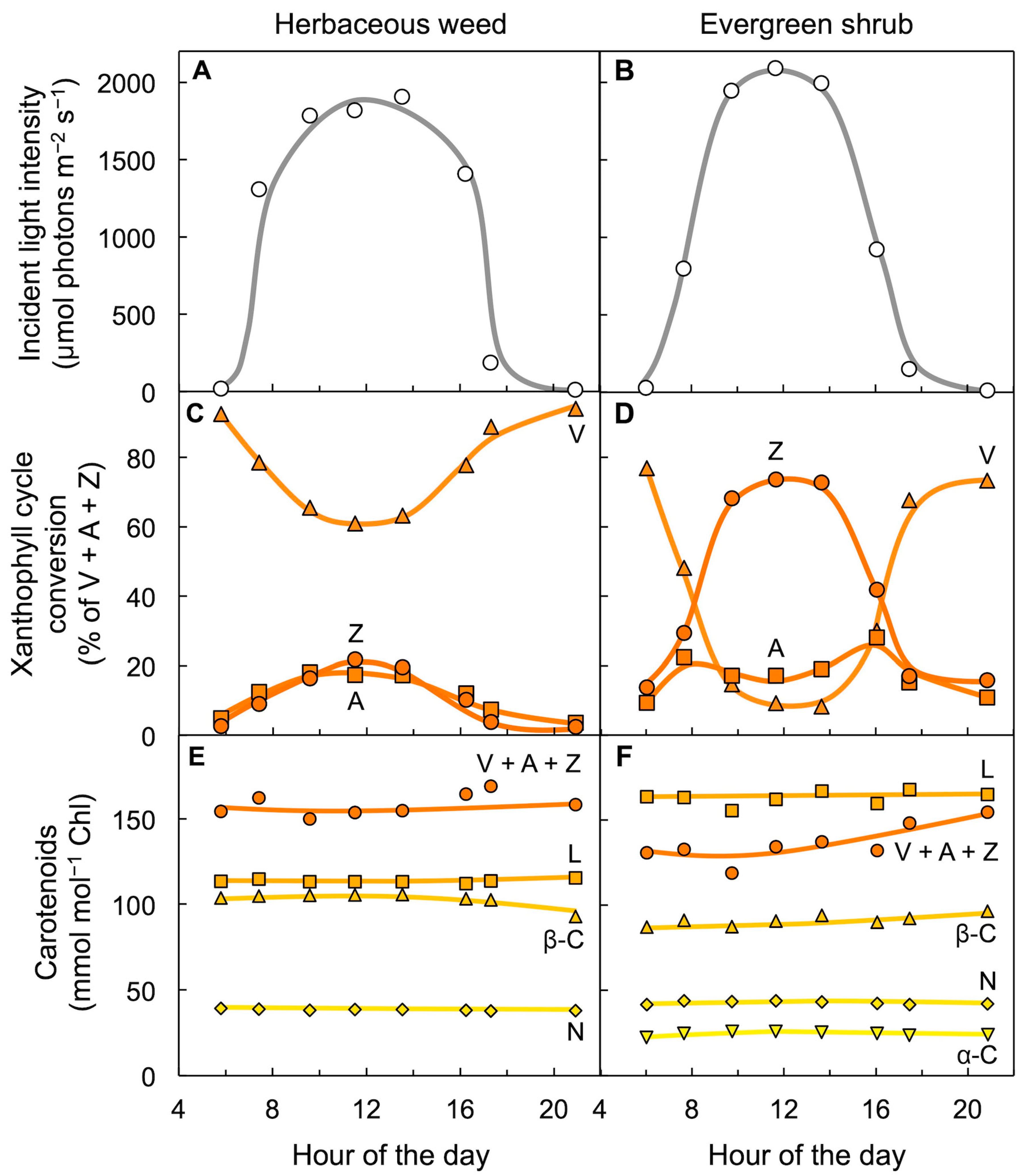
5. Leaves Track Changes in Light Level over the Course of the Day with Adjustments in Xanthophyll Cycle Conversion State
6. Xanthophyll Cycle Conversion State and Dissipation of Unused Light as Thermal Energy
6.1. Harmless Dissipation of Unused Light: The Non-Photochemical Route to Thermal Energy
6.2. Dissipation of Unused Light as Thermal Energy Tracks Xanthophyll Cycle Conversions in Sun-Exposed Plants under Otherwise Favorable Condition
6.3. Light Gradients from Shade to Sun Determine not only Xanthophyll Cycle Dynamics but also Thermal Energy Dissipation Capacity
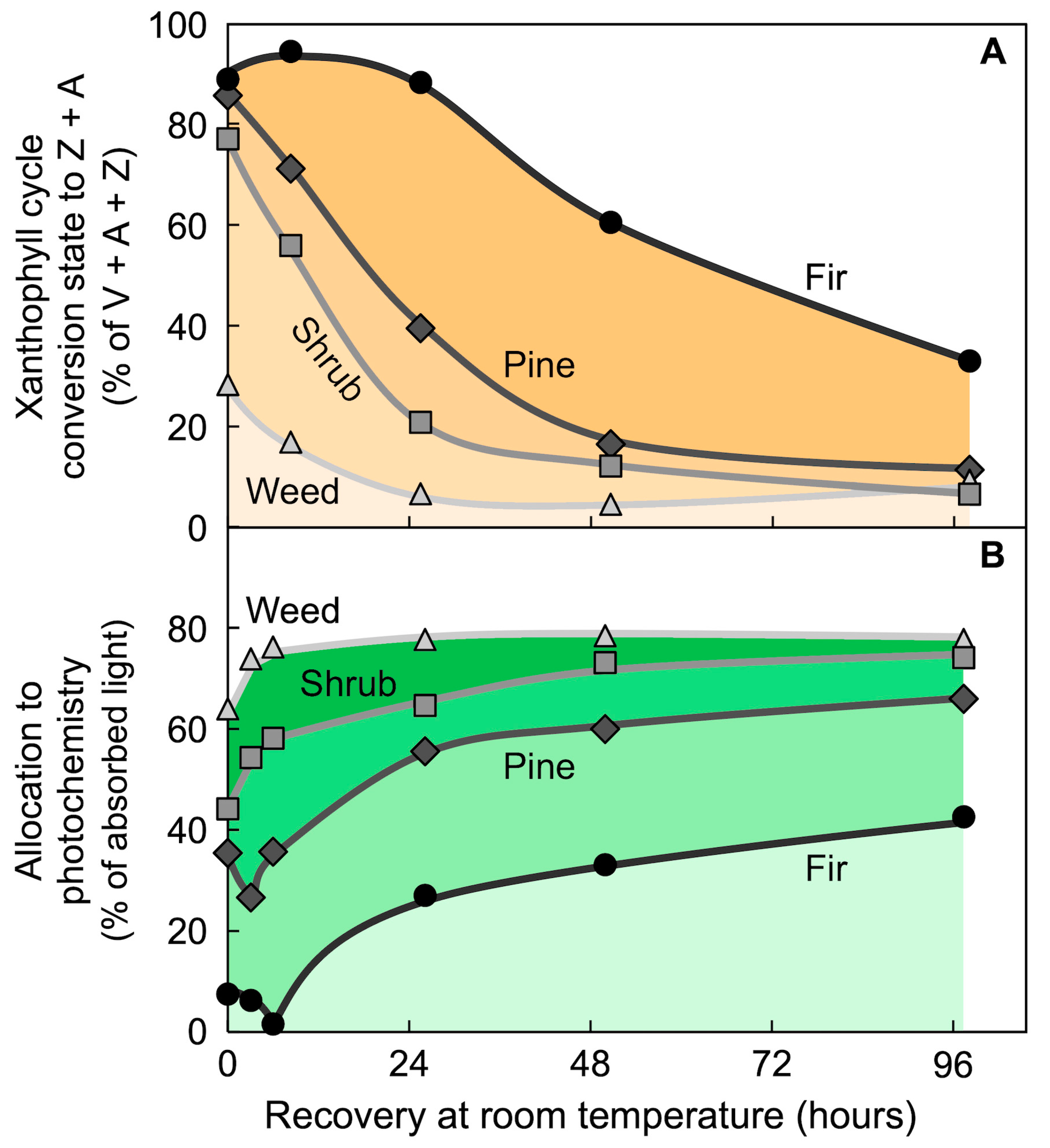
6.4. Thermal Energy Dissipation Activity Changes More Rapidly than Xanthophyll Cycle Conversions in Fluctuating Light Environments
6.5. Environmental Factors other than Light Can Dominate the Response: Mineral Nutrients
6.6. Environmental Factors other than Light Can Dominate the Response: Seasonal Cold
6.7. Environmental Factors other than Light Can Dominate the Response: Seasonal Heat/Aridity
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moreno, J.A.; Díaz-Gómez, J.; Fuentes-Font, L.; Angulo, L.F.; Sandmann, G.; Portero-Otin, M.; Capell, T.; Zhu, C.; Christou, P.; Nogareda, C. Poultry diets containing (keto)carotenoid-enriched maize improve egg yolk color and maintain quality. Anim. Feed Sci. Technol. 2020, 260, 114334. [Google Scholar] [CrossRef]
- Baseggio, J.; Murray, M.; Magallanes-Lundback, M.; Kaczmar, N.; Chamness, J.; Buckler, E.S.; Smith, M.E.; DellaPenna, D.; Tracy, W.F.; Gore, M.A. Natural variation for carotenoids in fresh kernels is controlled by uncommon variants in sweet corn. Plant Genome 2020, 13, e20008. [Google Scholar] [CrossRef]
- Khoo, H.-E.; Prasad, K.N.; Kong, K.-W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Saini, R.K.; Nile, S.H.; Park, S.W. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. Food Res. Intl. 2015, 76, 735–750. [Google Scholar] [CrossRef]
- Ohmiya, A. Diversity of carotenoid composition in flower petals. Jpn. Agric. Res. Q. JARQ 2011, 45, 163–171. [Google Scholar] [CrossRef]
- Ohmiya, A. Qualitative and quantitative control of carotenoid accumulation in flower petals. Sci. Hort. 2013, 163, 10–19. [Google Scholar] [CrossRef]
- Adams, W.W., III; Winter, K.; Schreiber, U.; Schramel, P. Photosynthesis and chlorophyll fluorescence characteristics in relationship to changes in pigment and element composition of leaves of Platanus occidentalis L. during autumnal leaf senescence. Plant Physiol. 1990, 93, 1184–1190. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Hernández, A.; Becerril, J.M. Antioxidant and pigment composition during autumnal leaf senescence in woody deciduous species differing in their ecological traits. Plant Biol. 2003, 5, 557–566. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; López-Pozo, M.; Stewart, J.J.; Adams, W.W., III. Zeaxanthin and lutein: Photoprotectors, anti-inflammatories, and brain food. Molecules 2020, 25, 3607. [Google Scholar] [CrossRef]
