Bioresearch of New 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones
Abstract
1. Introduction
- (1)
- The type and size of the alkoxy substituent in the 2-position of pyridine, 4-alkoxy derivatives have shown stronger analgesic properties than ethoxy analogs.
- (2)
- The role of the alkyl linker connecting the basic center of the arylamine with the cyclic imide system, 2-hydroxypropyl derivatives being the most active.
- (3)
- The importance of pharmacophoric groups in the phenyl substituent at N-4 of the piperazine ring for the direction and strength of their biological action. Phenyl homologs, unsubstituted, and also containing electron-withdrawing groups such as −CF3, −F, −Cl and −OCH3 were obtained. In some cases, the aryl ring has been replaced by a bioisosterictetrahydroisoquinoline moiety and the piperazine ring replaced by another cyclic amine.
2. Results
2.1. Chemistry
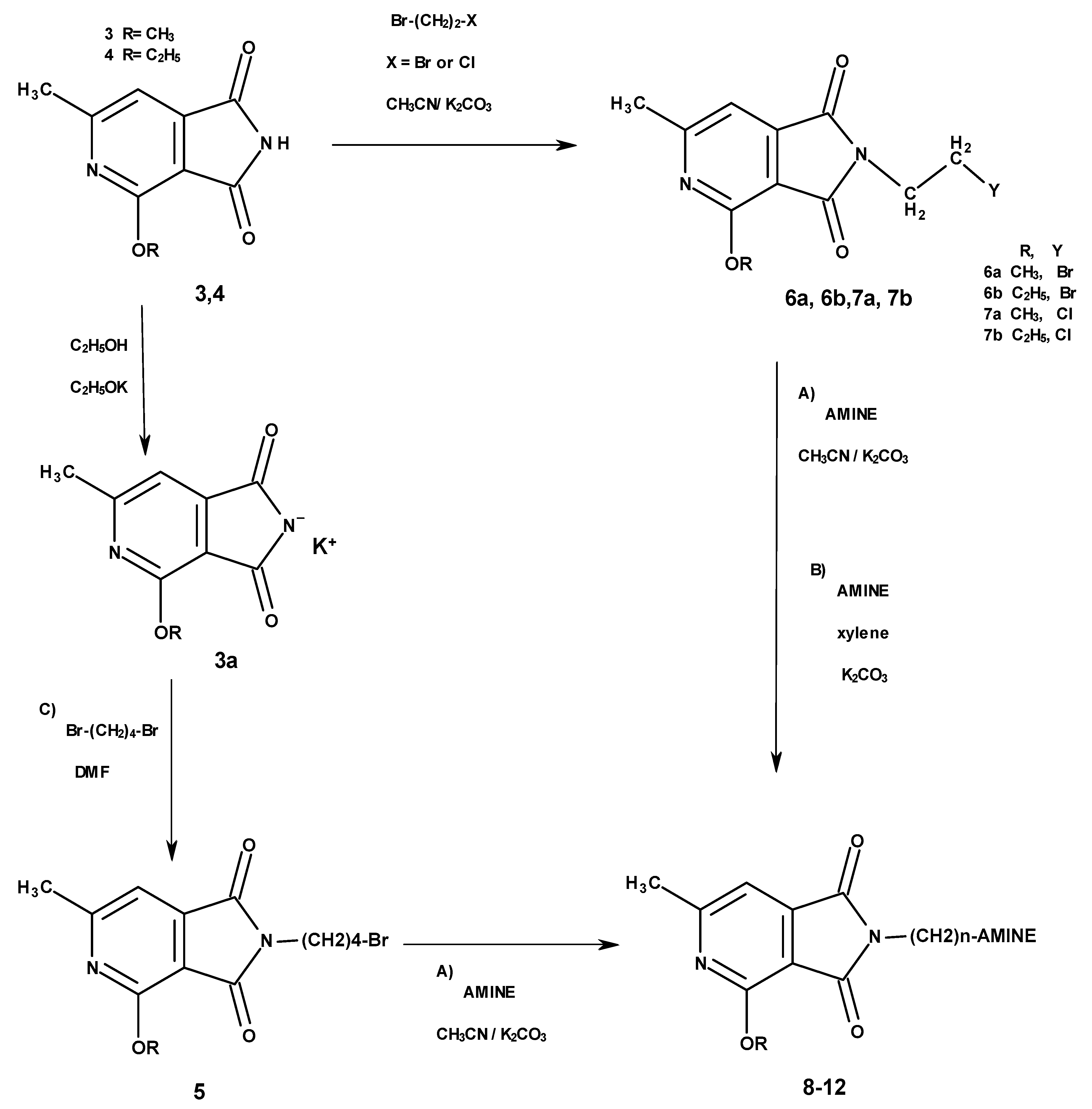
2.1.1. N-Substituted Derivatives of 4-alkoxy-6-methyl-1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones (5, 6a, 6b, 7a, 7b)
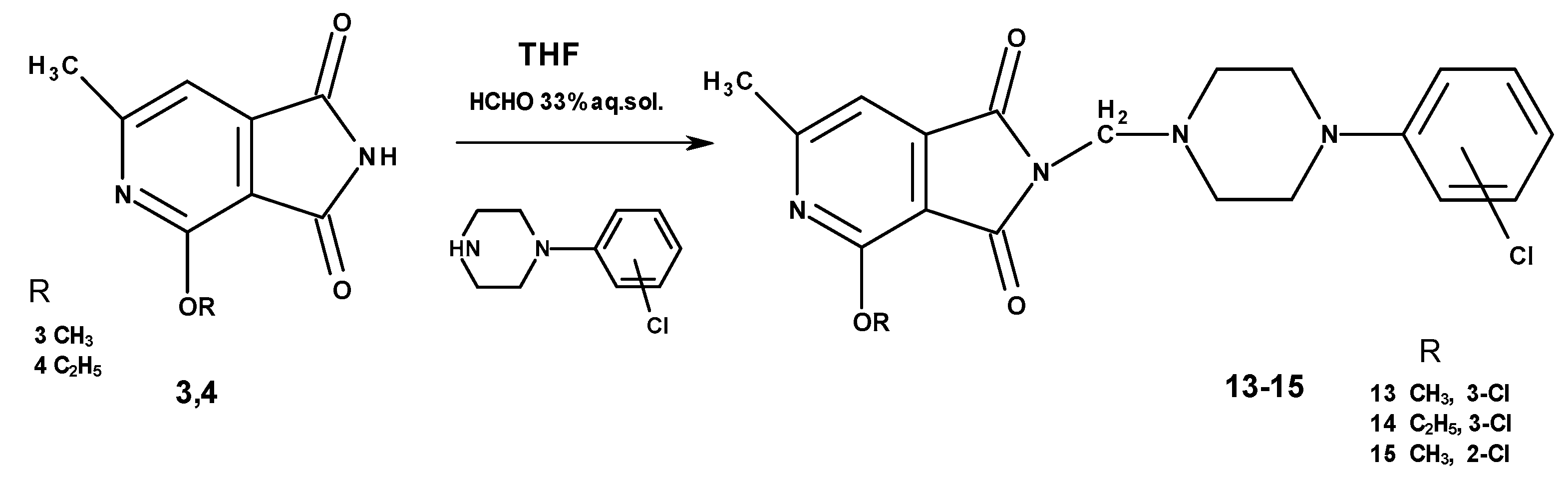
2.1.2. Synthesis of N-Aminomethyl Derivatives with Mannich Base Character (13–15)
2.2. Pharmacology
2.2.1. Toxicity
2.2.2. Analgesic and Sedative Activity
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. Procedure for Obtaining 4-methoxy-6-methyl-4-(N-morpholino)-butyl-1H-pyrrolo[3,4-c]pyridine-1,3-(2H)-dion(8)
4.1.2. Procedure for Obtaining 4-methoxy-/4-ethoxy-6-methyl-2-(2-bromoethylo)-1H-pyrrolo-[3,4-c]pyridine-1,3(2H)-diones (6a, 6b)
4.1.3. Procedure for Obtaining 4-methoxy-/4-ethoxy-6-methyl-2-(2-chloroethylo)-1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones (7a, 7b)
4.1.4. General Procedure for Obtaining Compounds 9–12
4.1.5. General Procedure for Obtaining Compounds 13–15
4.2. Materials and Methods of Pharmacology Experiments
4.2.1. Substances
4.2.2. Animals
4.3. Statistical Analysis
4.4. Acute Toxicity
4.5. Pain Reactivity
4.5.1. “Hot plate” Test
4.5.2. “Writhing” Test in Mice
4.6. Sedative Effect
