In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants
Abstract
:1. Introduction
2. Results
2.1. Design of the Drug Reservoirs and Printing of the Samples
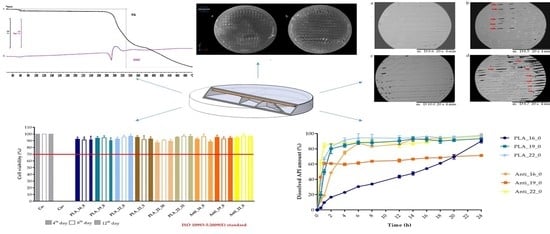
2.2. Content Uniformity and Weight Variation
2.3. PLA Degradation
2.4. Characterization
2.4.1. Thermogravimetric (TG) and Heatflow (Differential Scanning Analyis, DSC) Analysis
2.4.2. Contact Angle Measurement
2.4.3. Scanning Electron Microscopy
2.4.4. Microcomputed Tomography (MicroCT)
2.4.5. Raman Spectroscopy
2.5. In Vitro Dissolution Test
2.6. MTT Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Used Polymer Filaments
4.1.2. Diclofenac Sodium as a Model API
4.2. Methods
4.2.1. Design of the Drug Reservoirs and Printing of the Samples
4.2.2. Content Uniformity and Weight Variation
4.2.3. PLA Degradation
4.2.4. Characterization
Thermogravimetric (TG) and Heatflow (Differential Scanning Analyis, DSC) Analysis
Contact Angle
Scanning Electron Microscopy (SEM)
Microcomputed Tomography (MicroCT)
Raman Spectroscopy
4.2.5. In Vitro Dissolution Test
4.2.6. Cytotoxicity Experiments
Sterilization
Cell Culture
MTT Cell Viability Assay
4.2.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Sample | PLA_16_0 | PLA_19_0 | PLA_22_0 | PLA_22_5 | PLA_22_10 | PLA_22_15 | Anti_16_0 | Anti_19_0 | Anti_22_0 | PETG_16_0 | PETG_19_0 | PETG_22_0 | PMMA_16_0 | PMMA_19_0 | PMMA_22_0 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sampling time (h) | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD | Dissolved API (%) | ±SD |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 0.083 | 0.55 | 0.34 | 3.46 | 1.24 | 20.23 | 6.07 | 3.59 | 1.02 | 0.13 | 0.12 | 0.59 | 0.13 | 5.7 | 3.37 | 17.97 | 2.27 | 36.5 | 4.78 | 5.79 | 3.19 | 20.91 | 4.97 | 1.72 | 0.5 | 7.67 | 1.17 | 3.54 | 0.71 | 14.1 | 1.84 |
| 0.25 | 2.59 | 0.4 | 11.55 | 0.89 | 24.45 | 3.81 | 11.97 | 1.3 | 0.46 | 0.3 | 6.94 | 1.54 | 8.02 | 4.23 | 30.03 | 1.82 | 46.67 | 3.21 | 8.51 | 1.31 | 53.48 | 2.79 | 3.57 | 1.11 | 14.54 | 1.31 | 9.86 | 1.03 | 26.57 | 1.47 |
| 0.5 | 2.55 | 0.34 | 19.68 | 3.95 | 35.44 | 3.07 | 17.12 | 1.22 | 1.72 | 0.71 | 14.74 | 2.98 | 13.01 | 2.94 | 42.96 | 1.34 | 62.22 | 3.26 | 12.83 | 2.06 | 76.07 | 1.86 | 7.69 | 0.84 | 22.51 | 2 | 18.54 | 3.25 | 32.76 | 2.02 |
| 0.75 | 5.4 | 0.46 | 35.66 | 10.96 | 49.04 | 3.14 | 26.21 | 2.26 | 6.31 | 1.47 | 29.32 | 2.37 | 16.39 | 2.54 | 56.85 | 1.6 | 77.44 | 3.42 | 18.58 | 4.42 | 90.47 | 1.35 | 18.17 | 1.62 | 37.04 | 12.42 | 38.28 | 6.32 | 57.01 | 3.08 |
| 1 | 9.6 | 0.88 | 49.66 | 3.37 | 67.22 | 1.73 | 37.27 | 1.66 | 18.75 | 2.75 | 42.43 | 1.4 | 18.91 | 1.26 | 60.96 | 1.73 | 84.59 | 3.1 | 30.12 | 9.25 | 91.29 | 0.83 | 29.23 | 1.1 | 71.8 | 0.83 | 74.17 | 4.62 | 75.32 | 2.99 |
| 2 | 16.65 | 0.92 | 80.04 | 6.51 | 82.95 | 1.24 | 55.67 | 0.43 | 31.61 | 2.83 | 66.73 | 1.72 | 48.61 | 1.41 | 60.71 | 1.09 | 86.47 | 1.17 | 55.65 | 5.32 | 94.22 | 0.43 | 57.7 | 0.88 | 81.38 | 0.43 | 97.23 | 1.1 | 92.95 | 3.41 |
| 4 | 22.9 | 1.02 | 84.5 | 2.48 | 91.53 | 2.8 | 62.3 | 2.05 | 42.65 | 2.28 | 73.99 | 1.38 | 74.9 | 2.25 | 59.66 | 1.69 | 87.6 | 0.96 | 61.55 | 0.95 | 96.33 | 1.69 | 73.75 | 1.55 | 82.85 | 0.56 | 98.83 | 0.61 | 90.6 | 2.15 |
| 6 | 30.49 | 1.55 | 88.14 | 2.4 | 94.45 | 5.32 | 63.94 | 2.89 | 42.22 | 1.66 | 82.99 | 3.48 | 87.33 | 1.82 | 61.34 | 1.73 | 88.51 | 0.37 | 61.97 | 1.42 | 97.94 | 1.52 | 75.29 | 1.06 | 85.44 | 0.88 | 98.87 | 0.87 | 99.96 | 1.49 |
| 8 | 33.85 | 1.19 | 88.06 | 3.52 | 94.37 | 4.67 | 65.89 | 1.45 | 45.08 | 0.76 | 83.33 | 2.94 | 83.09 | 0.33 | 63.76 | 0.84 | 90.3 | 1.2 | 62.23 | 2.63 | 97.86 | 0.52 | 76.96 | 1.01 | 89.45 | 0.81 | 99.07 | 0.99 | 99.19 | 2.42 |
| 12 | 43.6 | 1.96 | 90.3 | 4.52 | 93.9 | 3.43 | 68.79 | 0.35 | 46.6 | 1.64 | 84.91 | 1.4 | 86.11 | 0.85 | 64.64 | 2.05 | 85.56 | 3.86 | 63.41 | 4.29 | 98.48 | 0.99 | 75.59 | 1.66 | 91.09 | 1.99 | 98.63 | 1.21 | 99.3 | 0.38 |
| 14 | 47.82 | 2.2 | 92.98 | 2.72 | 94.42 | 2.09 | 68.44 | 0.42 | 48.95 | 1.61 | 86.87 | 1.6 | 92.63 | 3.3 | 66.55 | 1.56 | 87.23 | 2.01 | 61.24 | 1.22 | 97.64 | 2.43 | 78.3 | 1.71 | 89.89 | 1.58 | 99.53 | 0.57 | 98.05 | 1.78 |