- Kromdijk, J.; Glowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Büchel, C. Fucoxanthin-chlorophyll-proteins and non-photochemical fluorescence quenching of diatoms. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 259–275. [Google Scholar] [CrossRef]
- Kirilovsky, D.; Kaňa, R.; Prášil, O. Mechanisms modulating energy arriving at reaction centers in cyanobacteria. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 471–501. [Google Scholar] [CrossRef]
- Lavaud, J.; Goss, R. The peculiar features of non-photochemical fluorescence quenching in diatoms and brown algae. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 421–443. [Google Scholar] [CrossRef]
- Morosinotto, T.; Bassi, R. Molecular mechanisms for the activation of non-photochemical fluorescence quenching from unicellular algae to mosses and higher plants. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 315–331. [Google Scholar] [CrossRef]
- Esteban, R.; Moran, J.F.; Becerril, J.M.; García-Plazaola, J.I. Versatility of carotenoids: An integrated view on diversity, evolution, functional roles and environmental interactions. Environ. Exp. Bot. 2015, 119, 63–75. [Google Scholar] [CrossRef]
- Sapozhnikov, D.I.; Krasovskaya, T.A.; Maevskaya, A.N. Change in the interrelationship of the basic carotenoids of the plastids of green leaves under the action of light. Dokl. Akad. Nauk. USSR 1957, 13, 465–467. [Google Scholar]
- Yamamoto, H.Y. A random walk to and through the xanthophyll cycle. In Photoprotection, Photoinhibition, Gene Regulation, and Environment, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Adams, W.W., III, Mattoo, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 21, pp. 1–10. [Google Scholar] [CrossRef]
- Hager, A. The reversible, light-induced conversions of xanthophylls in the chloroplast. In Pigments in Plants; Czygan, F.-C., Ed.; Fischer: Stuttgart, Germany, 1980; pp. 57–79. [Google Scholar]
- Demmig-Adams, B.; Adams, W.W., III. Light stress and photoprotection related to the xanthophyll cycle. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; Foyer, C., Mullineaux, P., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 105–126. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Matsubara, S.; Osmond, C.B. The lutein epoxide cycle in higher plants: Its relationships to other xanthophyll cycles and possible functions. Funct. Plant Biol. 2007, 34, 759–773. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, S.; Krause, G.H.; Aranda, J.; Virgo, A.; Beisel, K.G.; Jahns, P.; Winter, K. Sun-shade patterns of leaf carotenoid composition in 86 species of neotropical forest plants. Funct. Plant Biol. 2009, 36, 20–36. [Google Scholar] [CrossRef]
- Esteban, R.; García-Plazaola, J.I. Involvement of a second xanthophyll cycle in non-photochemical quenching of chlorophyll fluorescence: The lutein epoxide story. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 277–295. [Google Scholar] [CrossRef]
- Laza-Martínez, A.; Fernández-Marín, B.; García-Plazaola, J.I. Rapid colour changes in Euglena sanguinea (Euglenophyceae) caused by internal lipid globule migration. Eur. J. Phycol. 2019, 54, 91–101. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Gilmore, A.M.; Adams, W.W., III. In vivo functions of carotenoids in higher plants. FASEB J. 1996, 10, 403–412. [Google Scholar] [CrossRef]
