Theoretical Prediction on the New Types of Noble Gas Containing Anions OBONgO− and OCNNgO− (Ng=He, Ar, Kr and Xe)
Abstract
:1. Introduction
2. Methods
3. Results and Discussion
3.1. Structures
3.2. Energetics
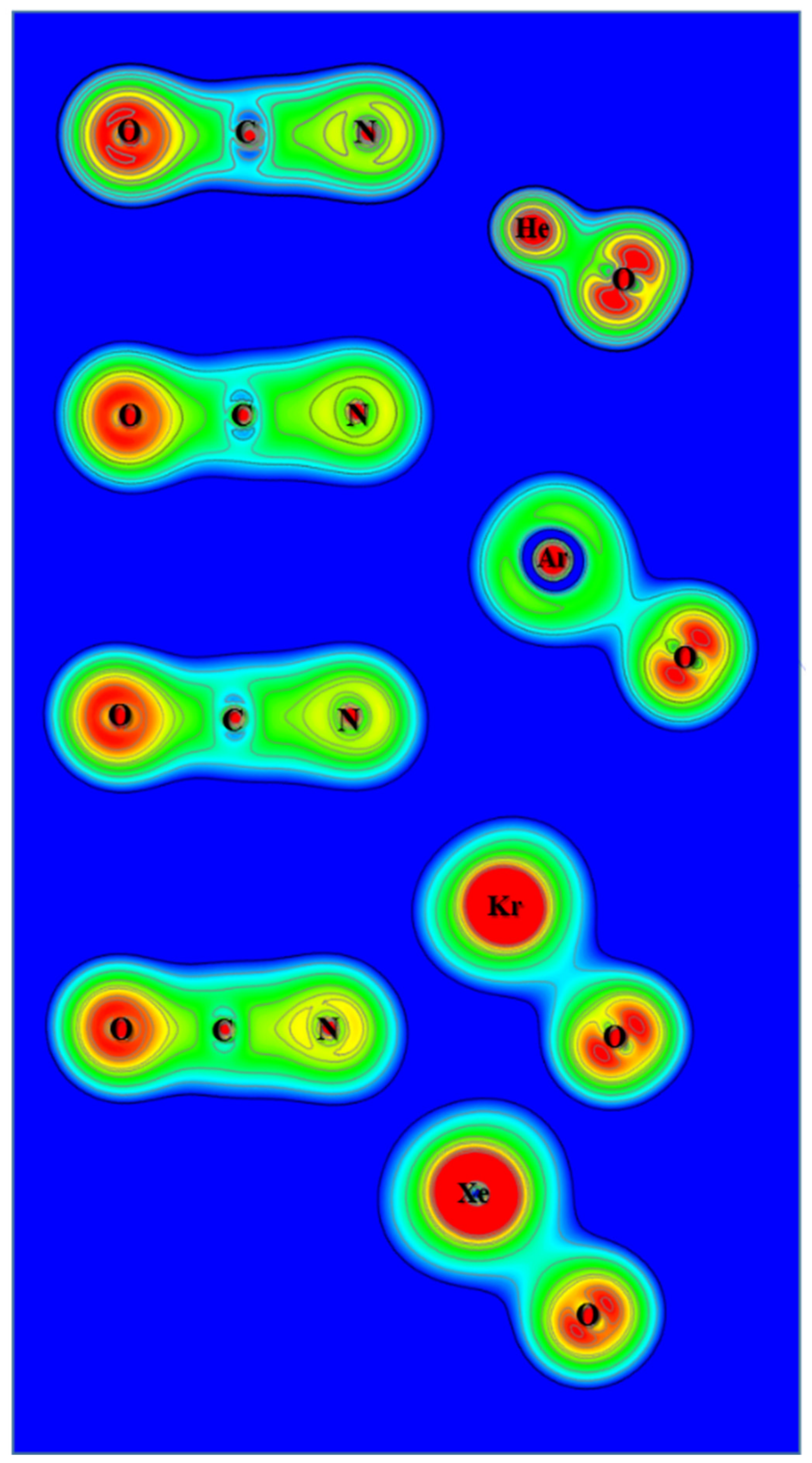
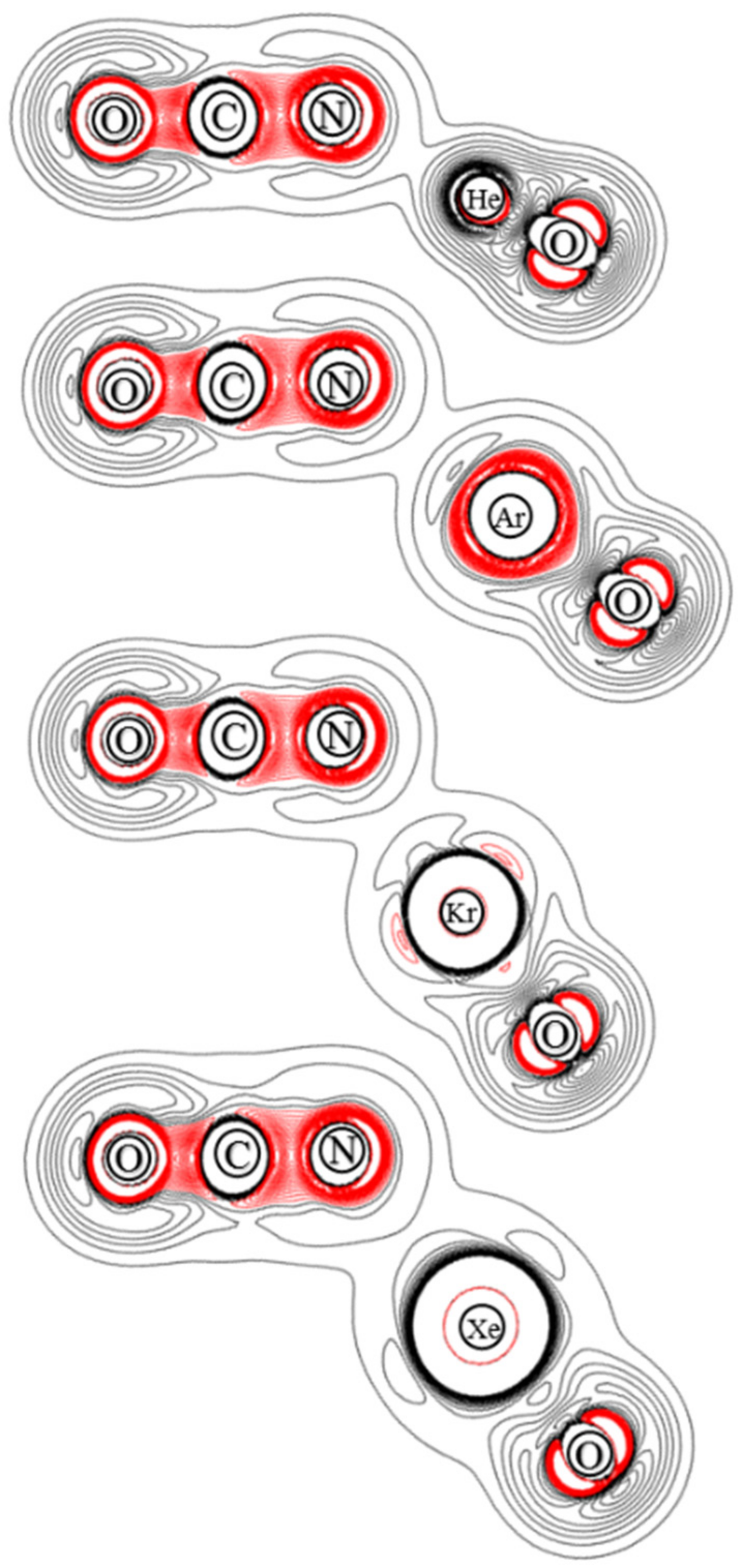
3.3. Electron Density
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bartlett, N. Xenon hexafluoroplatinate (V) Xe+ [PtF6]−. Proc. Chem. Soc. 1962, 218, 197–236. [Google Scholar]
- Agron, P.A.; Begun, G.M.; Levy, H.A.; Mason, A.A.; Jones, C.G.; Smith, D.F. Xenon Difluoride and the Nature of the Xenon-Fluorine Bond. Science 1963, 139, 842–844. [Google Scholar] [CrossRef] [PubMed]
- Claassen, H.H.; Selig, H.; Malm, J.G. Xenon Tetrafluoride. J. Am. Chem. Soc. 1962, 84, 3593. [Google Scholar] [CrossRef]
- Selig, H.; Claassen, H.H.; Chernick, C.L.; Malm, J.G.; Huston, J.L. Xenon Tetroxide: Preparation and Some Properties. Science 1964, 143, 1322–1323. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Lundell, J.; Räsänen, M.; Khriachtchev, L.Y. Neutral rare-gas containing charge-transfer molecules in solid matrices. III. HXeCN, HXeNC, and HKrCN in Kr and Xe. J. Chem. Phys. 1998, 109, 618–625. [Google Scholar] [CrossRef]
- Khriachtchev, L.; Pettersson, M.; Runeberg, N.; Lundell, J.; Räsänen, M. A stable argon compound. Nat. Cell Biol. 2000, 406, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, M.; Khriachtchev, L.Y.; Lignell, A.; Räsänen, M.; Bihary, Z.; Gerber, R.B. HKrF in solid krypton. J. Chem. Phys. 2002, 116, 2508–2515. [Google Scholar] [CrossRef]