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Palmer, R.H.C.; McGeary, J.E.; Knopik, V.S.; Bidwell, L.C.; Metrik, J.M. CNR1 and FAAH variation and affective states induced by marijuana smoking. Am. J. Drug Alcohol Abus. 2019, 45, 514–526. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Yoo, J.; Gong, X.; Liu, D.; Han, Q.; Luo, X.; Chang, W.; Chen, G.; Im, S.-T.; Kim, Y.H.; et al. Differential Inhibition of Nav1.7 and Neuropathic Pain by Hybridoma-Produced and Recombinant Monoclonal Antibodies that Target Nav1.7: Differential Activities of Nav1.7-Targeting Monoclonal Antibodies. Neurosci. Bull. 2018, 34, 22–41. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.A.; Bellampalli, S.S.; Dustrude, E.T.; Khanna, R. Mining the Nav1.7 interactome: Opportunities for chronic pain therapeutics. Biochem. Pharmacol. 2019, 163, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Dziubina, A.; Szkatuła, D.; Gdula-Argasińska, J.; Kotańska, M.; Filipek, B. Antinociceptive, antiedematous, and antiallodynic activity of 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-dione derivatives in experimental models of pain. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2019, 393, 813–827. [Google Scholar] [CrossRef] [PubMed]
- Sladowska, H.; Szkatula, D.; Filipek, B.; Maciag, D.; Sapa, J.; Zygmunt, M. ChemInform Abstract: Synthesis and Properties of 2-(4-Substituted)butyl Derivatives of Some 2,3-Dihydro-1,3-dioxo-1H-pyrrolo[3,4-c]pyridines. Chemin 2001, 32, 133–138. [Google Scholar] [CrossRef]
- Śladowska, H.; Filipek, B.; Szkatuła, D.; Sabiniarz, A.; Kardasz, M.; Potoczek, J.; Sieklucka-Dziuba, M.; Rajtar, G.; Kleinrok, Z.; Lis, T. Investigations on the synthesis and pharmacological properties of 4-alkoxy-2-[2-hydroxy-3-(4-aryl-1-piperazinyl)propyl]-6-methyl-1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Farmaco 2002, 57, 897–908. [Google Scholar] [CrossRef]
- Muszalska, I.; Śadowska, H.; Szkatuła, D. A validated spectrophotometric and liquid chromatography method for determination and purity evaluation of 4-methoxy-2-[2-hydroxy-3(4-phenyl-1-piperazinyl)]propyl-2,3-dihydro-6-methyl-1,3-dioxo-1H-pyrrolo[3,4-c]pyridine. Farmaco 2003, 58, 513–519. [Google Scholar] [CrossRef]
- Sladowska, H.; Filipek, B.; Szkatuła, D.; Sapa, J.; Bednarski, M.; Ciołkowska, M. Investigations on the synthesis and pharmacological properties of N-substituted derivatives of 4-alkoxy-6-methyl-1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Farmaco 2005, 60, 53–59. [Google Scholar] [CrossRef]