- Logan, B.A.; Barker, D.H.; Demmig-Adams, B.; Adams, W.W., III. Acclimation of leaf carotenoid composition and ascorbate levels to gradients in the light environment within an Australian rainforest. Plant Cell Environ. 1996, 19, 1083–1090. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992, 15, 411–419. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Chlorophyll and carotenoid composition in leaves of Euonymus kiautschovicus acclimated to different degrees of light stress in the field. Aust. J. Plant Physiol. 1996, 23, 649–659. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol. 1998, 39, 474–482. [Google Scholar] [CrossRef]
- Niinemets, Ü.; Bilger, W.; Kull, O.; Tenhunen, J.D. Acclimation to high irradiance in temperate deciduous trees in the field: Changes in xanthophyll cycle pool size and in photosynthetic capacity along a canopy light gradient. Plant Cell Environ. 1998, 21, 1205–1218. [Google Scholar] [CrossRef]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; Escobar, C.M.; López-Pozo, M.; Demmig-Adams, B. Growth and essential carotenoid micronutrients in Lemna gibba as a function of growth light intensity. Front. Plant Sci. 2020, 11, 480. [Google Scholar] [CrossRef]
- Stewart, J.J.; Adams, W.W., III; López-Pozo, M.; Garcia, N.D.; McNamara, M.; Escobar, C.M.; Demmig-Adams, B. Comparative assessment of the acclimation of duckweed (Lemna sp.) to light intensity. Cells 2021. manuscript in preparation. [Google Scholar]
- Adams, W.W., III; Demmig-Adams, B. Operation of the xanthophyll cycle in higher plants in response to diurnal changes in incident sunlight. Planta 1992, 186, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, A.S.; Adams, W.W., III; Demmig-Adams, B. The xanthophyll cycle and acclimation of Pinus ponderosa and Malva neglecta to winter stress. Oecologia 1999, 118, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Demmig-Adams, B.; Rosenstiel, T.N.; Brightwell, A.K.; Ebbert, V. Photosynthesis and photoprotection in overwintering plants. Plant Biol. 2002, 4, 545–557. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III; Grace, S.C. Antioxidants and xanthophyll cycle-dependent energy dissipation in Cucurbita pepo L. and Vinca major L. acclimated to four growth PPFDs in the field. J. Exp. Bot. 1998, 49, 1869–1879. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection in an ecological context: The remarkable complexity of thermal energy dissipation. New Phytol. 2006, 172, 11–21. [Google Scholar] [CrossRef] [PubMed]
- García-Plazaola, J.I.; Hernandez, H.; Fernández-Marín, B.; Esteban, R.; Peguero-Pina, J.J.; Verhoeven, A.; Cavender-Bares, J. Photoprotective mechanisms in the genus Quercus in response to winter cold and summer drought. In Oaks Physiological Ecology. Exploring the Functional Diversity of Genus Quercus L., Tree Physiology; Gil-Pelegrín, E., Peguero-Pina, J., Sancho-Knapik, D., Eds.; Springer: Cham, Switzerland, 2017; Volume 7, pp. 361–391. [Google Scholar] [CrossRef]