| 16 | 54.09 | 1.09 | 92.26 | 2.91 | 96.53 | 0.62 | 68.61 | 0.46 | 49.14 | 1.2 | 86.94 | 2.78 | 93.28 | 1.3 | 68.03 | 1.65 | 88.35 | 1.35 | 59.89 | 1.63 | 98.03 | 1.7 | 80.25 | 1.26 | 92.07 | 1.46 | 98.64 | 0.41 | 98.85 | 0.24 |
| 18 | 61.1 | 1.55 | 90.35 | 3.26 | 94.54 | 2.06 | 68.79 | 1.33 | 49.95 | 1.91 | 86.08 | 1.09 | 94.37 | 0.78 | 68.35 | 0.77 | 93.47 | 2.73 | 60.96 | 2.7 | 97.82 | 2.3 | 78.16 | 1.25 | 93.36 | 1.17 | 98.68 | 1.66 | 97.67 | 0.5 |
| 20 | 69.49 | 3.05 | 91.29 | 1.82 | 95.15 | 1.76 | 70.17 | 1.59 | 48.95 | 0.94 | 86.74 | 2.94 | 95.09 | 1.67 | 69.98 | 1.43 | 93.34 | 1.74 | 59.4 | 1.18 | 98.49 | 1.16 | 77.59 | 2.78 | 93.8 | 1.22 | 96.59 | 3.74 | 99.28 | 0.55 |
| 24 | 90.12 | 2.42 | 93.33 | 0.7 | 97.31 | 2.72 | 70.85 | 1.46 | 51.26 | 1.5 | 87.53 | 1.56 | 97.48 | 1.7 | 71.34 | 1.57 | 95.73 | 0.89 | 59.96 | 2.81 | 99.49 | 0.57 | 79.77 | 1.36 | 96.39 | 1.14 | 97.14 | 1.37 | 99.49 | 0.38 |
| Pairwise Comparison of Dissolution Profiles | |||
|---|---|---|---|
| Sample 1 vs. | Sample 2 | f1 | f2 (%) |
| PLA_16_0 | PLA_19_0 | 51.47 | 17.47 |
| PLA_16_0 | PLA_22_0 | 56.63 | 14.22 |
| PLA_19_0 | PLA_22_0 | 10.63 | 48.21 |
| PLA_22_0 | PLA_22_5 | 32.87 | 27.41 |
| PLA_22_0 | PLA_22_10 | 57.25 | 15.32 |
| PLA_22_0 | PLA_22_15 | 18.68 | 38.61 |
| PLA_22_5 | PLA_22_10 | 36.31 | 33.58 |
| PLA_22_5 | PLA_22_15 | 23.87 | 40.79 |
| PLA_22_10 | PLA_22_15 | 90.20 | 22.55 |
| Anti_16_0 | Anti_19_0 | 42.81 | 26.82 |
| Anti_16_0 | Anti_22_0 | 26.55 | 22.85 |
| Anti_19_0 | Anti_22_0 | 28.31 | 29.51 |
| PETG_16_0 | PETG_19_0 | 47.87 | 15.46 |
| PETG_16_0 | PETG_22_0 | 19.98 | 42.03 |
| PETG_19_0 | PETG_22_0 | 60.80 | 18.49 |
| PMMA_16_0 | PMMA_19_0 | 9.21 | 51.12 |
| PMMA_16_0 | PMMA_22_0 | 11.16 | 47.93 |
| PMMA_19_0 | PMMA_22_0 | 7.07 | 51.33 |
| PLA_16_0 | Anti_16_0 | 46.36 | 21.26 |
| PLA_16_0 | PETG_16_0 | 39.89 | 30.54 |
| PLA_16_0 | PMMA_16_0 | 53.23 | 16.39 |
| Anti_16_0 | PETG_16_0 | 40.30 | 29.59 |
| Anti_16_0 | PMMA_16_0 | 14.56 | 35.43 |
| PETG_16_0 | PMMA_16_0 | 34.99 | 26.13 |
| PLA_19_0 | Anti_19_0 | 37.73 | 30.33 |
| PLA_19_0 | PETG_19_0 | 22.72 | 26.15 |
| PLA_19_0 | PMMA_19_0 | 10.82 | 46.70 |
| Anti_19_0 | PETG_19_0 | 34.04 | 23.19 |
| Anti_19_0 | PMMA_19_0 | 37.22 | 24.35 |
| PETG_19_0 | PMMA_19_0 | 18.02 | 28.65 |
| PLA_22_0 | Anti_22_0 | 13.01 | 40.53 |
| PLA_22_0 | PETG_22_0 | 39.05 | 30.36 |
| PLA_22_0 | PMMA_22_0 | 5.85 | 61.29 |
| Anti_22_0 | PETG_22_0 | 47.96 | 22.59 |
| Anti_22_0 | PMMA_22_0 | 15.28 | 39.93 |
| PETG_22_0 | PMMA_22_0 | 31.10 | 26.85 |
References
- Tan, F.; Zhu, Y.; Ma, Z.; Al-Rubeai, M. Recent advances in the implant-based drug delivery in otorhinolaryngology. Acta Biomater. 2020, 108, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.A.; Alexander, M.R.; Irvine, D.J.; Wildman, R.D.; Wallace, M.J.; Sharpe, S.; Yoo, J.; Roberts, C.J. Extrusion 3D Printing of Paracetamol Tablets from a Single Formulation with Tunable Release Profiles Through Control of Tablet Geometry. AAPS PharmSciTech 2018, 19, 3403–3413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villanueva, K.; Martin, D.; Martinkovich, S.; Blomain, E.W. Treatment of a chronically infected nasal silicone prosthesis with continuous antibiotic irrigation and gentamicin-impregnated polymethylmethacrylate beads. JPRAS Open 2018, 15, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Tack, P.; Victor, J.; Gemmel, P.; Annemans, L. 3D-printing techniques in a medical setting: A systematic literature review. Biomed. Eng. Online 2016, 15, 115. [Google Scholar] [CrossRef] [Green Version]
- Ursan, I.; Chiu, L.; Pierce, A. Three-dimensional drug printing: A structured review. J. Am. Pharm. Assoc. 2013, 53, 136–144. [Google Scholar] [CrossRef]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Norman, J.; Madurawe, R.D.; Moore, C.M.V.; Khan, M.A.; Khairuzzaman, A. A new chapter in pharmaceutical manufacturing: 3D-printed drug products. Adv. Drug Deliv. Rev. 2017, 108, 39–50. [Google Scholar] [CrossRef]