- Muszalska, I.; Śladowska, H.; Szkatuła, D. Quantitative Determination of 4-Ethoxy-2-[2-Hydroxy -3-(4-Phenyl-1-Piperazinyl)]-Propyl -2,3-Dihydro-6-Methyl-1,3-Dioxo-1H-Pyrrolo-[3,4-c] Pyridine Applying High-Performance Liquid Chromatography Using UV Detection. Studies on Degradation Mechanism. Chem. Anal. 2005, 50, 875–886. [Google Scholar]
- Śladowska, H.; Sabiniarz, A.; Szkatuła, D.; Filipek, B.; Sapa, J. Synthesis and properties of 4-alkoxy-2-[2-hydroxy-3-(4-o,m,p-halogenoaryl-1 -piperazinyl)propyl]-6-methyl-1H-pyrrolo-[3,4-c]pyridine-1,3(2H)-diones with analgesic and sedative activities. Acta Pol. Pharm. -Drug Res. 2007, 63, 245–254. [Google Scholar]
- Muszalska, I.; Górski, P.; Sladowska, H.; Szkatuła, D.; Sabiniarz, A. Chromatographic Separation of Derivatives of 4?Alkoxy?6?methyl?1 H ?pyrrolo[3,4?c]pyridine?1,3(2H)?dione by TLC and HPLC. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 2103–2115. [Google Scholar] [CrossRef]
- Krzyżak, E.; Szkatuła, D.; Szczęśniak-Sięga, B.M.; Malinka, W. Synthesis and DSC study a new pyridinedicarboximide diones derivatives, obtained under various conditions. J. Therm. Anal. Calorim. 2014, 120, 847–853. [Google Scholar] [CrossRef]
- Muszalska, I.; Ciemniejewski, M.P.; Lesniewska, M.A.; Szkatuła, D.; Malinka, W. Forced Degradation and Photodegradation Studies of Pyrrolo[3,4-c]pyridine-1,3-dione Derivatives as Analgesic Active Compounds Using HPLC, UV and IR Spectrometry, and HPLC/MS Methods. J. AOAC Int. 2015, 98, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Krzyżak, E.; Szkatuła, D.; Wiatrak, B.; Gębarowski, T.; Marciniak, A. Synthesis, Cyclooxygenases Inhibition Activities and Interactions with BSA of N-substituted 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones Derivatives. Molecules 2020, 25, 2934. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T.; Wilcoxon, F. A simplified method of evaluating dose-effect experiments. J. Pharmacol. Exp. Ther. 1949, 96, 99–113. [Google Scholar] [PubMed]
- United States Patent Document. Vol. #4205173. Available online: htpp://chem.sis.nlm.nih.gov/chemidplus/[morphine] (accessed on 12 December 2020).
- Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the Most Relevant APIs, 5th ed.; Thieme: Leipzig, Germany, 2009. [Google Scholar]