- Adams, W.W., III; Muller, O.; Cohu, C.M.; Demmig-Adams, B. Photosystem II efficiency and non-photochemical quenching in the context of source-sink balance. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 503–529. [Google Scholar] [CrossRef]
- Adams, W.W., III; Stewart, J.J.; Demmig-Adams, B. Photosynthetic modulation in response to plant activity and environment. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration; Adams, W.W., III, Terashima, I., Eds.; Springer: Cham, Switzerland, 2018; Volume 44, pp. 493–563. [Google Scholar] [CrossRef]
- Adams, W.W., III; Volk, M.; Hoehn, A.; Demmig-Adams, B. Leaf orientation and the response of the xanthophyll cycle to incident light. Oecologia 1992, 90, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.H.; Adams, W.W., III. The xanthophyll cycle and energy dissipation in differently oriented faces of the cactus Opuntia macrorhiza. Oecologia 1997, 109, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Barker, D.H. Seasonal changes in xanthophyll cycle-dependent energy dissipation in Yucca glauca Nuttall. Plant Cell Environ. 1998, 21, 501–512. [Google Scholar] [CrossRef]
- Barker, D.H.; Logan, B.A.; Adams, W.W., III; Demmig-Adams, B. Photochemistry and xanthophyll cycle-dependent energy dissipation in differently oriented cladodes of Opuntia stricta during the winter. Aust. J. Plant Physiol. 1998, 25, 95–104. [Google Scholar] [CrossRef]
- Barker, D.H.; Adams, W.W., III; Demmig-Adams, B.; Logan, B.A.; Verhoeven, A.S.; Smith, S.D. Nocturnally retained zeaxanthin does not remain engaged in a state primed for energy dissipation during the summer in two Yucca species growing in the Mojave Desert. Plant Cell Environ. 2002, 25, 95–103. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Adams, W.W., III; Bilger, W. Context, quantification, and measurement guide for non-photochemical quenching of chlorophyll fluorescence. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 187–201. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Stewart, J.J.; Adams, W.W., III. Chloroplast photoprotection and the trade-off between abiotic and biotic defense. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Grace, S.C. Physiology of light tolerance in plants. Hort. Rev. 1997, 18, 215–246. [Google Scholar] [CrossRef]
- Verhoeven, A.S.; Adams, W.W., III; Demmig-Adams, B. Two forms of sustained xanthophyll cycle-dependent energy dissipation in overwintering Euonymus kiautschovicus. Plant Cell Environ. 1998, 21, 893–903. [Google Scholar] [CrossRef]
- Brooks, M.D.; Jansson, S.; Niyogi, K.K. PsbS-dependent non-photochemical quenching. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 297–314. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Garab, G.; Adams, W.W., III; Govindjee, U. (Eds.) Non-Photochemical Quenching and Thermal Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2014; Volume 40. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Photoinhibition and zeaxanthin formation in intact leaves. A possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol. 1987, 84, 218–224. [Google Scholar] [CrossRef]