- Azad, M.A.; Olawuni, D.; Kimbell, G.; Badruddoza, A.Z.M.; Hossain, M.S.; Sultana, T. Polymers for Extrusion-Based 3D Printing of Pharmaceuticals: A Holistic Materials—Process Perspective. Pharmaceutics 2020, 12, 124. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Sulkis, M.; Driver, J.; Saade-Castillo, A.; Thompson, A.; Koo, J.H. Multi-functional ULTEMTM1010 composite filaments for additive manufacturing using Fused Filament Fabrication (FFF). Addit. Manuf. 2018, 24, 298–306. [Google Scholar]
- Li, G.; Zhao, J.; Wu, W.; Jiang, J.; Wang, B.; Jiang, H.; Fuh, J.Y.H. Effect of ultrasonic vibration on mechanical properties of 3D printing non-crystalline and semi-crystalline polymers. Materials 2018, 11, 826. [Google Scholar] [CrossRef] [Green Version]
- Awad, A.; Trenfield, S.J.; Gaisford, S.; Basit, A.W. 3D printed medicines: A new branch of digital healthcare. Int. J. Pharm. 2018, 548, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sun, L.; Mei, Z.; Zhang, F.; He, M.; Fletcher, C.; Wang, F.; Yang, J.; Bi, D.; Jiang, Y.; et al. 3D printed biodegradable implants as an individualized drug delivery system for local chemotherapy of osteosarcoma. Mater. Des. 2020, 186, 108336. [Google Scholar] [CrossRef]
- Cho, H.; Jammalamadaka, U.; Tappa, K. Nanogels for pharmaceutical and biomedical applications and their fabrication using 3D printing technologies. Materials 2018, 11, 302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.H.; Kathuria, H.; Tan, J.J.Y.; Kang, L. 3D printed drug delivery and testing systems—A passing fad or the future? Adv. Drug Deliv. Rev. 2018, 132, 139–168. [Google Scholar] [CrossRef] [PubMed]
- Pérez, M.; Medina-Sánchez, G.; García-Collado, A.; Gupta, M.; Carou, D. Surface quality enhancement of fused deposition modeling (FDM) printed samples based on the selection of critical printing parameters. Materials 2018, 11, 1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacón, J.M.; Caminero, M.A.; García-Plaza, E.; Núñez, P.J. Additive manufacturing of PLA structures using fused deposition modelling: Effect of process parameters on mechanical properties and their optimal selection. Mater. Des. 2017, 124, 143–157. [Google Scholar] [CrossRef]
- Zidan, A.; Alayoubi, A.; Asfari, S.; Coburn, J.; Ghammraoui, B.; Cruz, C.N.; Ashraf, M. Development of mechanistic models to identify critical formulation and process variables of pastes for 3D printing of modified release tablets. Int. J. Pharm. 2019, 555, 109–123. [Google Scholar] [CrossRef]
- Maroni, A.; Melocchi, A.; Parietti, F.; Foppoli, A.; Zema, L.; Gazzaniga, A. 3D printed multi-compartment capsular devices for two-pulse oral drug delivery. J. Control. Release 2017, 268, 10–18. [Google Scholar] [CrossRef]
- Okwuosa, T.C.; Pereira, B.C.; Arafat, B.; Cieszynska, M.; Isreb, A.; Alhnan, M.A. Fabricating a Shell-Core Delayed Release Tablet Using Dual FDM 3D Printing for Patient-Centred Therapy. Pharm. Res. 2017, 34, 427–437. [Google Scholar] [CrossRef]
- Tan, D.K.; Maniruzzaman, M.; Nokhodchi, A. Advanced pharmaceutical applications of hot-melt extrusion coupled with fused deposition modelling (FDM) 3D printing for personalised drug delivery. Pharmaceutics 2018, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Kollamaram, G.; Croker, D.M.; Walker, G.M.; Goyanes, A.; Basit, A.W.; Gaisford, S. Low temperature fused deposition modeling (FDM) 3D printing of thermolabile drugs. Int. J. Pharm. 2018, 545, 144–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, B.; Cappetti, N.; Di Maio, L.; Scarfato, P.; Incarnato, L. 3D printing of PLA/clay nanocomposites: Influence of printing temperature on printed samples properties. Materials 2018, 11, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasrin, R.; Biswas, S.; Rashid, T.U.; Afrin, S.; Jahan, R.A.; Haque, P.; Rahman, M.M. Preparation of Chitin-PLA laminated composite for implantable application. Bioact. Mater. 2017, 2, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Salick, M.R.; Jing, X.; Jacques, B.R.; Crone, W.C.; Peng, X.F.; Turng, L.S. Characterization of thermoplastic polyurethane/polylactic acid (TPU/PLA) tissue engineering scaffolds fabricated by microcellular injection molding. Mater. Sci. Eng. C 2013, 33, 4767–4776. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Yang, Q.; Wang, Y.; Yu, H.; Chen, X.; Jing, X. Biodegradable electrospun poly(l-lactide) fibers containing antibacterial silver nanoparticles. Eur. Polym. J. 2006, 42, 2081–2087. [Google Scholar] [CrossRef]