- Hennies, H.H.; Friderichs, E.; Schneider, J. Receptor binding, analgesic and antitussive potency of tramadol and other selected opioids. Arzneimittelforschung 1988, 38, 877–880. [Google Scholar]
- Raffa, R.B.; Friderichs, E.; Reimann, W.; Shank, R.P.; E Codd, E.; Vaught, J.L. Opioid and nonopioid components independently contribute to the mechanism of action of tramadol, an ’atypical’ opioid analgesic. J. Pharmacol. Exp. Ther. 1992, 260, 275–285. [Google Scholar]
- Gillen, C.; Haurand, M.; Kobelt, D.J.; Wnendt, S. Affinity, potency and efficacy of tramadol and its metabolites at the cloned human µ-opioid receptor. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2000, 362, 116–121. [Google Scholar] [CrossRef]
- Luszczki, J.J.; Borowicz, K.K.; Swiader, M.; Czuczwar, S.J. Interactions Between Oxcarbazepine and Conventional Antiepileptic Drugs in the Maximal Electroshock Test in Mice: An Isobolographic Analysis. Epilepsia 2003, 44, 489–499. [Google Scholar] [CrossRef]
- Eddy, N.B.; Leimbach, D. Synthetic Analgesics. II. Dithienylbutenyl- and Dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953, 107, 385–393. [Google Scholar]
- Hendershot, L.C.; Forsaith, J. Antagonism of the frequency of phenylquinone-induced writhing in the mouse by weak analgesics and nonanalgesics. J. Pharmacol. Exp. Ther. 1959, 125, 237–240. [Google Scholar] [PubMed]

| Compound | R | n | Amine | Compound | R | n | Amine |
|---|---|---|---|---|---|---|---|
| 8 | CH3 | 4 |  | 11 | CH3 | 2 |  |
| 9 | CH3 | 2 | 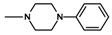 | 12 | C2H5 | 2 | 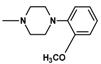 |
| 10 | C2H5 | 2 |  | ||||
| Compound | LD50(mg/kg) |
| 8 | >2000 |
| 9 | 1500 (1395.0–1710.0) |
| 10–15 | >2000 |
| ASA [15] | 167.0 |
| Morphine [16] | 140.0 |

| Compounds | R | n | Amine | ED50 (mg/kg) ± SEM |
|---|---|---|---|---|
| 8 | CH3 | 4 | 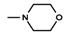 | 14.5 ± 0.03 (11.15–11.28) |
| 9 | CH3 | 2 | 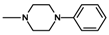 | 3.67 ± 0.49 (2.82–4.77) |
| 10 | C2H5 | 2 | 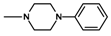 | 15.8 ± 0.91 (14.1–17.7) |
| 11 | CH3 | 2 | 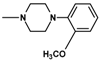 | 3.25 ± 0.80 (2.01–5.16) |
| 12 | C2H5 | 2 | 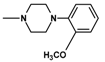 | 14.9 ± 2.01 (11.5–19.4) |
| 13 | CH3 | 1 | 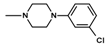 | 14.8 ± 1.40 (12.4–17.9) |