- Demmig, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Zeaxanthin and the heat dissipation of excess light energy in Nerium oleander exposed to a combination of high light and drought. Plant Physiol. 1988, 87, 17–24. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Lichtsctreß und Lichtschutz bei Pflanzen. Naturewissenschaften 1989, 76, 262–267. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Winter, K.; Meyer, A.; Schreiber, U.; Pereira, J.; Krüger, A.; Czygan, F.-C.; Lange, O.L. Photochemical efficiency of photosystem II, photon yield of O2 evolution, photosynthetic capacity, and carotenoid composition during the midday depression of net CO2 uptake in Arbutus unedo growing in Portugal. Planta 1989, 177, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Light response of CO2 assimilation, dissipation of excess excitation energy, and zeaxanthin content of sun and shade leaves. Plant Physiol. 1989, 90, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Zeaxanthin and the induction and relaxation kinetics of the dissipation of excess excitation energy in leaves in 2% O2, 0% CO2. Plant Physiol. 1989, 90, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Winter, K.; Krüger, A.; Czygan, F.-C. Zeaxanthin synthesis, energy dissipation, and photoprotection of photosystem II at chilling temperatures. Plant Physiol. 1989, 90, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Polivka, T.; Frank, H.A. Spectroscopic investigation of carotenoids involved in non-photochemical fluorescence quenching. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 203–227. [Google Scholar] [CrossRef]
- Bennet, D.I.G.; Amamath, K.; Park, S.; Steen, C.J.; Morris, J.M.; Fleming, G.R. Models and mechanisms of the rapidly reversible regulation of photosynthetic light harvesting. Open Biol. 2019, 9, 190043. [Google Scholar] [CrossRef]
- Park, S.; Steen, C.J.; Lyska, D.; Fischer, A.L.; Endelman, B.; Iwai, M.; Niyogi, K.K.; Fleming, G.R. Chlorophyll–carotenoid excitation energy transfer and charge transfer in Nannochloropsis oceanica for the regulation of photosynthesis. Proc. Natl. Acad. Sci. USA 2019, 116, 3385–3390. [Google Scholar] [CrossRef]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef]
- Havaux, M.; Niyogi, K.K. The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc. Natl. Acad. Sci. USA 1999, 96, 8762–8767. [Google Scholar] [CrossRef]
- Havaux, M.; García-Plazaola, J.I. Beyond non-photochemical fluorescence quenching: The overlapping antioxidant functions of zeaxanthin and tocopherols. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 583–603. [Google Scholar] [CrossRef]
- Grudzinski, W.; Nierzwicki, L.; Welc, R.; Reszczynska, E.; Luchowski, R.; Czub, J.; Gruszecki, W.I. Localization and orientation of xanthophylls in a lipid bilayer. Sci. Rep. 2017, 7, 9619. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W., III; Barker, D.H.; Logan, B.A.; Bowling, D.R.; Verhoeven, A.S. Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol. Plant. 1996, 98, 253–264. [Google Scholar] [CrossRef]
