Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Promotes Lipolysis and Thermogenesis In Vitro and In Vivo
Abstract
:1. Introduction
2. Results
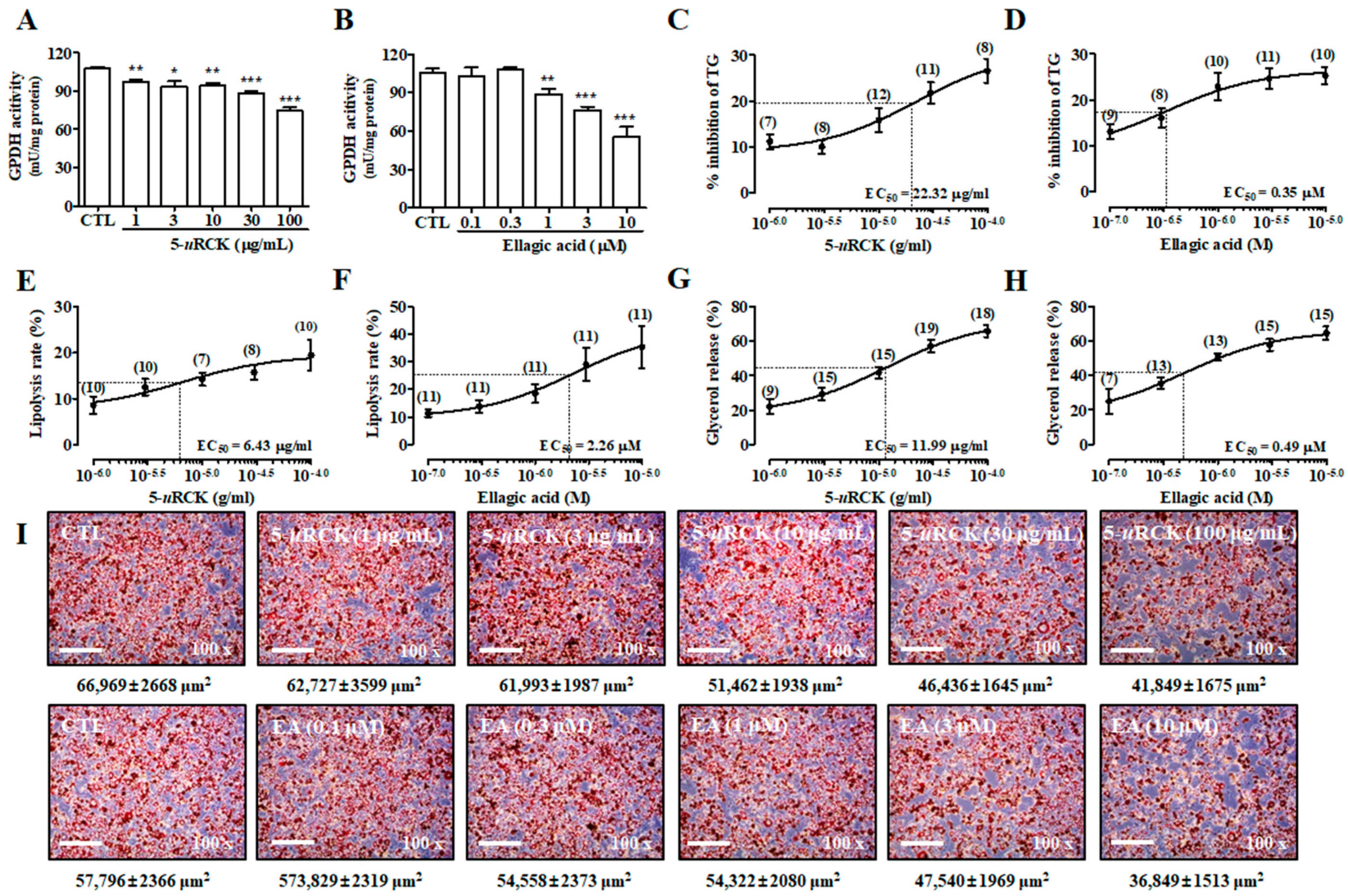
2.1. 5-uRCK and Ellagic Acid Improve Lipolysis in Mature Adipocytes
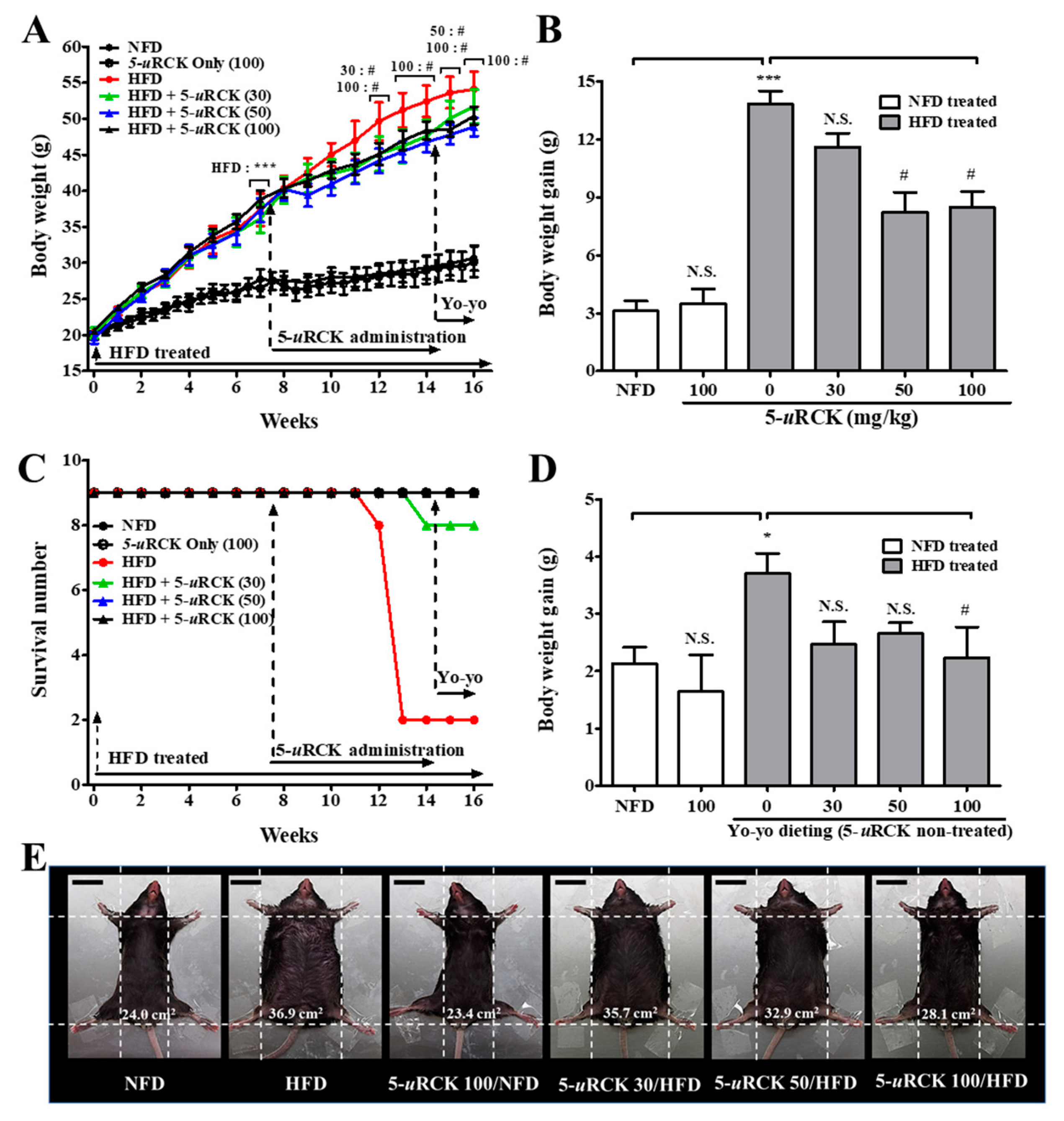
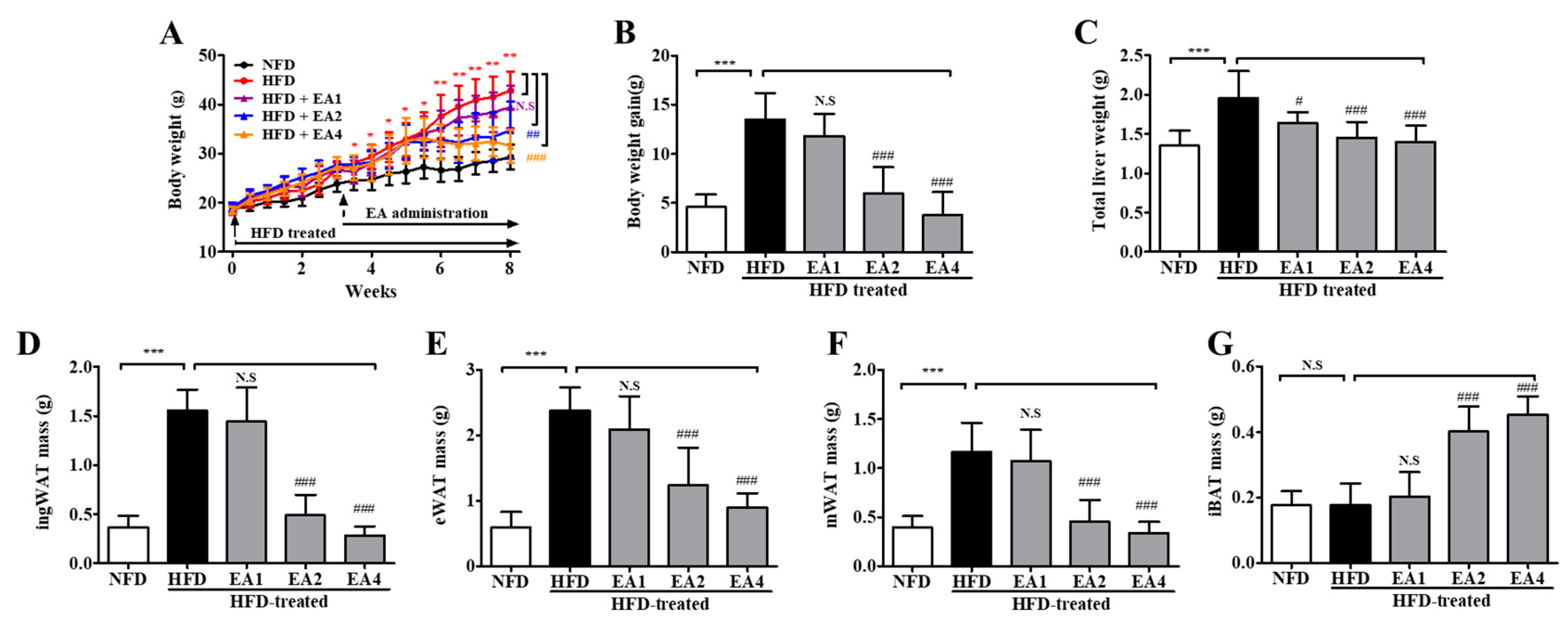
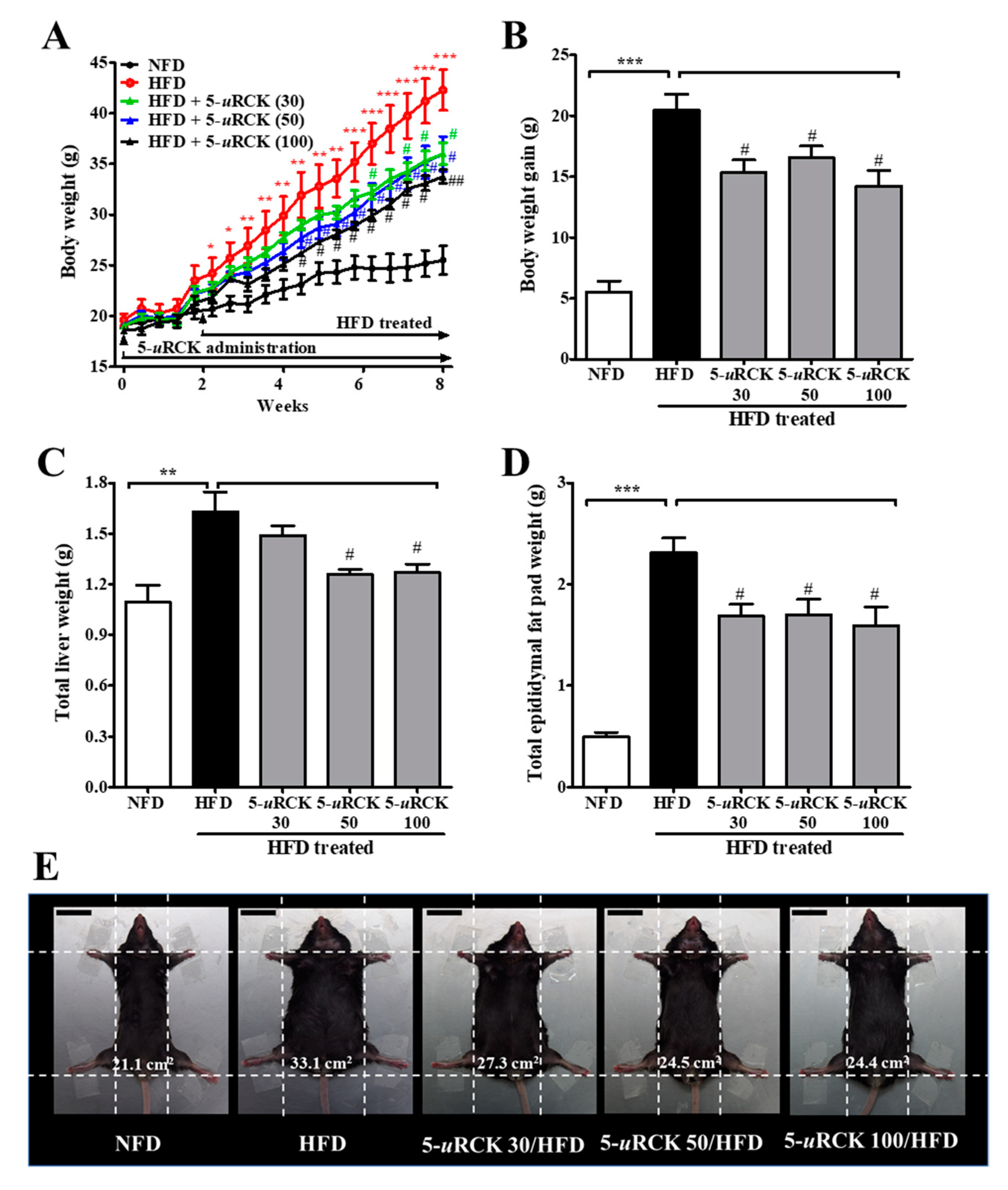
2.2. 5-uRCK Reduces Body Weight Gain in 16-Week Long-Term HFD-Fed Mice
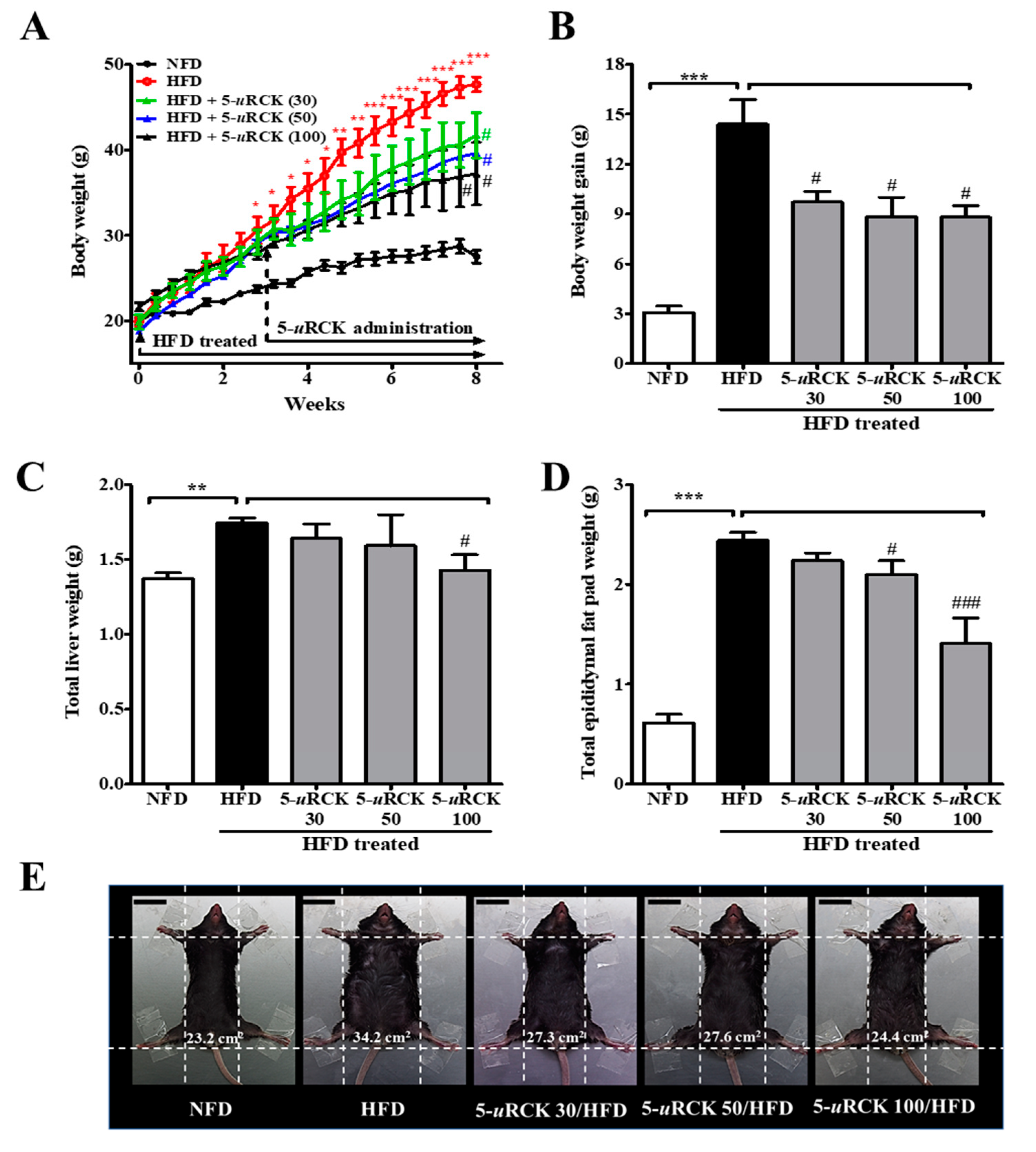
2.3. Post-Treatment with 5-uRCK Reduced Body Weight Gain and Fat Accumulation in HFD-Fed Mice at 8 Weeks
2.4. Post-Treatment with 5-uRCK Ameliorated the Lipid Profile in Mice after 8 Weeks of HFD Feeding
2.5. Post-Treatment with 5-uRCK Lowers Biochemical Parameters of Serum at 8 Weeks of HFD Feeding
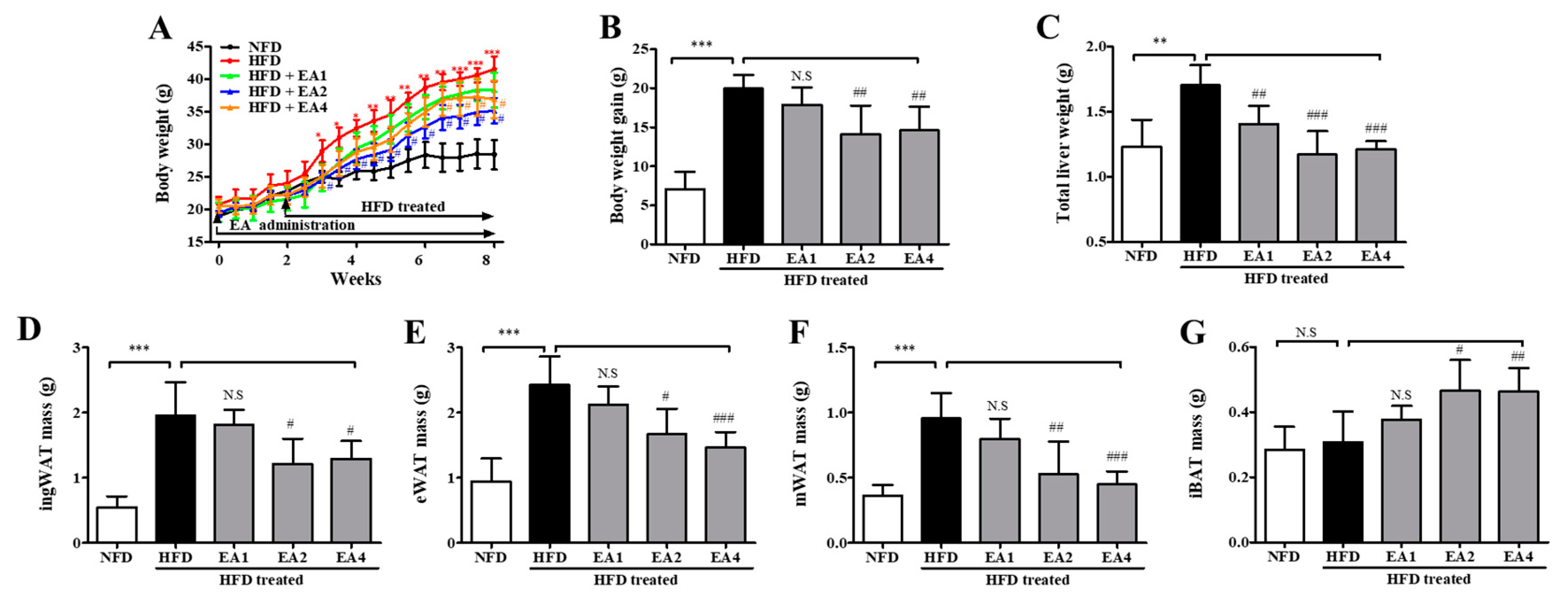
2.6. Effects of Ellagic Acid on Reducing Body Weight Gain in HFD-Fed Mice at 8 Weeks
2.7. Effects of Ellagic Acid on Serum Lipid Levels and Biochemical Parameters in Mice Fed an HFD
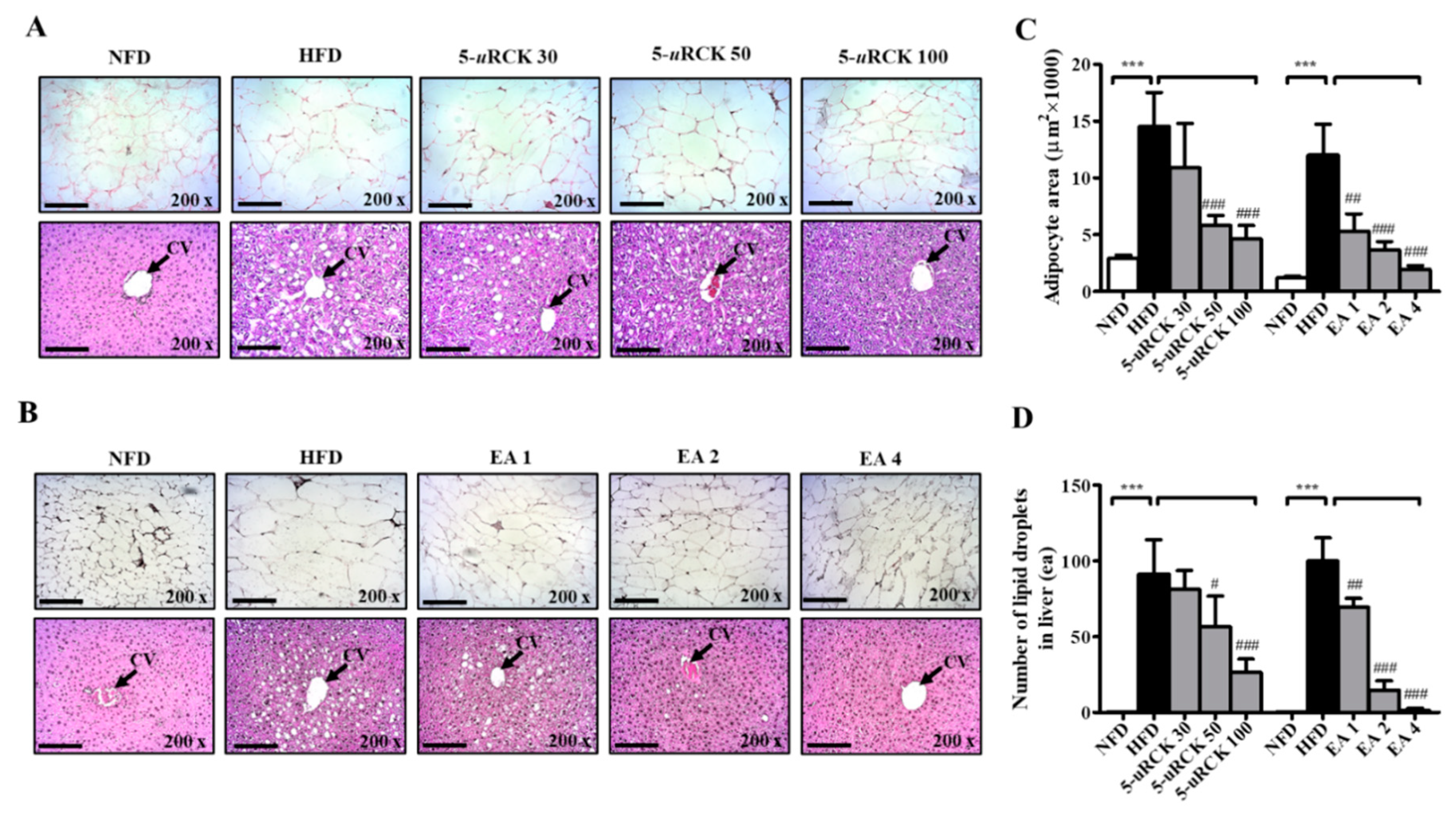
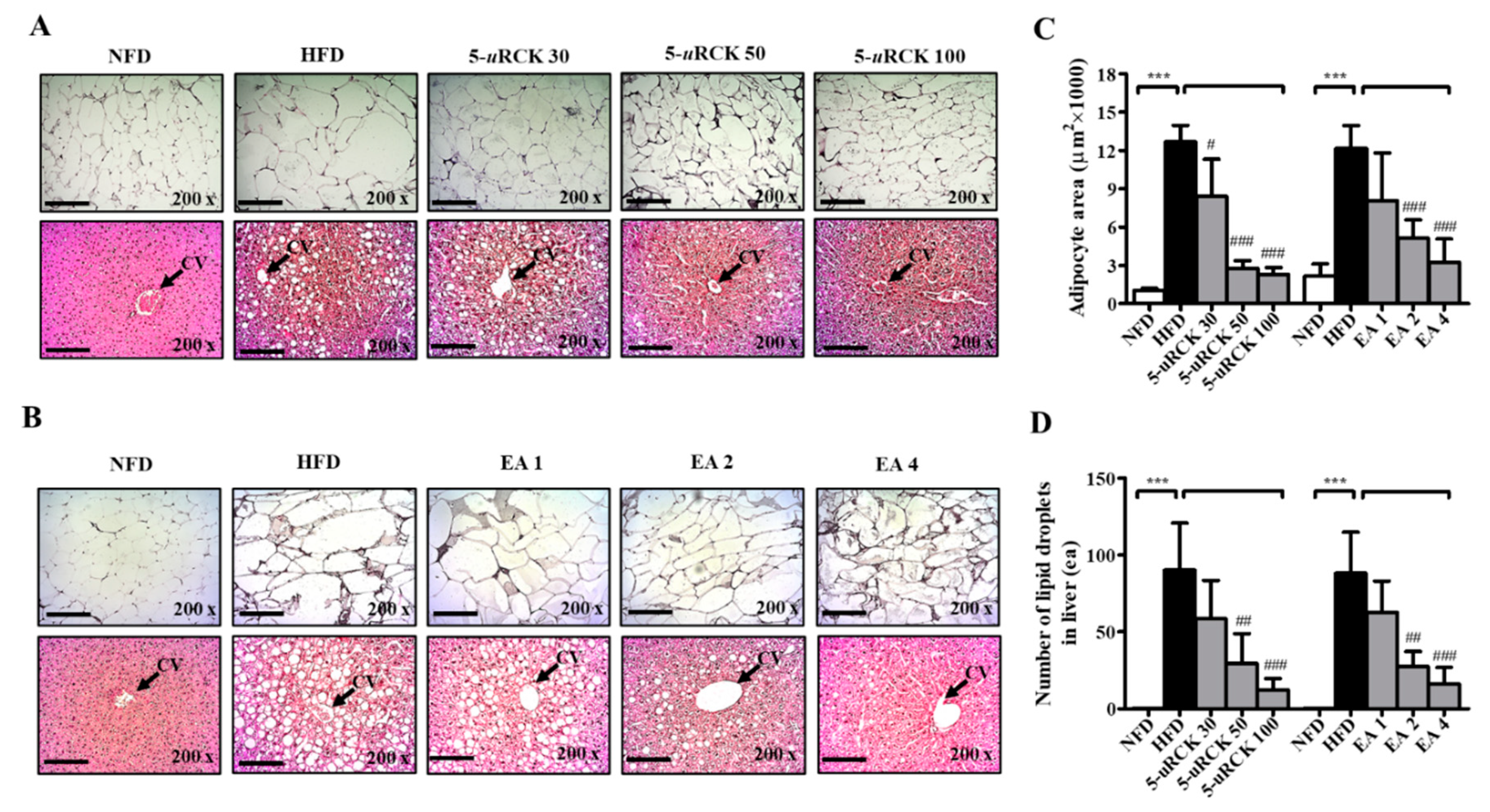
2.8. Effects of 5-uRCK and Ellagic Acid on Histological Changes in Adipose Tissue and Liver
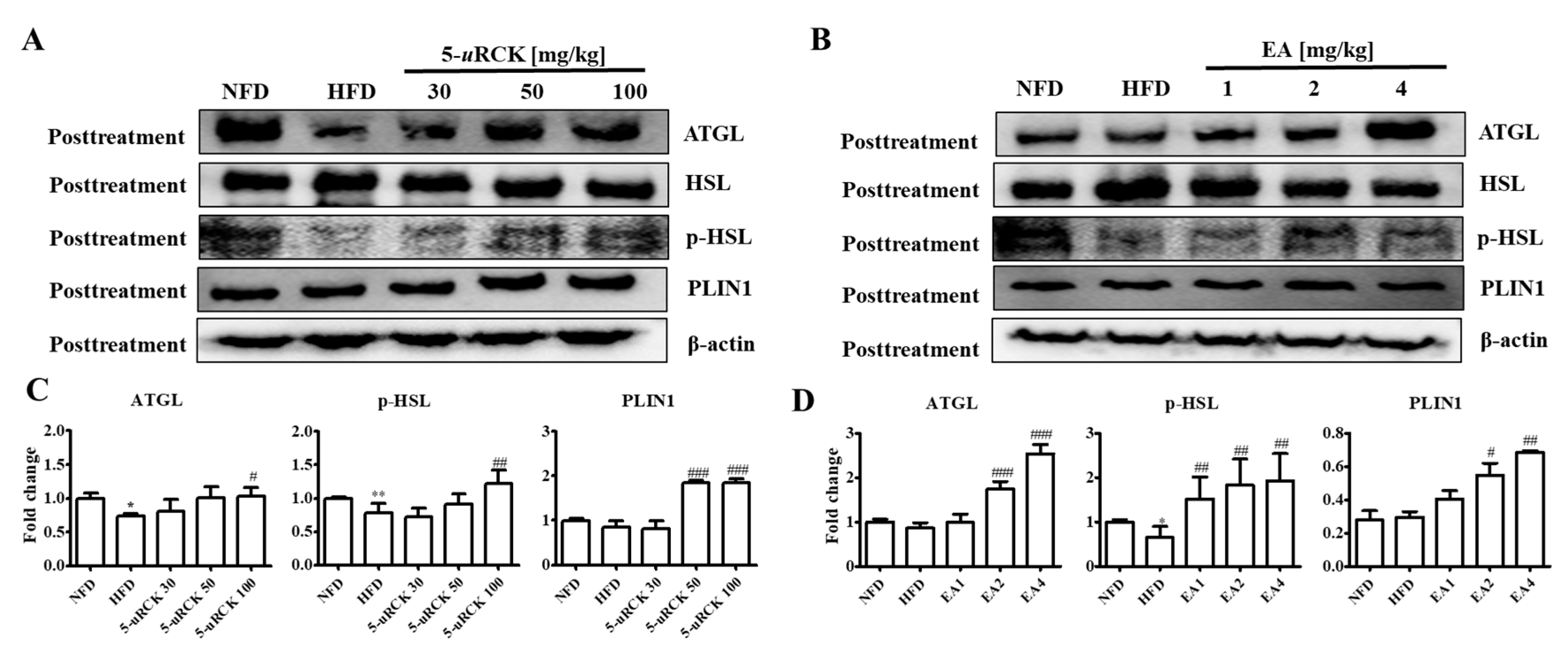
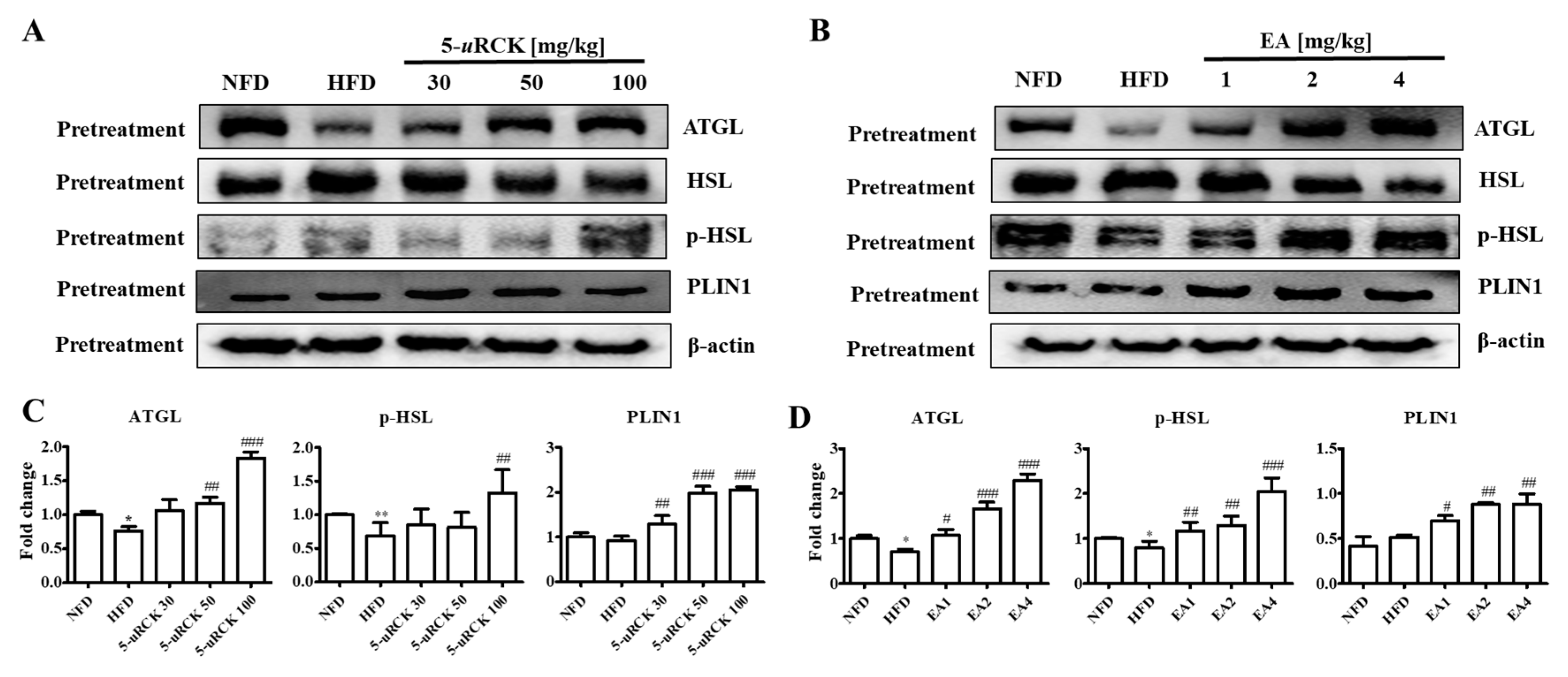
2.9. Protein Expression Levels for Lipolysis-Related Proteins in Visceral eWAT
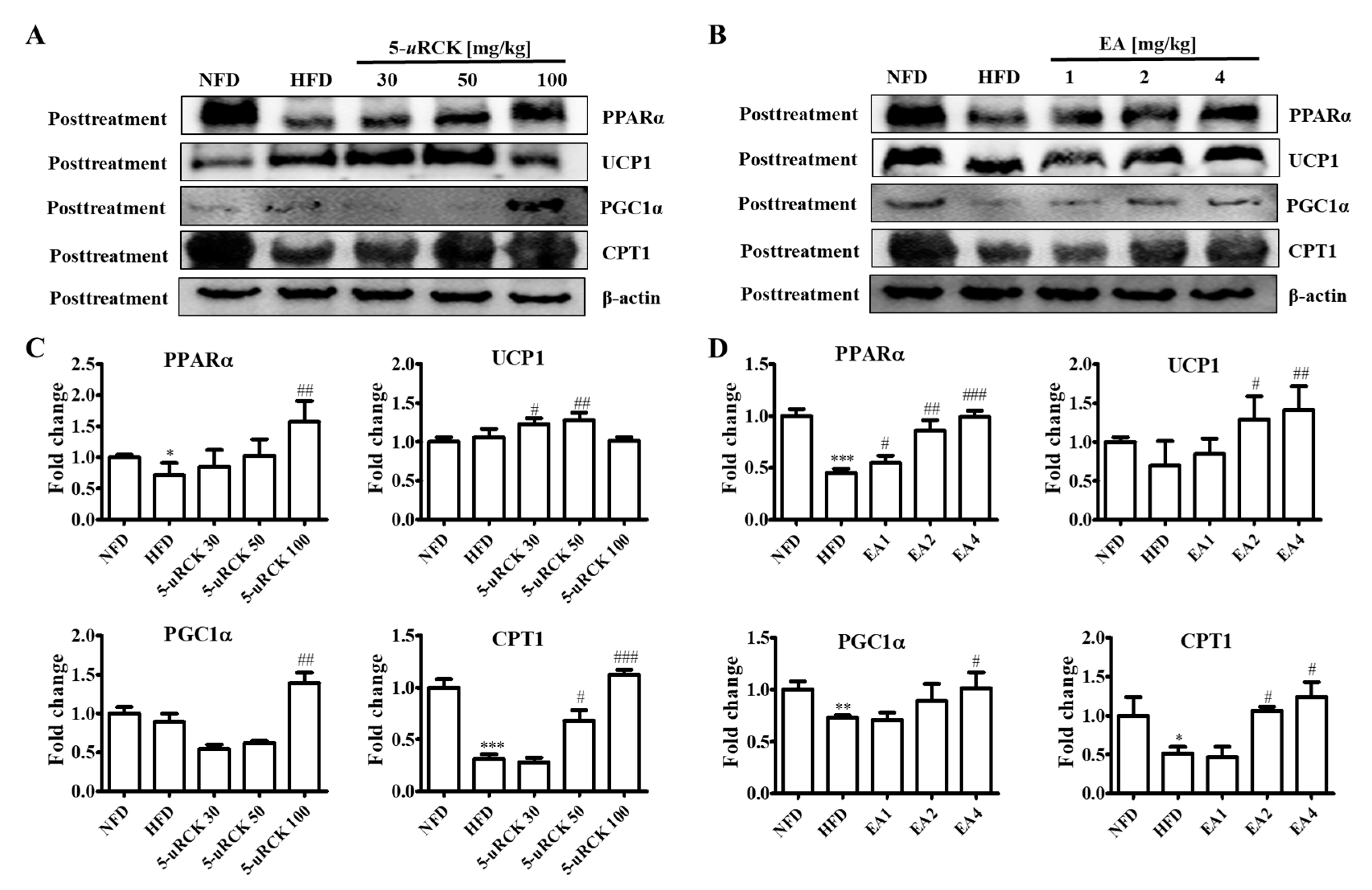
2.10. Protein Expression Levels for Browning-Related Proteins in Subcutaneous ingWAT