- Gao, M.; Sun, L.; Guo, Y.; Shi, J.; Zhang, J. Modification of polyethylene terephthalate (PET) films surface with gradient roughness and homogenous surface chemistry by dielectric barrier discharge plasma. Chem. Phys. Lett. 2017, 689, 179–184. [Google Scholar] [CrossRef]
- Durgashyam, K.; Indra Reddy, M.; Balakrishna, A.; Satyanarayana, K. Experimental investigation on mechanical properties of PETG material processed by fused deposition modeling method. Mater. Today Proc. 2019, 18, 2052–2059. [Google Scholar] [CrossRef]
- Ridwan-Pramana, A.; Marcián, P.; Borák, L.; Narra, N.; Forouzanfar, T.; Wolff, J. Structural and mechanical implications of PMMA implant shape and interface geometry in cranioplasty—A finite element study. J. Cranio-Maxillofac. Surg. 2016, 44, 34–44. [Google Scholar] [CrossRef]
- Assemany, L.P.F.; Júnior, O.R.; da Silva, E.; Maria da Penha, A.P. Evaluation of 3D printing filaments for construction of a pediatric phantom for dosimetry in CBCT. Radiat. Phys. Chem. 2019, 167, 108227. [Google Scholar] [CrossRef]
- Sadhasivam, B.; Ramamoorthy, D.; Dhamodharan, R. Scale-up of non-toxic poly(butylene adipate-co-terephthalate)-Chitin based nanocomposite articles by injection moulding and 3D printing. Int. J. Biol. Macromol. 2020, 165, 3145–3155. [Google Scholar] [CrossRef]
- Ortíz-Palacios, J.; Rodríguez-Alba, E.; Avelar, M.; Martínez, A.; Del Pilar Carreón-Castro, M.; Rivera, E. Synthesis and characterization of novel dendrons bearing amino-nitro-substituted azobenzene units and oligo(ethylene glycol) spacers: Thermal, optical properties, langmuir blodgett films and liquid-crystalline behaviour. Molecules 2013, 18, 1502–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito Corcione, C.; Gervaso, F.; Scalera, F.; Padmanabhan, S.K.; Madaghiele, M.; Montagna, F.; Sannino, A.; Licciulli, A.; Maffezzoli, A. Highly loaded hydroxyapatite microsphere/ PLA porous scaffolds obtained by fused deposition modelling. Ceram. Int. 2018, 45, 2803–2810. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Wang, Z.; Qi, Y.; Li, L.; Zhang, P.; Chen, X.; Huang, Y. Composite PLA/PEG/nHA/Dexamethasone Scaffold Prepared by 3D Printing for Bone Regeneration. Macromol. Biosci. 2018, 18, 1800068. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Yu, B.; Xiao, D.; Zhang, M.; Wu, X.; Li, G. Biomimetic superhydrophobic hollowed-out pyramid surface based on self-assembly. Materials 2018, 11, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Yang, S.; Wang, Y.; Yu, Z.; Ao, H.; Zhang, H.; Qin, L.; Guillaume, O.; Eglin, D.; Richards, R.G.; et al. Anti-infective efficacy, cytocompatibility and biocompatibility of a 3D-printed osteoconductive composite scaffold functionalized with quaternized chitosan. Acta Biomater. 2016, 46, 112–128. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, A.; Sperling, P.; Beerlink, A.; Tshabalala, L.; Hoosain, S.; Mathe, N.; le Roux, S.G. Standard method for microCT-based additive manufacturing quality control 2: Density measurement. MethodsX 2018, 5, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Frosch, T.; Wyrwich, E.; Yan, D.; Popp, J.; Frosch, T. Fiber-Array-Based Raman Hyperspectral Imaging for Simultaneous, Chemically-Selective Monitoring of Particle Size and Shape of Active Ingredients in Analgesic Tablets. Molecules 2019, 24, 4381. [Google Scholar] [CrossRef] [Green Version]
- Boetker, J.; Water, J.J.; Aho, J.; Arnfast, L.; Bohr, A.; Rantanen, J. Modifying release characteristics from 3D printed drug-eluting products. Eur. J. Pharm. Sci. 2016, 90, 47–52. [Google Scholar] [CrossRef]
- van Tonder, A.; Joubert, A.M.; Cromarty, A.D. Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Res. Notes 2015, 8, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Arany, P.; Róka, E.; Mollet, L.; Coleman, A.W.; Perret, F.; Kim, B.; Kovács, R.; Kazsoki, A.; Zelkó, R.; Gesztelyi, R.; et al. Fused Deposition Modeling 3D Printing: Test Platforms for Evaluating Post-Fabrication Chemical Modifications and In-Vitro Biological Properties. Pharmaceutics 2019, 11, 277. [Google Scholar] [CrossRef] [Green Version]