- Khriachtchev, L.Y.; Räsänen, M.; Gerber, R.B. Noble-Gas Hydrides: New Chemistry at Low Temperatures. Acc. Chem. Res. 2009, 42, 183–191. [Google Scholar] [CrossRef]
- Arppe, T.; Khriachtchev, L.Y.; Lignell, A.; Domanskaya, A.; Räsänen, M. Halogenated Xenon Cyanides ClXeCN, ClXeNC, and BrXeCN. Inorg. Chem. 2012, 51, 4398–4402. [Google Scholar] [CrossRef]
- Fernandez, I.; Frenking, G. Neutral noble gas compounds exhibiting a Xe–Xe bond: Structure, stability and bonding situation. Phys. Chem. Chem. Phys. 2012, 14, 14869–14877. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wang, X. Infrared Spectra of NgBeS (Ng=Ne, Ar, Kr, Xe) and BeS2 in Noble-Gas Matrices. J. Phys. Chem. A 2013, 117, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Samanta, D. Prediction of Superhalogen-Stabilized Noble Gas Compounds. J. Phys. Chem. Lett. 2014, 5, 3151–3156. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.; Jana, G.; Ravell, E.; Zarate, X.; Osorio, E.; Merino, G.; Chattaraj, P.K. Stable NCNgNSi (Ng=Kr, Xe, Rn) Compounds with Covalently Bound C-Ng-N Unit: Possible Isomerization of NCNSi through the Release of the Noble Gas Atom. Chem. Eur. J. 2018, 24, 2879–2887. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.Y.; Li, J.-F.; Zhao, R.F.; Luo, L.; Wang, Y.-C.; Yin, B. Exploring the structure, bonding and stability of noble gas compounds promoted by superhalogens. A case study on HNgMX3 (Ng=Ar–Rn, M=Be–Ca, X=F–Br) via combined high-level ab initio and DFT calculations. Phys. Chem. Chem. Phys. 2019, 21, 19104–19114. [Google Scholar] [CrossRef]
- Abdeveiszadeh, Z.; Noorizadeh, S. Theoretical investigation on the structure and stability of some neutral noble gas compounds containing Xe–Xe bond. Int. J. Quantum Chem. 2020, 120, e26185. [Google Scholar] [CrossRef]