2.11. Pretreatment with 5-uRCK Inhibits HFD-Induced Body Weight Gain
2.12. Pretreatment with Ellagic Acid Inhibits Body Weight Gain in HFD-Fed Mice
2.13. 5-uRCK and Ellagic Acid Inhibited Serum Lipid Profile Increases in HFD-Induced Obese Mice
2.14. Pretreatment with 5-uRCK or Ellagic Acid Improves Serum Biochemical Parameters in HFD-Fed Mice
2.15. 5-uRCK and Ellagic Acid Suppress Histological Changes in the eWAT and Liver Tissues of HFD-Fed Mice
2.16. Protein Expression Levels for Lipolysis-Related Proteins in Visceral eWAT
2.17. Protein Expression Levels for Browning-Related Proteins in Subcutaneous ingWAT
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Extracts
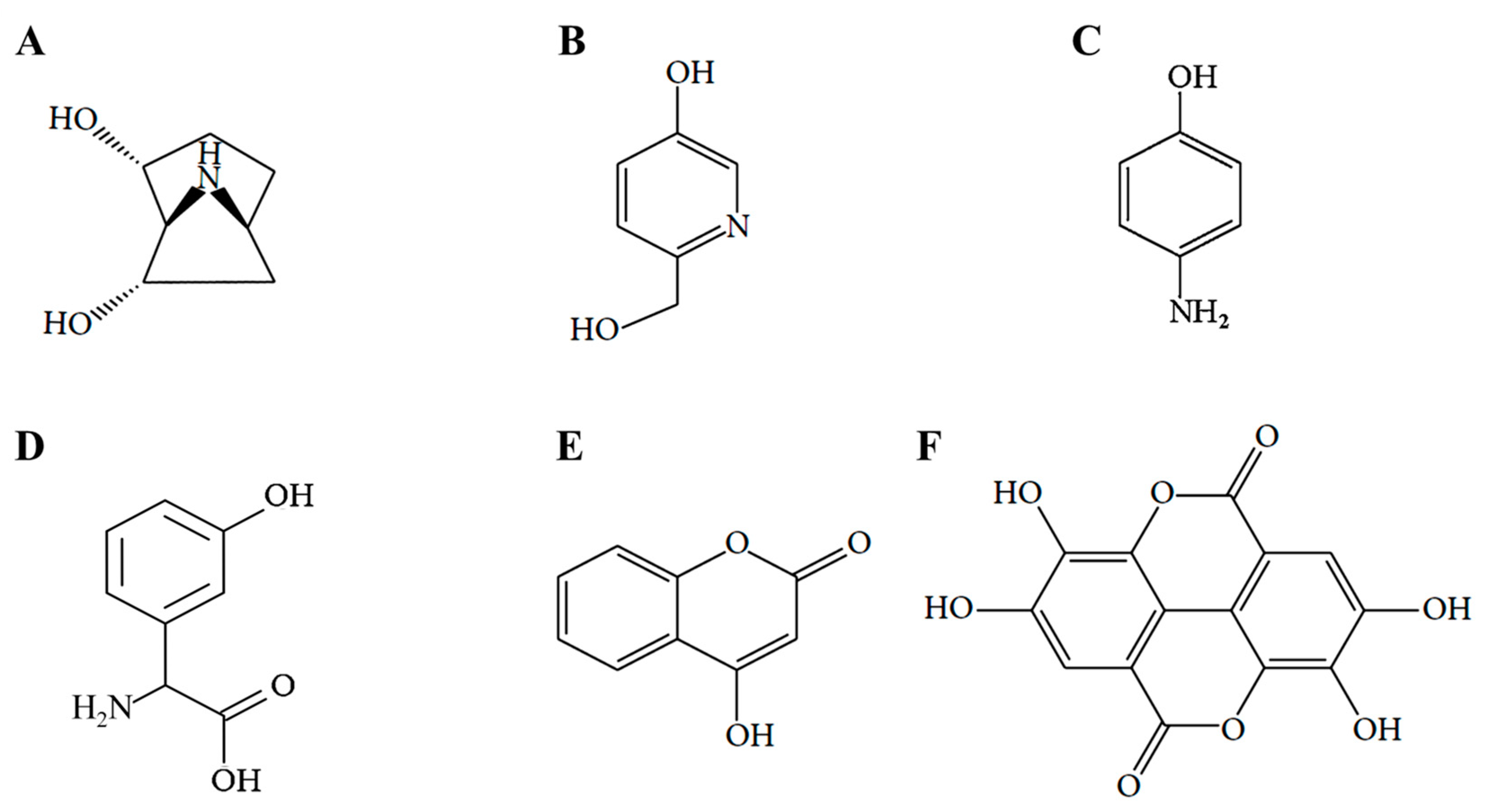
4.3. Isolation of Ellagic Acid
4.4. Cell Culture
4.5. Lipid Accumulation, GPDH Activity, and Triglyceride Measurement
4.6. Measurement of Free Glycerol Release
4.7. Animals
4.8. Experimental Groups and Administration
4.9. Preparation of Blood Samples and Biochemical Characterization
4.10. Histological Analysis of the Liver and Adipose Tissue
4.11. Protein Extraction and Immunoblot Assays
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Joyal, S.V. A perspective on the current strategies for the treatment of obesity. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 341–356. [Google Scholar] [CrossRef]
- Krentz, A.J.; Fujioka, K.; Hompesch, M. Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes Obes. Metab. 2016, 18, 558–570. [Google Scholar] [CrossRef]