| 14 | C2H5 | 1 | 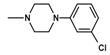 | 18.4 ± 1.73 (15.3–22.1) |
| 15 | CH3 | 1 |  | 19.2 ± 2.14 (14.3–22.7) |
| ASA | 39.15 ± 4.84 (29.1–48.1) | |||
| Morphine | 2.44 ± 0.97 (1.18–5.02) |
| Compounds | Dose (mg/kg) | Prolonged Time (%) | Time of Reaction to Pain Stimulus (s) ± SEM |
|---|---|---|---|
| Control | 0 | 9.57 ± 1.8 | |
| 8 | 200 | 27.48 | 12.2 ± 1.4 |
| 100 | 3.87 | 10.2 ± 1.8 | |
| Control | 0 | 19.5 ± 2.6 | |
| 9 | 300 | 105.1 **** | 40.0 ± 4.4 **** |
| 150 | 101.0 *** | 39.2 ± 5 *** | |
| 75 | 55.38 * | 30.3 ± 2.7 * | |
| Control | 0 | 17.2 ± 2.1 | |
| 10 | 200 | 34.88 | 23.2 ± 2.6 |
| 100 | 6.98 | 18.4 ± 1.7 | |
| Control | 0 | 19.5 ± 2.6 | |
| 11 | 400 | 105.1 ** | 40.0 ± 8.5 ** |
| 200 | 50.7 | 29.4 ± 5.0 | |
| 100 | 23.0 | 24.0 ± 5.0 | |
| Control | 0 | 17.2 ± 2.1 | |
| 12 | 200 | 52.33 | 26.2 ± 3.1 |
| 100 | 28.46 | 22.1 ± 2.7 | |
| Control | 0 | 17.2 ± 2.1 | |
| 13 | 200 | 11.62 | 19.2 ± 1.8 |
| 100 | 4.07 | 17.9 ± 2.4 | |
| 14 | 200 | 16.82 | 20.1 ± 2.3 |
| 100 | 2.32 | 17.6 ± 2.4 | |
| 15 | 200 | 15.69 | 19.9 ± 3.9 |
| 100 | 17.0 ± 2.8 | ||
| Control | 0 | 14.5 ± 3.6 | |
| ASA | 400 | 115.86 ** | 31.3 ± 1.2 ** |
| 200 | 35.17 | 19.6 ± 4.1 | |
| 100 | 11.72 | 16.2 ± 4.9 | |
| Morphine | 6 | 111.10 ** | 30.6 ± 3.9 ** |
| 3 | 104.13 * | 29.6 ± 6 * | |
| 1 | 33.79 | 19.4 ± 2.1 |

| Compounds | R | n | Amine | ED50 (mg/kg) ± SEM |
|---|---|---|---|---|
| 8 | CH3 | 4 |  | 34.2 ± 8.50 (21.37–54.72) |
| 9 | CH3 | 2 | 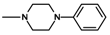 | 18.8 ± 4.00 (12.5–28.2) |
| 10 | C2H5 | 2 |  | 84.0 ± 5.10 (75–95) |
| 11 | CH3 | 2 | 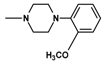 | 19.7 ± 4.89 (12.3 – 31.5) |
| 12 | C2H5 | 2 | 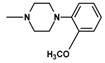 | 85.0 ± 4.20 (77–93.5) |
| 13 | CH3 | 1 |  | 164.0 ± 28.72 (117–229.6) |
| 14 | C2H5 | 1 | 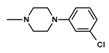 | 98.0 ± 13.26 (75.4–127.4) |
| 15 | CH3 | 1 | 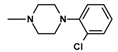 | 89.1 ± 4.46 (80–97.5) |
| Compounds | Dose (mg/kg) | Prolongation (%) | Duration of Anesthesia ± SEM (min) |
|---|---|---|---|
| Control | 0 | 51.5 ±11.2 | |
| 9 | 37.5 | 157.1 ** | 132.4 ± 27.8 ** |
| 18.75 | 96.5 * | 101.2 ± 28.4 * | |
| 9.375 | 34.8 | 69.4 ± 12 | |
| 11 | 50 | 140.8 *** | 124 ± 16.2 *** |
| 25 | 16.11 ** | 50.8 ± 14.2 ** | |
| 12.5 | 36.3 | 70.2 ± 24 |