- Wang, X.; Andrews, L.; Li, J.; Bursten, B.E. Significant Interactions between Uranium and Noble-Gas Atoms: Coordination of the UO2+ Cation by Ne, Ar, Kr, and Xe Atoms. Angew. Chem. Int. Ed. 2004, 43, 2554–2557. [Google Scholar] [CrossRef]
- Borocci, S.; Bronzolino, N.; Giordani, M.; Grandinetti, F. Cationic noble gas hydrides: A theoretical investigation of dinuclear HNgFNgH+ (Ng=He–Xe). J. Phys. Chem. A. 2010, 114, 7382–7390. [Google Scholar] [CrossRef]
- Ghosh, A.; Manna, D.; Ghanty, T.K. Theoretical Prediction of Noble Gas Inserted Thioformyl Cations: HNgCS+ (Ng=He, Ne, Ar, Kr, and Xe). J. Phys. Chem. A 2015, 119, 2233–2243. [Google Scholar] [CrossRef]
- Ghosh, A.; Gupta, A.; Gupta, R.; Ghanty, T.K. Noble gas hydrides in the triplet state: HNgCCO+ (Ng=He, Ne, Ar, Kr, and Xe). Phys. Chem. Chem. Phys. 2018, 20, 20270–20279. [Google Scholar] [CrossRef]
- Li, T.H.; Mou, C.H.; Chen, H.R.; Hu, W.P. Theoretical prediction of noble gas containing anions FNgO− (Ng=He, Ar, and Kr). Am. Chem. Soc. 2005, 127, 9241–9245. [Google Scholar] [CrossRef]
- Antoniotti, P.; Borocci, S.; Bronzolino, N.; Cecchi, P.; Grandinetti, F. Noble Gas Anions: A Theoretical Investigation of FNgBN− (Ng=He–Xe). J. Phys. Chem. A 2007, 111, 10144–10151. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-Y.; Yang, C.-Y.; Sun, Y.-L.; Hu, W.-P. Theoretical prediction on the structures and stability of the noble-gas containing anions FNgCC− (Ng=He, Ar, Kr, and Xe). J. Chem. Phys. 2012, 137, 194303. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Hong, J.T.; Hu, W.P. Theoretical Prediction of Stable Noble-Gas Anions XeNO2− and XeNO3− with very Short Xenon − Nitrogen Bond Lengths. J. Phys. Chem. A 2010, 114, 9359–9367. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Ghanty, T.K. Quantum chemical prediction of a superelectrophilic dianion and its binding with noble gas atoms. Chem. Commun. 2019, 55, 14379–14382. [Google Scholar] [CrossRef]
- Joshi, M.; Ghanty, T.K. Unprecedented stability enhancement of multiple charged anion through decoration with negative electron affinity noble gases. Phys. Chem. Chem. Phys. 2020, 22, 13368–13372. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Bai, X.; He, F.; Zhang, X.; Wang, Z.; Zhang, W. Theoretical investigation of the noble gas molecular anions XAuNgX− and HAuNgX− (X=F, Cl, Br; Ng=Xe, Kr, Ar). Struct. Chem. 2012, 23, 1693–1710. [Google Scholar] [CrossRef]
- Borocci, S.; Bronzolino, N.; Grandinetti, F. Noble gas–sulfur anions: A theoretical investigation of FNgS− (Ng=He, Ar, Kr, Xe). Chem. Phys. Lett. 2008, 458, 48–53. [Google Scholar] [CrossRef]
- Rohdenburg, M.; Mayer, M.; Grellmann, M.; Jenne, C.; Borrmann, T.; Kleemiss, F.; Azov, V.A.; Asmis, K.R.; Grabowsky, S.; Warneke, J. Superelectrophilic Behavior of an Anion Demonstrated by the Spontaneous Binding of Noble Gases to [B12Cl11]−. Angew. Chem. Int. Ed. 2017, 56, 7980–7985. [Google Scholar] [CrossRef]
- Mayer, M.; Rohdenburg, M.; van Lessen, V.; Nierstenhöfer, M.C.; Aprà, E.; Grabowsky, S.; Asmis, K.R.; Jenne, C.; Warneke, J. First steps towards a stable neon compound: Observation and bonding analysis of [B12(CN)11Ne]−. Chem. Commun. 2020, 56, 4591–4594. [Google Scholar] [CrossRef] [Green Version]