- González-Castejón, M.; Rodriguez-Casado, A. Dietary phytochemicals and their potential effects on obesity: A review. Pharmacol. Res. 2011, 64, 438–455. [Google Scholar] [CrossRef]
- Tung, Y.C.; Hsieh, P.H.; Pan, M.H.; Ho, C.T. Cellular models for the evaluation of the antiobesity effect of selected phytochemicals from food and herbs. J. Food Drug Anal. 2017, 25, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Masood, S.; Sultana, S.; Hadda, T.B.; Bader, A.; Zafar, M. Report: Antioxidant and nutraceutical value of wild medicinal Rubus berries. Pak. J. Pharm. Sci. 2015, 28, 241–247. [Google Scholar] [PubMed]
- Moon, G.S. Constituents and Uses of Medicinal Herbs; Ilweolseogak: Seoul, Korea, 1991; pp. 310–311. [Google Scholar]
- Yang, J.W.; Choi, I.S. Comparison of the phenolic composition and antioxidant activity of Korean black raspberry, Bokbunja, (Rubus coreanus Miquel) with those of six other berries. CyTA J. Food 2017, 15, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Bhandary, B.; Lee, G.H.; Marahatta, A.; Lee, H.Y.; Kim, S.Y.; So, B.O.; Kwon, J.W.; Song, J.Y.; Lee, H.K.; Kim, H.R.; et al. Water extracts of immature Rubus coreanus regulate lipid metabolism in liver cells. Biol. Pharm. Bull. 2012, 35, 1907–1913. [Google Scholar] [CrossRef] [Green Version]
- Choung, M.G.; Lim, J.D. Antioxidant, anticancer and immune activation of anthocyanin fraction from Rubus coreanus Miquel fruits (Bokbunja). Korean J. Med. Crop Sci. 2012, 20, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Ward, L.; Brown, L. Ellagic acid attenuates high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2013, 52, 559–568. [Google Scholar] [CrossRef]