- The International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; The International Organization for Standardization: Geneva, Switzerland, 2009; Volume 3, p. 34. [Google Scholar]
- Mohseni, M.; Hutmacher, D.W.; Castro, N.J. Independent evaluation of medical-grade bioresorbable filaments for fused deposition modelling/fused filament fabrication of tissue engineered constructs. Polymers 2018, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speer, I.; Lenhart, V.; Preis, M.; Breitkreutz, J. Prolonged release from orodispersible films by incorporation of diclofenac-loaded micropellets. Int. J. Pharm. 2019, 554, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Eleftheriadis, G.K.; Monou, P.K.; Bouropoulos, N.; Fatouros, D.G. In vitro evaluation of 2D-printed edible films for the buccal delivery of diclofenac sodium. Materials 2018, 11, 864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.B.; Kim, S.N.; Lee, S.H.; Huh, B.K.; Shin, B.H.; Lee, C.; Cho, Y.C.; Heo, C.Y.; Choy, Y. Bin Soft implantable device with drug-diffusion channels for the controlled release of diclofenac. J. Control. Release 2020, 318, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Aquino, R.P.; Barile, S.; Grasso, A.; Saviano, M. Envisioning smart and sustainable healthcare: 3D Printing technologies for personalized medication. Futures 2018, 103, 35–50. [Google Scholar] [CrossRef]
- Sommer, A.C.; Blumenthal, E.Z. Implementations of 3D printing in ophthalmology. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1815–1822. [Google Scholar] [CrossRef]
- Ahmed, K.K.; Tamer, M.A.; Ghareeb, M.M.; Salem, A.K. Recent advances in polymeric implants. AAPS PharmSciTech 2019, 20, 300. [Google Scholar] [CrossRef]
- Yang, N.; Chen, H.; Han, H.; Shen, Y.; Gu, S.; He, Y.; Guo, S. 3D printing and coating to fabricate a hollow bullet-shaped implant with porous surface for controlled cytoxan release. Int. J. Pharm. 2018, 552, 91–98. [Google Scholar] [CrossRef]
- Stewart, S.A.; Domínguez-Robles, J.; Donnelly, R.F.; Larrañeta, E. Implantable polymeric drug delivery devices: Classification, manufacture, materials, and clinical applications. Polymers 2018, 10, 1379. [Google Scholar] [CrossRef] [Green Version]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tidau, M.; Kwade, A.; Finke, J.H. Influence of high, disperse api load on properties along the fused-layer modeling process chain of solid dosage forms. Pharmaceutics 2019, 11, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasvári, G.; Csontos, B.; Sovány, T.; Regdon, G.; Bényei, A.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; Fehér, P.; Sinka, D.; et al. Development and Characterisation of Modified Release Hard Gelatin Capsules, Based on In Situ Lipid Matrix Formation. AAPS PharmSciTech 2018, 19, 3165–3176. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Ma, L.; Fan, J.; Chen, Q.; Zhang, L.; Li, B.Q. Wetting behaviors of a nano-droplet on a rough solid substrate under perpendicular electric field. Nanomaterials 2018, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Tham, C.Y.; Abdul Hamid, Z.A.; Ahmad, Z.; Ismail, H. Surface Modification of Poly(lactic acid) (PLA) via Alkaline Hydrolysis Degradation. Adv. Mater. Res. 2014, 970, 324–327. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergence of 3D Printed Dosage Forms: Opportunities and Challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- Sevim, K.; Pan, J. A model for hydrolytic degradation and erosion of biodegradable polymers. Acta Biomater. 2018, 66, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef] [Green Version]
- Samaha, D.; Shehayeb, R.; Kyriacos, S. Modeling and comparison of dissolution profiles of diltiazem modified-release formulations. Dissolution Technol. 2009, 16, 41–46. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol. Pharm. Drug Res. 2010, 67, 217–223. [Google Scholar]