- Mayer, M.; Van Lessen, V.; Rohdenburg, M.; Hou, G.-L.; Yang, Z.; Exner, R.M.; Aprà, E.; Azov, V.A.; Grabowsky, S.; Xantheas, S.S.; et al. Rational design of an argon-binding superelectrophilic anion. Proc. Natl. Acad. Sci. USA 2019, 116, 8167–8172. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Head-Gordon, M.; Pople, J.A. A direct MP2 gradient method. Chem. Phys. Lett. 1990, 166, 275–280. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Dunning, T.H., Jr.; Peterson, K.A.; Wilson, A.K. Gaussian basis sets for use in correlated molecular calculations. X. The atoms aluminum through argon revisited. J. Chem. Phys. 2001, 114, 9244–9253. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 89, 11623–11627. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. A 2004, 108, 6908–6918. [Google Scholar] [CrossRef]
- Tsai, C.C.; Tsai, Z.Y.; Hu, W.P. A New Database and Benchmark of the Bond Energies of Noble-Gas Containing Molecules. Authorea 2019, 26238. [Google Scholar] [CrossRef]
- Purvis, G.D.; Bartlett, R.J. A full coupled-cluster singles and doubles model: The inclusion of disconnected triples. J. Chem. Phys. 1982, 76, 1910–1918. [Google Scholar] [CrossRef]
- Peterson, K.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically convergent basis sets with relativistic pseudopotentials. II. Small-core pseudopotentials and correlation consistent basis sets for the post-d group 16–18 elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef] [Green Version]