- Bobinaitė, R.; Viškelis, P.; Venskutonis, P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012, 132, 1495–1501. [Google Scholar] [CrossRef]
- Yang, H.M.; Oh, S.M.; Lim, S.S.; Shin, H.K.; Oh, Y.S.; Kim, J.K. Antiinflammatory activities of Rubus coreanus depend on the degree of fruit ripening. Phytother. Res. 2008, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Akune, T.; Ohba, S.; Kamekura, S.; Yamaguchi, M.; Chung, U.I.; Kubota, N.; Terauchi, Y.; Harada, Y.; Azuma, Y.; Nakamura, K.; et al. PPARγ insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Investig. 2004, 113, 846–855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Fu, M.; Cui, T.; Xiong, C.; Xu, K.; Zhong, W.; Xiao, Y.; Floyd, D.; Liang, J.; Li, E.; et al. Selective disruption of PPARγ 2 impairs the development of adipose tissue and insulin sensitivity. Proc. Natl. Acad. Sci. USA 2004, 101, 10703–10708. [Google Scholar] [CrossRef] [Green Version]
- Smink, J.J.; Leutz, A. Instruction of mesenchymal cell fate by the transcription factor C/EBPβ. Gene 2012, 497, 10–17. [Google Scholar] [CrossRef]
- Freytag, S.O.; Paielli, D.L.; Gilbert, J.D. Ectopic expression of the CCAAT/enhancer-binding protein α promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 1994, 8, 1654–1663. [Google Scholar] [CrossRef] [Green Version]