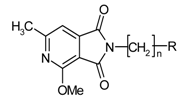
| Compound | R | n | ED50 (mg/kg) |
|---|---|---|---|
| 1H |  | 3 | 1.03 |
| 1P | 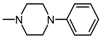 | 4 | 4.5 |
| 9 |  | 2 | 3.67 |
| 1K |  | 1 | 2.55 |
| 1I | 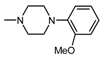 | 3 | 0.67 |
| 1R |  | 4 | 6.8 |
| 11 | 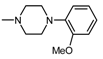 | 2 | 3.25 |
| 1L | 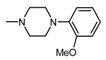 | 1 | 6.53 |
| 1N | 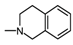 | 4 | 0.72 |
| 1M |  | 1 | 12.7 |
| 8 |  | 4 | 1.5 |
| 1Q |  | 1 | 13.66 |
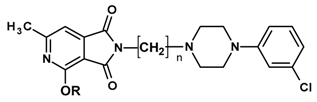
| Comp. | R | n | ED50 (mg/kg) |
|---|---|---|---|
| 1J | CH3 | 3 | 8.8 |
| 2J | C2H5 | 3 | 8.7 |
| 13 | CH3 | 1 | 14.8 |
| 14 | C2H5 | 1 | 18.4 |
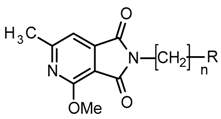
| Compound | R, n | Influence on the Locomotor Activity | Thiopental Anesthesia | |||
|---|---|---|---|---|---|---|
| ED50 (mg/kg) | Dose (mg/kg) | % Inh. | Dose (mg/kg) | % Prolong. | ||
| 1H |  , 3 , 3 | 15.17 | 50 25 12.5 | 66.55 *** 53.84 ** 47.67 * | 50 25 | 194 *** 118 * |
| 1P | 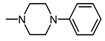 , 4 , 4 | - | 100 50 | 86.10 ** 67.14 * | 100 50 | 242.13 *** 140.25 *** |
| 9 |  , 2 , 2 | 18.8 | 37.5 18.75 9.37 | 67.18 *** 45.90 ** 42.79 * | 37.5 18.75 | 157.1 ** 96.5* |
| 1K | 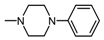 , 1 , 1 | 2.28 | 4.5 2.25 | 61.77 *** 49.23 ** | 4.5 2.25 | 199.1 *** 134.1 * |
| 1I |  3 3 | 11.8 | 50 25 12.5 | 76.69 **** 72.77 **** 53.91 ** | 50 25 12.5 | 207.2 ** 140.3 * 123.3 * |
| 1R | 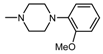 , 4 , 4 | - | 100 | 82.32 ** | 100 50 | 255.97 *** 159.12 *** |
| 11 | 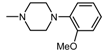 , 2 , 2 | 19.7 | 50 25 12.5 | 68.74 *** 55.85 ** 55.43 * | 50 25 | 140.8 *** 16.11 ** |
| 1L | 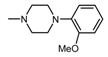 , 1 , 1 | 25.2 | 50 25 12.5 | 68.25 *** 40.84 * 37.81 * | 50 25 12.5 | 184.3 *** 133.2 ** 111.6 * |
| 1N | 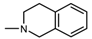 , 4 , 4 | 46 | 87.48 * | 46 23 | 286.5 *** 100.0 * | |
| 1M | 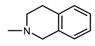 , 1 , 1 | 29.5 | 50 25 | 59.21 *** 47.25 ** | 50 25 | 195.1 *** 146.6 ** |
| 8 | 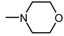 , 4 , 4 | 34.2 | 50 25 12.5 | 61.64 **** 35.36 ** 32.76 ** | - | Not tested |