- Peterson, K.A.; Figgen, D.; Dolg, M.; Stoll, H. Energy-consistent relativistic pseudopotentials and correlation consistent basis sets for the 4 d elements Y–Pd. J. Chem. Phys. 2007, 126, 124101. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Chen, J.-L.; Yang, C.-Y.; Lin, H.-J.; Hu, W.-P. Theoretical prediction of new noble-gas molecules FNgBNR (Ng=Ar, Kr, and Xe; R=H, CH3, CCH, CHCH2, F, and OH). Phys. Chem. Chem. Phys. 2013, 15, 9701–9709. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, R.J. Computing and Graphing Highest Density Regions. Am. Stat. 1996, 50, 120–126. [Google Scholar]
- Matta, C.F.; Gillespie, R.J. Understanding and Interpreting Molecular Electron Density Distributions. J. Chem. Educ. 2002, 79, 1141. [Google Scholar] [CrossRef]
- Fux, S.; Kiewisch, K.; Jacob, C.R.; Neugebauer, J.; Reiher, M. Analysis of electron density distributions from subsystem density functional theory applied to coordination bonds. Chem. Phys. Lett. 2008, 461, 353–359. [Google Scholar] [CrossRef]
- Thomas, J.; Walker, N.R.; Cooke, S.A.; Gerry, M.C.L. Microwave Spectra and Structures of KrAuF, KrAgF, and KrAgBr;83Kr Nuclear Quadrupole Coupling and the Nature of Noble Gas−Noble Metal Halide Bonding. J. Am. Chem. Soc. 2004, 126, 1235–1246. [Google Scholar] [CrossRef]
- Lin, T.-Y.; Hsu, J.-B.; Hu, W.-P. Theoretical prediction of new noble-gas molecules OBNgF (Ng=Ar, Kr, and Xe). Chem. Phys. Lett. 2005, 402, 514–518. [Google Scholar] [CrossRef]
- Borocci, S.; Grandinetti, F.; Nunzi, F.; Sanna, N. Classifying the chemical bonds involving the noble-gas atoms. New J. Chem. 2020, 44, 14536–14550. [Google Scholar] [CrossRef]
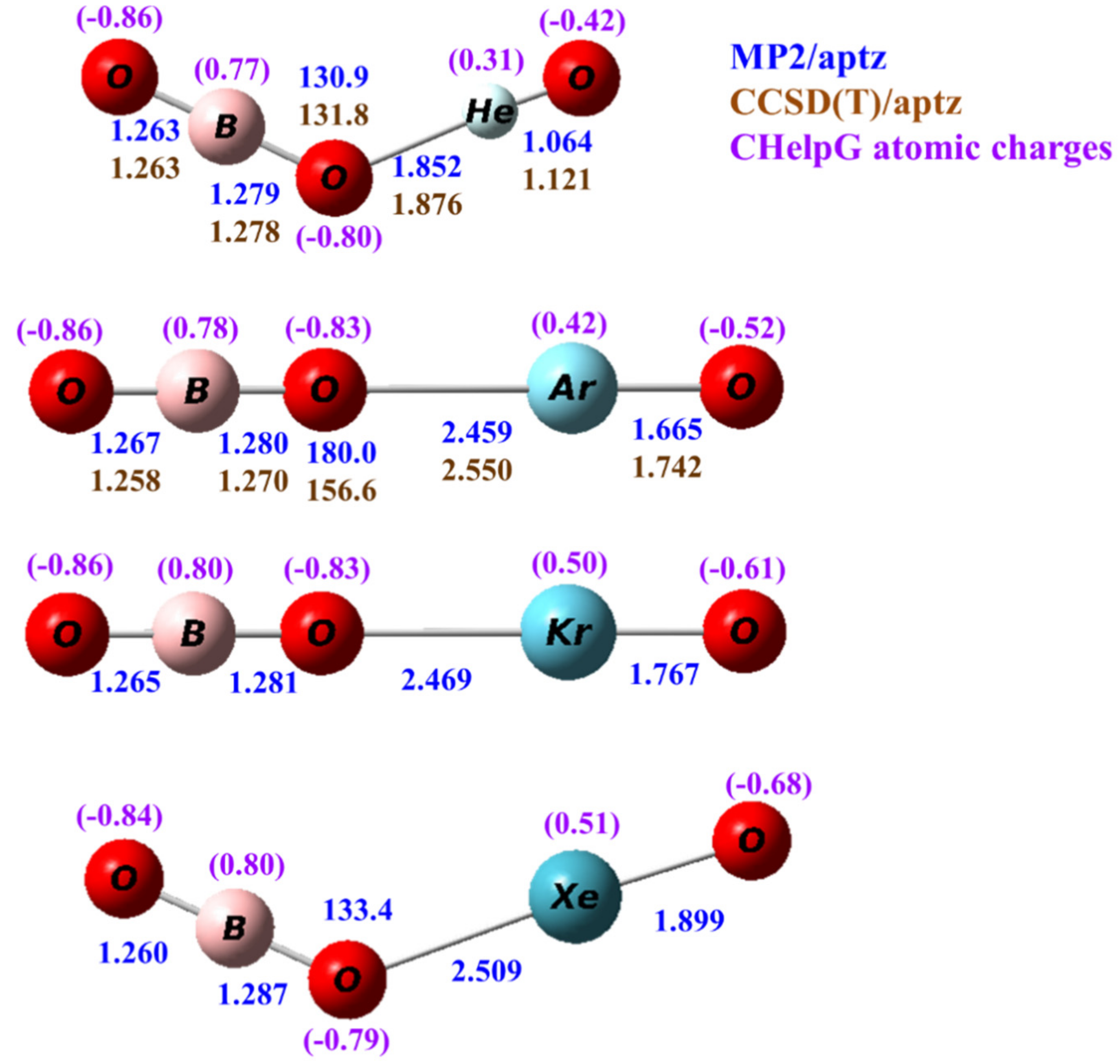


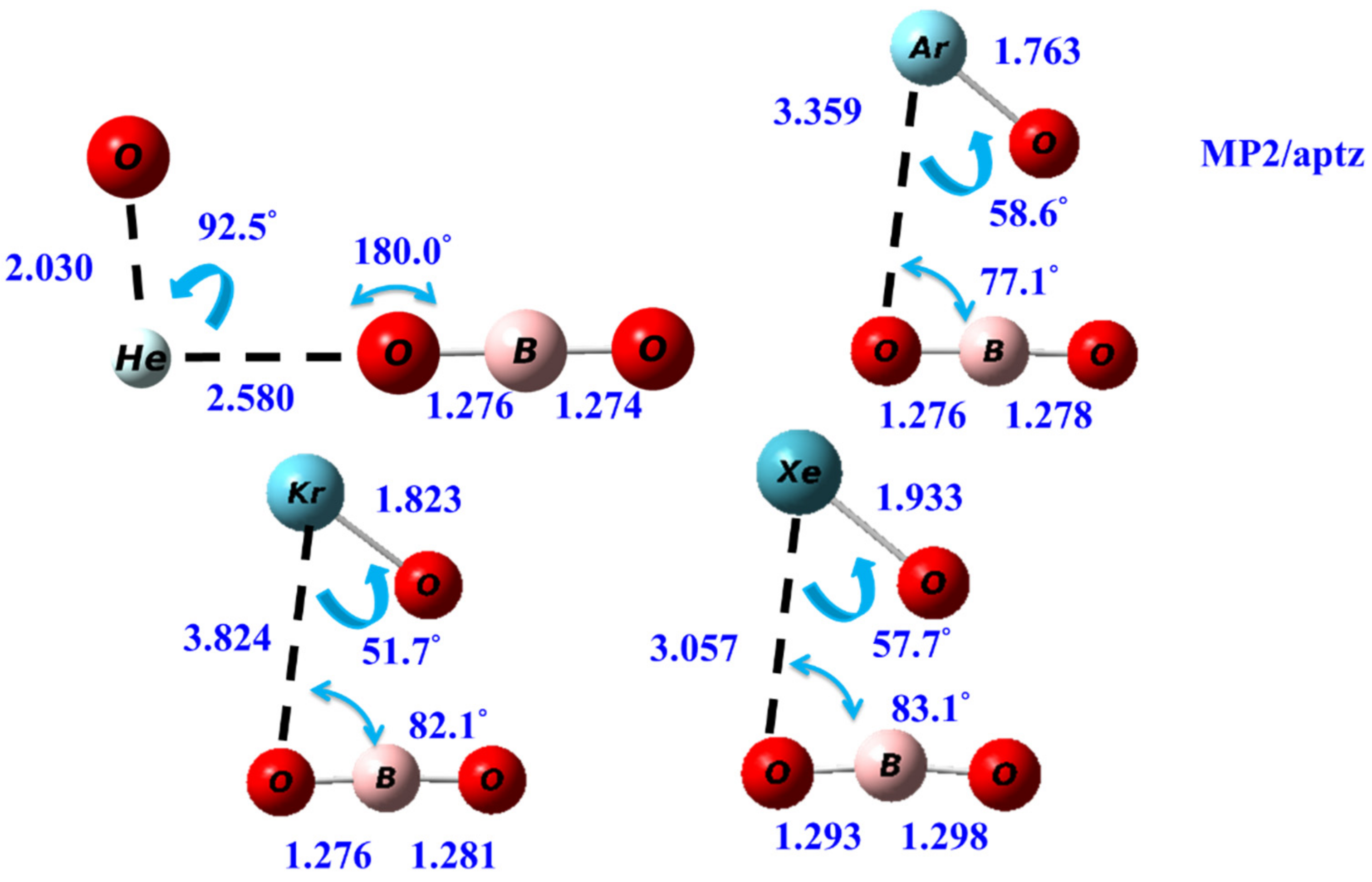
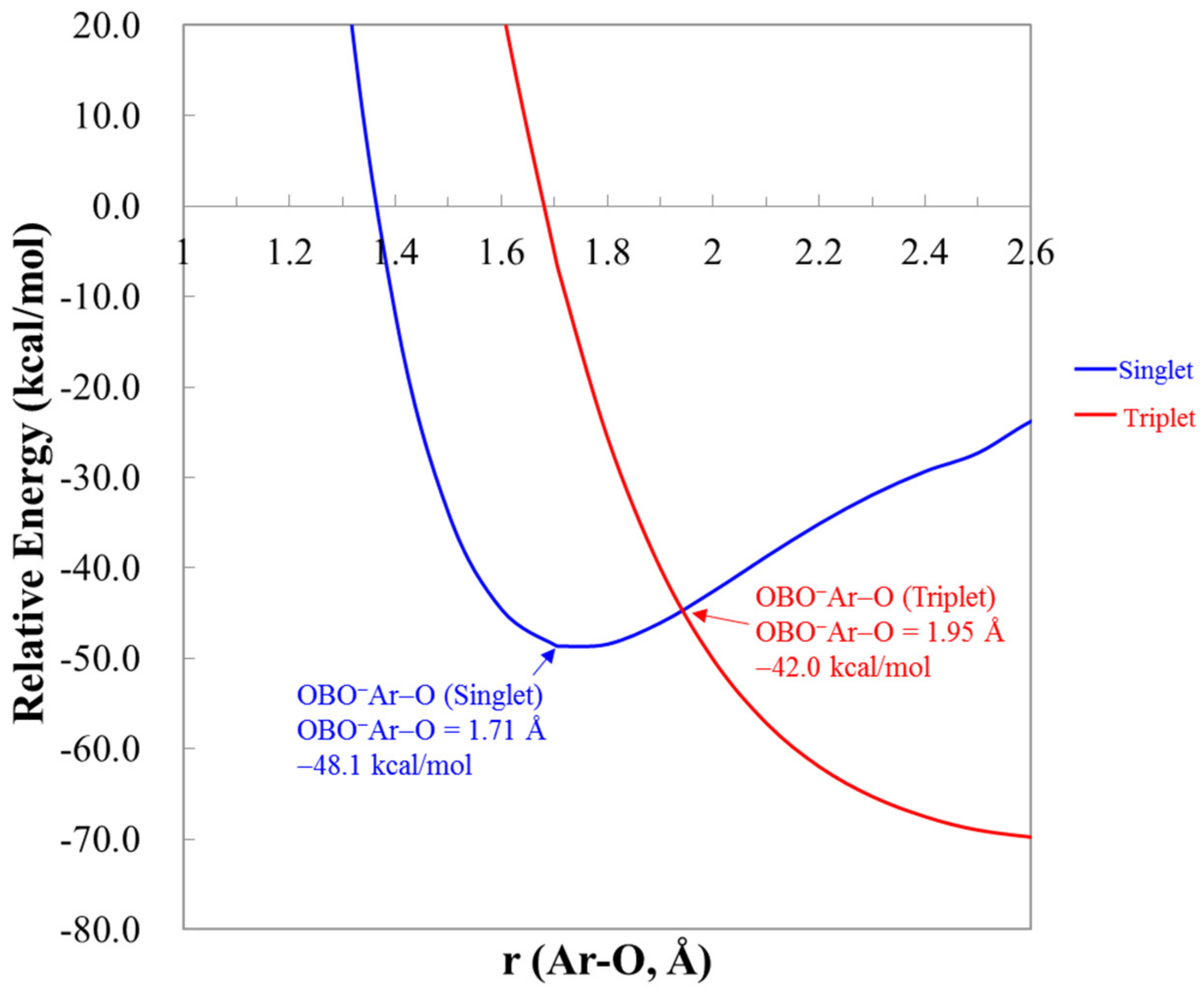

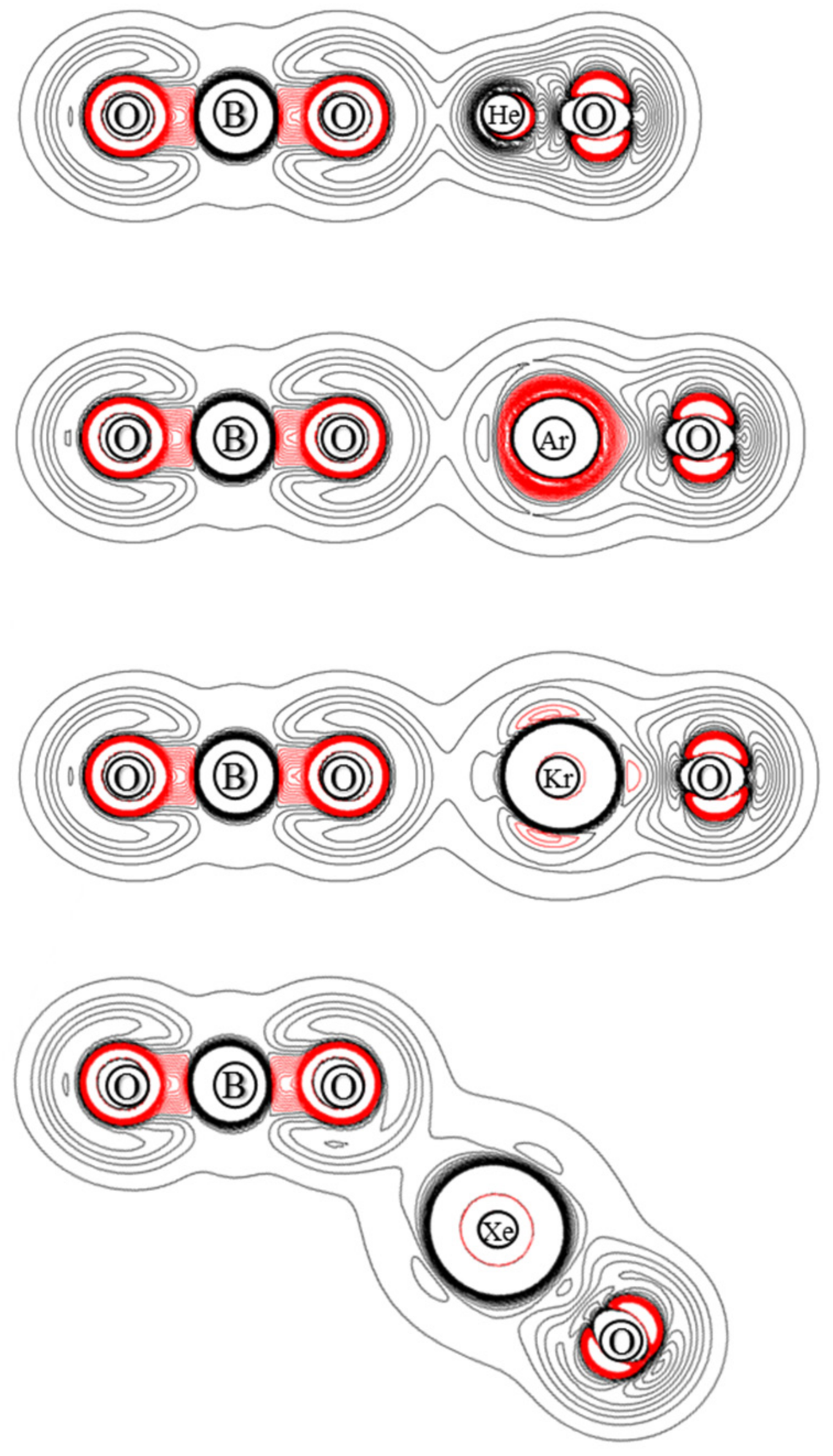