- Linhart, H.G.; Ishimura-Oka, K.; DeMayo, F.; Kibe, T.; Repka, D.; Poindexter, B.; Bick, R.J.; Darlington, G.J. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc. Natl. Acad. Sci. USA 2001, 98, 12532–12537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.D.; Finegold, M.J.; Bradley, A.; Ou, C.N.; Abdelsayed, S.V.; Wilde, M.D.; Taylor, L.R.; Wilson, D.R.; Darlington, G.J. Impaired energy homeostasis in C/EBPα knockout mice. Science 1995, 269, 1108–1112. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPβ and/or C/EBPδ gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef] [Green Version]
- Hagen, R.M.; Rodriguez-Cuenca, S.; Vidal-Puig, A. An allostatic control of membrane lipid composition by SREBP1. FEBS Lett. 2010, 584, 2689–2698. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.-A.; Cho, S.-H.; Lee, S.Y.; Kim, J.H.; Kim, Y.-S.; Oh, K.; Yoo, G.; Lee, D.-W.; Kim, S. Anti-obesity effects on unripe Rubus coreanus Miquel extract in high fat diet-induced obese mice. Int. J. Biochem. Res. Rev. 2014, 5, 20–26. [Google Scholar] [CrossRef]
- Oh, D.-R.; Kim, Y.; Choi, E.-J.; Lee, H.-; Jung, M.-A.; Bae, D.; Jo, A.; Kim, Y.R.; Kim, S. Antiobesity effects of unripe Rubus coreanus Miquel and its constituents: An in vitro and in vivo characterization of the underlying mechanism. Evid.-Based Complement. Altern. Med. 2016, 2016, 4357656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, M.-A.; Cho, S.-H.; Lee, S.-Y.; Kim, J.H.; Oh, K.; Kim, Y.-S.; Yoo, G.; Lee, D.-W.; Kim, S. Effects of unripe Rubus coreanus Miquel extract on improvement of lipid metabolism in C57BL/6 mice fed a high cholesterol diet. J. Korean Soc. Food Sci. Nutr. 2014, 43, 650–655. [Google Scholar] [CrossRef]