- Solorio, L.; Exner, A.A. Effect of the Subcutaneous Environment on Phase-Sensitive in Situ-Forming Implant Drug Release, Degradation, and Microstructure. J. Pharm. Sci. 2015, 104, 4322–4328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsawy, M.A.; Kim, K.H.; Park, J.W.; Deep, A. Hydrolytic degradation of polylactic acid (PLA) and its composites. Renew. Sustain. Energy Rev. 2017, 79, 1346–1352. [Google Scholar] [CrossRef]
- Eyal, A.M.; Canari, R. pH Dependence of Carboxylic and Mineral Acid Extraction by Amine-Based Extractants: Effects of pKa, Amine Basicity, and Diluent Properties. Ind. Eng. Chem. Res. 1995, 34, 1789–1798. [Google Scholar] [CrossRef]
- Göpferich, A. Mechanisms of polymer degradation and erosion1. Biomater. Silver Jubil. Compend. 1996, 17, 117–128. [Google Scholar]
- Schliecker, G.; Schmidt, C.; Fuchs, S.; Kissel, T. Characterization of a homologous series of D,L-lactic acid oligomers; a mechanistic study on the degradation kinetics in vitro. Biomaterials 2003, 24, 3835–3844. [Google Scholar] [CrossRef]
- Pizzoferrato, A.; Ciapetti, G.; Stea, S.; Cenni, E.; Arciola, C.R.; Granchi, D.; Savarino, L. Cell culture methods for testing biocompatibility. Clin. Mater. 1994, 15, 173–190. [Google Scholar] [CrossRef]
- Kinnari, T.J.; Soininen, A.; Esteban, J.; Zamora, N.; Alakoski, E.; Kouri, V.P.; Lappalainen, R.; Konttinen, Y.T.; Gomez-Barrena, E.; Tiainen, V.M. Adhesion of staphylococcal and Caco-2 cells on diamond-like carbon polymer hybrid coating. J. Biomed. Mater. Res. Part A 2008, 86, 760–768. [Google Scholar] [CrossRef]
- Chessa, D.; Ganau, G.; Spiga, L.; Bulla, A.; Mazzarello, V.; Campus, G.V.; Rubino, S. Staphylococcus aureus and Staphylococcus epidermidis virulence strains as causative agents of persistent infections in breast implants. PLoS ONE 2016, 11, e0146668. [Google Scholar] [CrossRef]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Gaucher, S.; Jarraya, M. Technical note: Comparison of the PrestoBlue and LDH release assays with the MTT assay for skin viability assessment. Cell Tissue Bank. 2015, 16, 325–329. [Google Scholar] [CrossRef]
- Wortelen, B.; Unni, A.; Rieger, J.W.; Lüdtke, A.; Osterloh, J. Cognitive Infocommunications, Theory and Applications; Springer International Publishing: Cham, Switzerland, 2019; Volume 13, ISBN 978-3-319-95995-5. [Google Scholar]
- Zichar, M.; Papp, I. Interaction between 3D Printing and Geometry Studies. In Proceedings of the ICGG 2018—Proceedings of the 18th International Conference on Geometry and Graphics, Milan, Italy, 3 August 2018; Cocchiarella, L., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1177–1190. [Google Scholar]
- Khaled, S.A.; Burley, J.C.; Alexander, M.R.; Yang, J.; Roberts, C.J. 3D printing of tablets containing multiple drugs with defined release profiles. Int. J. Pharm. 2015, 494, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Yao, Y.; Yin, G.; Huang, Z.; Liao, X.; Xu, X.; Zhao, G. A study on the in vitro degradation properties of poly(l-lactic acid)/β-tricalcuim phosphate(PLLA/β-TCP) scaffold under dynamic loading. Med. Eng. Phys. 2009, 31, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Regdon, G.; Hegyesi, D.; Pintye-Hódi, K. Thermal study of ethyl cellulose coating films used for modified release (MR) dosage forms. J. Therm. Anal. Calorim. 2012, 108, 347–352. [Google Scholar] [CrossRef]
- Vasvári, G.; Haimhoffer, Á.; Horváth, L.; Budai, I.; Trencsényi, G.; Béresová, M.; Dobó-Nagy, C.; Váradi, J.; Bácskay, I.; Ujhelyi, Z.; et al. Development and Characterisation of Gastroretentive Solid Dosage Form Based on Melt Foaming. AAPS PharmSciTech 2019, 20, 290. [Google Scholar] [CrossRef]
- Tarasco, M.; Cordelières, F.P.; Cancela, M.L.; Laizé, V. ZFBONE: An ImageJ toolset for semi-automatic analysis of zebrafish bone structures. Bone 2020, 138, 115480. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, S.; Szostak, R. Quantitative determination of diclofenac sodium in solid dosage forms by FT-Raman spectroscopy. J. Pharm. Biomed. Anal. 2008, 48, 814–821. [Google Scholar] [CrossRef]
- Goyanes, A.; Buanz, A.B.M.; Basit, A.W.; Gaisford, S. Fused-filament 3D printing (3DP) for fabrication of tablets. Int. J. Pharm. 2014, 476, 88–92. [Google Scholar] [CrossRef]