| OBONgO− | OBO− + Ng + O | Ng + BO3− | Barrier | S–T Gap |
|---|---|---|---|---|
| Ng=He | ||||
| MP2/apdz | 15.4 (11.8) | −96.7 (−95.9) | 13.6 (10.4) | 82.5 |
| MP2/aptz | 19.8 (16.1) | −94.7 (−94.0) | 17.6 (14.3) | 95.7 |
| CCSD(T)/aptz | 6.4 (3.8) | −72.6 (−71.8) | N.A. b | 66.3 |
| CCSD(T)/aptz a | 6.1 | −72.1 | 5.2 | 91.7 |
| CCSD(T)/apqz a | 7.4 | −70.3 | 6.4 | 80.2 |
| Ng=Ar | ||||
| MP2/apdz | 32.6 (31.7) | −79.5 (−76.0) | 21.2 (20.4) | 39.4 |
| MP2/aptz | 41.3 (39.5) | −73.2 (−70.6) | 23.6 (22.8) | 42.6 |
| CCSD(T)/aptz | 26.7 (25.0) | −74.8 (−73.5) | N.A. b | 30.4 |
| CCSD(T)/aptz a | 25.5 | −52.6 | 18.1 | 41.1 |
| CCSD(T)/apqz a | 26.5 | −51.2 | 18.5 | 41.3 |
| Ng=Kr | ||||
| MP2/apdz | 51.6 (50.0) | −60.4 (−57.8) | 28.0 (27.4) | 50.5 |
| MP2/aptz | 60.7 (59.0) | −53.7 (−51.0) | 30.0 (29.3) | 62.4 |
| CCSD(T)/aptz a | 42.5 | −35.7 | 24.3 | 58.9 |
| CCSD(T)/apqz a | 43.6 | −34.1 | 24.7 | 49.8 |
| Ng=Xe | ||||
| MP2/apdz | 76.2 (74.4) | −35.9 (−33.3) | 35.7 (34.9) | 67.1 |
| MP2/aptz | 86.8 (85.5) | −27.6 (−24.6) | 37.6 (37.3) | 69.2 |
| CCSD(T)/aptz a | 65.8 | −12.3 | 31.9 | 55.5 |
| CCSD(T)/apqz a | 67.4 | −10.3 | 32.4 | 55.4 |
| OCNNgO− | OCN− + Ng + O | Ng + NC(OO)− | Barrier | S–T Gap |
|---|---|---|---|---|
| Ng=He | ||||
| MP2/apdz | 15.8 (12.1) | −55.7 (−57.9) | 14.0 (11.1) | 85.9 |
| MP2/aptz | 17.4 (16.5) | −58.2 (−57.6) | 17.5 (14.5) | 88.0 |
| CCSD(T)/aptz | 7.0 (3.9) | −54.1 (−55.4) | N.A. b | 69.3 |
| CCSD(T)/aptz a | 6.6 | −53.8 | 5.6 | 115.6 |
| CCSD(T)/apqz a | 7.2 | −53.1 | 11.4 | 89.4 |
| Ng=Ar | ||||
| MP2/apdz | 33.3 (32.1) | −38.3 (−37.9) | 22.1 (21.2) | 41.1 |