- Lee, K.H.; Jeong, E.-S.; Jang, G.; Na, J.-R.; Park, S.; Kang, W.S.; Kim, E.; Choi, H.; Kim, J.S.; Kim, S. Unripe Rubus coreanus Miquel extract containing ellagic acid regulates AMPK, SREBP-2, HMGCR and INSIG-1 signaling and cholesterol metabolism in vitro and in vivo. Nutrients 2020, 12, 610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.M.; Chae, J.; Jeong, S.R.; Moon, M.J.; Ha, K.-C.; Kim, S.; Lee, J.H. The cholesterol-lowering effect of unripe Rubus coreanus is associated with decreased oxidized LDL and apolipoprotein B levels in subjects with borderline-high cholesterol levels: A randomized controlled trial. Lipids Health Dis. 2020, 19, 166. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef]
- Kim, H.-L.; Jung, Y.; Park, J.; Youn, D.-H.; Kang, J.; Lim, S.; Lee, B.S.; Jeong, M.-Y.; Choe, S.-K.; Park, R.; et al. Farnesol Has an Anti-obesity Effect in High-Fat Diet-Induced Obese Mice and Induces the Development of Beige Adipocytes in Human Adipose Tissue Derived-Mesenchymal Stem Cells. Front. Pharmacol. 2017, 8, 654. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, T.; Hata, K.; Hiwatashi, K.; Hori, K.; Suzuki, N.; Itoh, H. Suppression of murine preadipocyte differentiation and reduction of visceral fat accumulation by a Petasites japonicus ethanol extract in mice fed a high-fat diet. Biosci. Biotechnol. Biochem. 