- Seyednejad, H.; Gawlitta, D.; Kuiper, R.V.; De Bruin, A.; Van Nostrum, C.F.; Vermonden, T.; Dhert, W.J.A.; Hennink, W.E. In vivo biocompatibility and biodegradation of 3D-printed porous scaffolds based on a hydroxyl-functionalized poly(ε-caprolactone). Biomaterials 2012, 33, 4309–4318. [Google Scholar] [CrossRef]
- Nemes, D.; Kovács, R.; Nagy, F.; Mező, M.; Poczok, N.; Ujhelyi, Z.; Pető, A.; Fehér, P.; Fenyvesi, F.; Váradi, J.; et al. Interaction between different pharmaceutical excipients in liquid dosage forms—Assessment of cytotoxicity and antimicrobial activity. Molecules 2018, 23, 1827. [Google Scholar] [CrossRef] [Green Version]
- Nemes, D.; Ujhelyi, Z.; Arany, P.; Peto, A.; Feher, P.; Varadi, J.; Fenyvesi, F.; Vecsernyes, M.; Bacskay, I. Biocompatibility investigation of different pharmaceutical excipients used in liquid dosage forms. Pharmazie 2018, 73, 16–18. [Google Scholar]




















| Sample | Weight | Content Uniformity | ||
|---|---|---|---|---|
| Average (mg) | ±SD | Average (mg) | ±SD | |
| PLA_16_0 | 346.93 | 5.39 | 29.45 | 0.89 |
| PLA_19_0 | 476.44 | 2.74 | 29.87 | 0.75 |
| PLA_22_0 | 608.79 | 1.45 | 29.60 | 0.83 |
| PLA_22_5 | 635.87 | 1.65 | 29.94 | 0.47 |
| PLA_22_10 | 646.04 | 4.81 | 29.75 | 0.73 |
| PLA_22_15 | 690.85 | 5.34 | 29.86 | 0.86 |
| Anti_16_0 | 344.08 | 4.00 | 29.53 | 0.92 |
| Anti_19_0 | 468.19 | 3.48 | 29.78 | 1.0 |
| Anti_22_0 | 617.77 | 3.74 | 29.56 | 0.82 |
| PETG_16_0 | 354.17 | 2.40 | 29.72 | 0.45 |
| PETG_19_0 | 513.55 | 0.54 | 29.99 | 0.67 |
| PETG_22_0 | 648.54 | 1.86 | 29.68 | 0.81 |
| PMMA_16_0 | 366.49 | 3.61 | 29.66 | 0.36 |
| PMMA_19_0 | 527.96 | 2.57 | 29.53 | 0.63 |
| PMMA_22_0 | 615.87 | 2.84 | 29.91 | 0.78 |
| Sample | Measured Weight (mg) ± SD | |||||
|---|---|---|---|---|---|---|
| After Printing | 1st Week | 2nd Week | 4th Week | 6th Week | 8th Week | |
| PLA_16_0 | 315.44 ± 0.57 | 315.32 ± 0.42 | 314.82 ± 0.22 | 314.59 ± 0.84 | 315.93 ± 1.34 | 314.75 ± 0.56 |
| PLA_19_0 | 446.87 ± 0.98 | 446.47 ± 0.88 | 446.53 ± 1.18 | 446.43 ± 0.58 | 445.86 ± 0.18 | 445.42 ± 0.76 |
| PLA_22_0 | 576.96 ± 1.45 | 577.05 ± 0.72 | 576.37 ± 0.48 | 577.04 ± 0.33 | 577.04 ± 0.33 | 576.53 ± 0.18 |
| PLA_22_5 | 603.65 ± 0.12 | 602.72 ± 0.27 | 602.27 ± 0.34 | 603.74 ± 0.37 | 602.72 ± 1.05 | 603.52 ± 1.05 |
| PLA_22_10 | 615.98 ± 2.41 | 615.49 ± 0.38 | 615.92 ± 1.58 | 615.63 ± 0.81 | 615.07 ± 0.23 | 615.07 ± 0.45 |
| PLA_22_15 | 660.57 ± 1.34 | 659.59 ± 1.14 | 660.39 ± 0.56 | 659.79 ± 0.34 | 660.82 ± 1.54 | 660.03 ± 0.72 |
| Anti_16_0 | 313.82 ± 1.21 | 312.28 ± 0.51 | 313.03 ± 0.93 | 313.75 ± 0.63 | 312.34 ± 0.25 | 312.06 ± 0.57 |
| Anti_19_0 | 437.19 ± 0.97 | 436.97 ± 0.84 | 437.03 ± 0.14 | 436.13 ± 0.59 | 436.94 ± 0.37 | 437.42 ± 0.70 |
| Anti_22_0 | 586.82 ± 0.75 | 586.42 ± 0.55 | 585.97 ± 1.25 | 585.36 ± 0.74 | 586.22 ± 1.16 | 586.34 ± 0.04 |
| Sample | Weight of the Measured Diclofenac (mg) in the Cavity | Total Weight (mg) of Diclofenac | |||||
|---|---|---|---|---|---|---|---|
| 1st Cavity | 2nd Cavity | 3rd Cavity | 4th Cavity | 5th Cavity | 6th Cavity | ||
| PLA_16_0 | 28.04 ± 0.15 | - | - | - | - | - | 28.04 ± 0.15 |
| PLA_16_5 | 4.73 ± 0.04 | 7.87 ± 0.18 | 11.32 ± 0.71 | 2.61 ± 0.31 | 0.19 ± 0.005 | 1.34 ± 0.09 | 28.06 ± 0.22 |
| PLA_16_10 | 10.89 ± 0.22 | 5.58 ± 0.16 | 11.76 ± 0.29 | 2.32 ± 0.17 | 1.87 ± 0.04 | 0.29 ± 0.13 | 32.71 ± 0.17 |
| PLA_16_15 | 12.12 ± 0.10 | 6.41 ± 0.05 | 6.08 ± 0.004 | 3.10 ± 0.14 | 1.85 ± 0.20 | 0.29 ± 0.31 | 29.85 ± 0.13 |
| Sample | Diffusion Rate (µg·mL−1·h−1) | Flux (µg·cm−2·h−1) | Dissolved API Amount (%) | t-Test Result | |||
|---|---|---|---|---|---|---|---|
| Average 0–2 h | Average 2–24 h | 0–2 h | 2–24 h | 2 h | 24 h | 2 h vs. 24 h | |
| PLA_16_0 | 2.57 | 1.08 | 0.49 | 0.21 | 16.64 | 90.12 | **** |
| PLA_19_0 | 13.00 | 0.21 | 1.83 | 0.03 | 80.04 | 93.33 | * |
| PLA_22_0 | 21.70 | 0.25 | 2.33 | 0.03 | 82.95 | 97.31 | ** |
| PLA_22_5 | 10.15 | 0.25 | 1.09 | 0.03 | 55.66 | 70.85 | **** |
| PLA_22_10 | 4.26 | 0.33 | 0.46 | 0.04 | 31.61 | 51.26 | *** |