- Logan, B.A.; Barker, D.H.; Adams, W.W., III; Demmig-Adams, B. The response of xanthophyll cycle-dependent energy dissipation in Alocasia brisbanensis to sunflecks in a subtropical rainforest. Aust. J. Plant Physiol. 1997, 24, 27–33. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B.; Logan, B.A.; Barker, D.H.; Osmond, C.B. Rapid changes in xanthophyll cycle-dependent energy dissipation and photosystem II efficiency in two vines, Stephania japonica and Smilax australis, growing in the understory of an open Eucalyptus forest. Plant Cell Environ. 1999, 22, 125–136. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Atherton, J.; Olascoaga, B.; Kolari, P.; Porcar-Castell, A.; García-Plazaola, J.I. When the sun never sets: Daily changes in pigment composition in three subarctic woody plants during the summer solstice. Trees 2018, 32, 615–630. [Google Scholar] [CrossRef]
- Niyogi, K.K.; Grossman, A.R.; Björkman, O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 1998, 10, 1121–1143. [Google Scholar] [CrossRef] [PubMed]
- Külheim, C.; Ågren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Moeller, D.L.; Logan, B.A.; Adams, W.W., III. Positive correlation between levels of retained zeaxanthin plus antheraxanthin and degree of photoinhibition in shade leaves of Schefflera arboricola (Hayata) Merrill. Planta 1998, 205, 367–374. [Google Scholar] [CrossRef]
- Logan, B.A.; Demmig-Adams, B.; Rosenstiel, T.N.; Adams, W.W., III. Effect of nitrogen limitation on foliar antioxidants in relationship to other metabolic characteristics. Planta 1999, 209, 213–220. [Google Scholar] [CrossRef]
- Krapp, A.; Stitt, M. An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: Changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 1995, 195, 313–323. [Google Scholar] [CrossRef]
- Verhoeven, A.S.; Demmig-Adams, B.; Adams, W.W., III. Enhanced employment of the xanthophyll cycle and thermal energy dissipation in spinach exposed to high light and nitrogen stress. Plant Physiol. 1997, 113, 817–824. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, J.; Abadía, A. Thermal energy dissipation in plant under unfavorable soil conditions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 605–630. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Chlorophyll fluorescence as a tool to monitor plant response to the environment. In Chlorophyll a Fluorescence: A Signature of Photosynthesis, Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 583–604. [Google Scholar] [CrossRef]
- Adams, W.W., III; Zarter, C.R.; Mueh, K.E.; Amiard, V.; Demmig-Adams, B. Energy dissipation and photoinhibition: A continuum of photoprotection. In Photoprotection, Photoinhibition, Gene Regulation, and Environment, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Adams, W.W., III, Mattoo, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 21, pp. 49–64. [Google Scholar] [CrossRef]
- Zarter, C.R.; Adams, W.W., III; Ebbert, V.; Cuthbertson, D.; Adamska, I.; Demmig-Adams, B. Winter down-regulation of intrinsic photosynthetic capacity coupled with up-regulation of Elip-like proteins and persistent energy dissipation in a subalpine forest. New Phytol. 2006, 172, 272–282. [Google Scholar] [CrossRef]