2010, 74, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Gavrilova, O.; Pack, S.; Jou, W.; Mullen, S.; Sumner, A.E.; Cushman, S.W.; Periwal, V. Hypertrophy and/or Hyperplasia: Dynamics of Adipose Tissue Growth. PLoS Comput. Biol. 2009, 5, e1000324. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.; Sekhon-Loodu, S.; Mantso, T.; Panayiotidis, M.I. Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss. Pharmacol. Ther. 2016, 165, 153–163. [Google Scholar] [CrossRef]
- Balaji, M.; Ganjayi, M.S.; Hanuma Kumar, G.E.; Parim, B.N.; Mopuri, R.; Dasari, S. A review on possible therapeutic targets to contain obesity: The role of phytochemicals. Obes. Res. Clin. Pract. 2016, 10, 363–380. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Takahashi, N.; Kawada, T.; Miyashita, K. Fucoxanthin and its metabolite, fucoxanthinol, suppress adipocyte differentiation in 3T3-L1 cells. Int. J. Mol. Med. 2006, 18, 147–152. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Wang, Y.; You, J.S.; Mir, P.S.; McAllister, T.A. Effect of a seaweed extract on fatty acid accumulation and glycerol-3-phosphate dehydrogenase activity in 3T3-L1 adipocytes. Lipids 2009, 44, 125–132. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweiger, M.; Schreiber, R.; Haemmerle, G.; Lass, A.; Fledelius, C.; Jacobsen, P.; Tornqvist, H.; Zechner, R.; Zimmermann, R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J. Biol. Chem. 2006, 281, 40236–40241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lass, A.; Zimmermann, R.; Oberer, M.; Zechner, R. Lipolysis—A highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 2011, 50, 14–27. [Google Scholar] [CrossRef] [Green Version]