| 1Q | 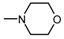 , 1 , 1 | 44 | 42.65 * | 44 | 141.7 ** | |
| Compound | Formula (mol. wt.) | M.p. (°C) Solvent | Yield (%)/Method | IR Absorptions in KBr (cm−1) | ||
|---|---|---|---|---|---|---|
| C=O | CH2 | Mono- and Disubst. Benzene | ||||
| 6a | C11H11BrN2O3 299.13 | 113–114 n-hexane | 42 | 1718 1771 | 2920 2950 | - |
| 6b | C12H13BrN2O3 313.10 | 100–102 Cyclohexane | 45 | 1720 1770 | 2900 2950 | - |
| 7a | C11H11ClN2O3 254.67 | 110–112 Cyclohexane | 79 | 1740 1770 | 2920 2950 | - |
| 7b | C12H13ClN2O3 268.69 | 102–103 Cyclohexane | 81 | 1730 1780 | 2900 2980 | - |
| 8 | C17H23N3O4 333.38 | 92–93 ethanol | 51 | 1720 1780 | 2800 2980 | - |
| 9 | C21H24N4O3 380.43 | 150–152 Cyclohexane | 51.7/A 29/B | 1717 1770 | 2820 2950 | 690,756 |
| 10 | C22H26N4O3 394.47 | 118–120 Cyclohexane | 55/A 35/B | 1715 1770 | 2840 2940 | 690,750 |
| 11 | C22H26N4O4 410.46 | 175–176 Cyclohexane | 48.5/A 32/B | 1714 1769 | 2930 2950 | 748 |
| 12 | C23H28N4O4 424.50 | 165–167 Ethanol/cyhlohexane | 55/A 37/B | 1715 1765 | 2820 2940 | 750 |
| 13 | C20H21ClN4O3 400.5 | 157–160 n-heptane | 65 | 1720 1770 | - | 690,750 |
| 14 | C21H23ClN4O3 414.50 | 127–129 n-heptane | 70 | 1715 1775 | - | 695,750 |
| 15 | C20H21ClN4O3 400.5 | 162–164 n-heptane | 52 | 1720 1770 | - | 750 |
Sample Availability: Samples of the compounds 8–15 are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkatuła, D.; Krzyżak, E.; Mogilski, S.; Sapa, J.; Filipek, B.; Świątek, P. Bioresearch of New 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Molecules 2020, 25, 5883. https://doi.org/10.3390/molecules25245883
Szkatuła D, Krzyżak E, Mogilski S, Sapa J, Filipek B, Świątek P. Bioresearch of New 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Molecules. 2020; 25(24):5883. https://doi.org/10.3390/molecules25245883
Chicago/Turabian StyleSzkatuła, Dominika, Edward Krzyżak, Szczepan Mogilski, Jacek Sapa, Barbara Filipek, and Piotr Świątek. 2020. "Bioresearch of New 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones" Molecules 25, no. 24: 5883. https://doi.org/10.3390/molecules25245883
APA StyleSzkatuła, D., Krzyżak, E., Mogilski, S., Sapa, J., Filipek, B., & Świątek, P. (2020). Bioresearch of New 1H-pyrrolo[3,4-c]pyridine-1,3(2H)-diones. Molecules, 25(24), 5883. https://doi.org/10.3390/molecules25245883