- Zarter, C.R.; Adams, W.W., III; Ebbert, V.; Adamska, I.; Jansson, S.; Demmig-Adams, B. Winter acclimation of PsbS and related proteins in the evergreen Arctostaphylos uva-ursi as influenced by altitude and light environment. Plant Cell Environ. 2006, 29, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Cohu, C.M.; Muller, O.; Adams, W.W., III. Modulation of photosynthetic energy conversion efficiency in nature: From seconds to seasons. Photosynth. Res. 2012, 113, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Koh, S.C.; Cohu, C.M.; Muller, O.; Stewart, J.J.; Adams, W.W., III. Non-photochemical quenching in contrasting plant species and environments. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 531–552. [Google Scholar] [CrossRef]
- Adams, W.W., III; Demmig-Adams, B. Lessons from nature: A personal perspective. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria, Advances in Photosynthesis and Respiration; Demmig-Adams, B., Garab, G., Adams, W.W., III, Govindjee, U., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 40, pp. 45–72. [Google Scholar] [CrossRef]
- Zarter, C.R.; Demmig-Adams, B.; Ebbert, V.; Adamska, I.; Adams, W.W., III. Photosynthetic capacity and light harvesting efficiency during the winter-to-spring transition in subalpine conifers. New Phytol. 2006, 172, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.W., III; Zarter, C.R.; Ebbert, V.; Demmig-Adams, B. Photoprotective strategies of overwintering evergreens. BioScience 2004, 54, 41–49. [Google Scholar] [CrossRef]
- Adams, W.W., III; Watson, A.M.; Mueh, K.E.; Amiard, V.; Turgeon, R.; Ebbert, V.; Logan, B.A.; Combs, A.F.; Demmig-Adams, B. Photosynthetic acclimation in the context of structural constraints to carbon export from leaves. Photosynth. Res. 2007, 94, 455–466. [Google Scholar] [CrossRef]
- Adams, W.W., III; Muller, O.; Cohu, C.M.; Demmig-Adams, B. May photoinhibition be a consequence, rather than a cause, of limited plant productivity? Photosynth. Res. 2013, 117, 31–44. [Google Scholar] [CrossRef]
- Monson, R.K.; Turnipseed, A.A.; Sparks, J.P.; Harley, P.C.; Scott-Denton, L.E.; Sparks, K.; Huxman, T.E. Carbon sequestration in a high-elevation, subalpine forest. Glob. Chang. Biol. 2002, 8, 459–478. [Google Scholar] [CrossRef]
- Míguez, F.; Fernández-Marín, B.; Becerril, J.M.; García-Plazaola, J.I. Diversity of winter photoinhibitory responses: A case study in co-occurring lichens, mosses, herbs and woody plants from subalpine environments. Physiol. Plant. 2017, 160, 282–296. [Google Scholar] [CrossRef]
- Verhoeven, A.; García-Plazaola, J.I.; Fernández-Marín, B. Shared mechanisms of photoprotection in photosynthetic organisms tolerant to desiccation or to low temperature. Environ. Exp. Bot. 2018, 154, 66–79. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Nadal, M.; Gago, J.; Fernie, A.R.; López-Pozo, M.; Artetxe, U.; García-Plazaola, J.I.; Verhoeven, A. Born to revive: Molecular and physiological mechanisms of double tolerance in a paleotropical and resurrection plant. New Phytol. 2020, 226, 741–759. [Google Scholar] [CrossRef]