| PLA_22_15 | 10.07 | 0.36 | 1.08 | 0.04 | 66.73 | 87.53 | *** |
| Anti_16_0 | 7.42 | 0.85 | 1.42 | 0.16 | 48.61 | 97.48 | **** |
| Anti_19_0 | 19.63 | 0.18 | 2.76 | 0.02 | 60.71 | 71.34 | *** |
| Anti_22_0 | 31.15 | 0.19 | 3.35 | 0.02 | 86.47 | 95.73 | *** |
| PETG_16_0 | 9.43 | 0.06 | 1.80 | 0.01 | 55.65 | 59.96 | ns |
| PETG_19_0 | 28.62 | 0.08 | 4.03 | 0.01 | 94.22 | 99.49 | *** |
| PETG_22_0 | 7.76 | 0.40 | 0.83 | 0.04 | 57.70 | 79.77 | **** |
| PMMA_16_0 | 17.56 | 0.26 | 3.36 | 0.05 | 81.38 | 96.39 | **** |
| PMMA_19_0 | 17.18 | 0.00 | 2.42 | 0.00 | 97.23 | 97.14 | ns |
| PMMA_22_0 | 21.77 | 0.12 | 2.34 | 0.01 | 92.95 | 99.48 | * |
| Sample | Zero-Order Kinetics | First-Order Kinetics | Zero-Order Kinetics | First-Order Kinetics | * Zero-Order Kinetics | * First-Order Kinetics |
|---|---|---|---|---|---|---|
| 0–24 h | 0–24 h | 0–2 h | 0–2 h | 0–X h | 0–X h | |
| PLA_16_0 | 0.98 | 0.85 | 0.98 | 0.97 | - | - |
| PLA_19_0 | 0.56 | 0.71 | 0.98 | 0.99 | - | - |
| PLA_22_0 | 0.52 | 0.72 | 0.89 | 0.98 | - | - |
| PLA_22_5 | 0.60 | 0.68 | 0.97 | 0.99 | - | - |
| PLA_22_10 | 0.68 | 0.71 | 0.93 | 0.94 | - | - |
| PLA_22_15 | 0.61 | 0.73 | 0.98 | 0.99 | - | - |
| Anti_16_0 | 0.73 | 0.93 | 0.97 | 0.94 | - | - |
| * Anti_19_0 | 0.43 | 0.60 | 0.65 | 0.68 | 0.91 | 1.00 |
| * Anti_22_0 | 0.36 | 0.65 | 0.64 | 0.80 | 0.85 | 0.98 |
| PETG_16_0 | 0.51 | 0.49 | 0.99 | 0.97 | - | - |
| * PETG_19_0 | 0.29 | 0.67 | 0.60 | 0.77 | 0.95 | 1.00 |
| PETG_22_0 | 0.61 | 0.67 | 0.98 | 0.97 | - | - |
| PMMA_16_0 | 0.54 | 0.80 | 0.88 | 0.90 | - | - |
| PMMA_19_0 | 0.45 | 0.40 | 0.93 | 0.95 | - | - |
| PMMA_22_0 | 0.47 | 0.45 | 0.90 | 0.98 | - | - |
| Properties | Method | PLA | Antibacterial PLA | PETG | PMMA |
|---|---|---|---|---|---|
| Specific gravity (g/cm3) | D792 | 1.24 | 1.24 | 1.29 | 1.17 |
| Heat distortion temperature at 0.45 MPa (°C) | D790 | 55 | 80‒90 | 68 | 106 |
| Glass Trans. temperature (°C) | D3418 | 55‒60 | 55‒60 | 80 | 105 |
| Tensile strength (MPa) | ISO 527 | 60 | 66 | 53 | 90 |
| Tensile elongation (%) | ISO 527 | 6.00 | 3.31 | 4.01 | 15.0 |
| Tensile modulus (MPa) | ISO 527 | 3800 | 4400 | 2040 | 2900 |
| Notched Izod impact (kJ/m2) | ISO 180 | 16 | 118 | 4.5 | 6.4 |
| Properties | Method | PLA | Antibacterial PLA | PETG | PMMA |
|---|---|---|---|---|---|
| Tensile strength (MPa) | ISO 527 | 31.6 | 33.0 | 43.0 | 83.0 |
| Tensile modulus (MPa) | ISO 527 | 1800 | 2300 | 2800 | 3200 |
| Notched Izod impact (kJ/m2) | ISO 180 | 2.6 | 3.8 | 9.4 | 2.0 |
| Filament Type | PLA | Antibacterial PLA | PETG | PMMA |
|---|---|---|---|---|
| Filament Diameter (mm) | 1.75 | 1.75 | 1.75 | 1.75 |
| Extruder Nozzle Diameter (µm) | 400 | 400 | 400 | 400 |
| Infill Percentage (%) | 0, 5, 10, 15 | 0, 5, 10, 15 | 0, 5, 10, 15 | 0, 5, 10, 15 |
| Extrusion Temperature (℃) | 215 | 215 | 250 | 270 |
| Bed Temperature (℃) | 60 | 60 | 90 | 110 |
| Layer Thickness (µm) | 200 | 200 | 200 | 200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arany, P.; Papp, I.; Zichar, M.; Csontos, M.; Elek, J.; Regdon, G., Jr.; Budai, I.; Béres, M.; Gesztelyi, R.; Fehér, P.; et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules 2020, 25, 5889. https://doi.org/10.3390/molecules25245889
Arany P, Papp I, Zichar M, Csontos M, Elek J, Regdon G Jr., Budai I, Béres M, Gesztelyi R, Fehér P, et al. In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules. 2020; 25(24):5889. https://doi.org/10.3390/molecules25245889
Chicago/Turabian StyleArany, Petra, Ildikó Papp, Marianna Zichar, Máté Csontos, János Elek, Géza Regdon, Jr., István Budai, Mónika Béres, Rudolf Gesztelyi, Pálma Fehér, and et al. 2020. "In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants" Molecules 25, no. 24: 5889. https://doi.org/10.3390/molecules25245889
APA StyleArany, P., Papp, I., Zichar, M., Csontos, M., Elek, J., Regdon, G., Jr., Budai, I., Béres, M., Gesztelyi, R., Fehér, P., Ujhelyi, Z., Vasvári, G., Haimhoffer, Á., Fenyvesi, F., Váradi, J., Miklós, V., & Bácskay, I. (2020). In Vitro Tests of FDM 3D-Printed Diclofenac Sodium-Containing Implants. Molecules, 25(24), 5889. https://doi.org/10.3390/molecules25245889