| MP2/aptz | 42.3 (40.5) | −33.3 (−33.7) | 24.9 (24.0) | 55.8 |
| CCSD(T)/aptz | 27.3 (25.0) | −36.9 (−34.3) | N.A. b | 34.4 |
| CCSD(T)/aptz a | 26.2 | −34.2 | 19.1 | 42.6 |
| CCSD(T)/apqz a | 26.5 | −33.9 | 19.5 | 42.8 |
| Ng=Kr | ||||
| MP2/apdz | 53.1 (51.0) | −18.5 (−19.0) | 30.0 (29.1) | 52.5 |
| MP2/aptz | 62.6 (60.7) | −13.3 (−13.4) | 32.1 (31.3) | 64.5 |
| CCSD(T)/aptz a | 43.6 | −16.8 | 26.3 | 51.3 |
| CCSD(T)/apqz a | 43.9 | −16.4 | 26.6 | 51.4 |
| Ng=Xe | ||||
| MP2/apdz | 78.3 (76.2) | 6.8 (6.2) | 38.7 (37.9) | 61.6 |
| MP2/aptz | 89.7 (87.7) | 14.1 (13.6) | 41.1 (40.2) | 71.4 |
| CCSD(T)/aptz a | 67.0 | 6.4 | 34.1 | 56.8 |
| CCSD(T)/apqz a | 67.8 | 7.4 | 32.4 | 55.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, C.-C.; Lu, Y.-W.; Hu, W.-P. Theoretical Prediction on the New Types of Noble Gas Containing Anions OBONgO− and OCNNgO− (Ng=He, Ar, Kr and Xe). Molecules 2020, 25, 5839. https://doi.org/10.3390/molecules25245839
Tsai C-C, Lu Y-W, Hu W-P. Theoretical Prediction on the New Types of Noble Gas Containing Anions OBONgO− and OCNNgO− (Ng=He, Ar, Kr and Xe). Molecules. 2020; 25(24):5839. https://doi.org/10.3390/molecules25245839
Chicago/Turabian StyleTsai, Cheng-Cheng, Yu-Wei Lu, and Wei-Ping Hu. 2020. "Theoretical Prediction on the New Types of Noble Gas Containing Anions OBONgO− and OCNNgO− (Ng=He, Ar, Kr and Xe)" Molecules 25, no. 24: 5839. https://doi.org/10.3390/molecules25245839
APA StyleTsai, C.-C., Lu, Y.-W., & Hu, W.-P. (2020). Theoretical Prediction on the New Types of Noble Gas Containing Anions OBONgO− and OCNNgO− (Ng=He, Ar, Kr and Xe). Molecules, 25(24), 5839. https://doi.org/10.3390/molecules25245839