- Verhoeven, A.S.; Adams, W.W., III; Demmig-Adams, B. Close relationship between the state of the xanthophyll cycle pigments and photosystem II efficiency during recovery from winter stress. Physiol. Plant. 1996, 96, 567–576. [Google Scholar] [CrossRef]
- Havaux, M.; Tardy, F.; Ravenel, J.; Chanu, D.; Parot, P. Thylakoid membrane stability to heat stress studied by flash spectroscopic measurements of the electrochromic shift in intact potato leaves: Influence of the xanthophyll content. Plant Cell Environ. 1996, 19, 1359–1368. [Google Scholar] [CrossRef]
- Havaux, M. Carotenoids as membrane stabilizers in chloroplasts. Trends Plant Sci. 1998, 3, 147–151. [Google Scholar] [CrossRef]
- Davison, P.A.; Hunter, C.N.; Horton, P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature 2002, 418, 203–206. [Google Scholar] [CrossRef]
- García-Plazaola, J.I.; Esteban, R.; Hormaetxe, K.; Fernández-Marín, B.; Becerril, J.M. Photoprotective responses of Mediterranean and Atlantic trees to the extreme heat-wave of summer 2003 in Southwestern Europe. Trees 2008, 22, 385–392. [Google Scholar] [CrossRef]
- Ebbert, V.; Adams, W.W., III; Mattoo, A.K.; Sokolenko, A.; Demmig-Adams, B. Up-regulation of a photosystem II core protein phosphatase inhibitor and sustained D1 phosphorylation in zeaxanthin-retaining, photoinhibited needles of overwintering Douglas fir. Plant Cell Environ. 2005, 28, 232–240. [Google Scholar] [CrossRef]
- Wang, X.; Peng, Y.H.; Singer, J.W.; Fessehaie, A.; Krebs, S.I.; Arora, R. Seasonal changes in photosynthesis, antioxidant systems and ELIP expression in a thermonastic and non-thermonastic Rhododendron species: A comparison of photoprotective strategies in overwintering plants. Plant Sci. 2009, 177, 607–617. [Google Scholar] [CrossRef]
- Malnoë, A. Photoinhibition or photoprotection of photosynthesis? Update on the (newly termed) sustained quenching component qH. Environ. Exp. Bot. 2018, 154, 123–133. [Google Scholar] [CrossRef]












Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Demmig-Adams, B.; Stewart, J.J.; López-Pozo, M.; Polutchko, S.K.; Adams, W.W., III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules 2020, 25, 5825. https://doi.org/10.3390/molecules25245825
Demmig-Adams B, Stewart JJ, López-Pozo M, Polutchko SK, Adams WW III. Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules. 2020; 25(24):5825. https://doi.org/10.3390/molecules25245825
Chicago/Turabian StyleDemmig-Adams, Barbara, Jared J. Stewart, Marina López-Pozo, Stephanie K. Polutchko, and William W. Adams, III. 2020. "Zeaxanthin, a Molecule for Photoprotection in Many Different Environments" Molecules 25, no. 24: 5825. https://doi.org/10.3390/molecules25245825
APA StyleDemmig-Adams, B., Stewart, J. J., López-Pozo, M., Polutchko, S. K., & Adams, W. W., III. (2020). Zeaxanthin, a Molecule for Photoprotection in Many Different Environments. Molecules, 25(24), 5825. https://doi.org/10.3390/molecules25245825