- Miyoshi, H.; Perfield, J.W., 2nd; Obin, M.S.; Greenberg, A.S. Adipose triglyceride lipase regulates basal lipolysis and lipid droplet size in adipocytes. J. Cell. Biochem. 2008, 105, 1430–1436. [Google Scholar] [CrossRef] [Green Version]
- Garcia, A.; Subramanian, V.; Sekowski, A.; Bhattacharyya, S.; Love, M.W.; Brasaemle, D.L. The Amino and Carboxyl Termini of Perilipin A Facilitate the Storage of Triacylglycerols. J. Biol. Chem. 2004, 279, 8409–8416. [Google Scholar] [CrossRef] [Green Version]
- Guzmán, M.; Lo Verme, J.; Fu, J.; Oveisi, F.; Blázquez, C.; Piomelli, D. Oleoylethanolamide stimulates lipolysis by activating the nuclear receptor peroxisome proliferator-activated receptor α (PPAR-α). J. Biol. Chem. 2004, 279, 27849–27854. [Google Scholar] [CrossRef] [Green Version]
- Dulloo, A.G.; Seydoux, J.; Jacquet, J. Adaptive thermogenesis and uncoupling proteins: A reappraisal of their roles in fat metabolism and energy balance. Physiol. Behav. 2004, 83, 587–602. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Yu, X.; Gonzales, F.; Mangelsdorf, D.J.; Wang, M.-Y.; Richardson, C.; Witters, L.A.; Unger, R.H. PPARα is necessary for the lipopenic action of hyperleptinemia on white adipose and liver tissue. Proc. Natl. Acad. Sci. USA 2002, 99, 11848–11853. [Google Scholar] [CrossRef] [Green Version]
- Tseng, Y.-H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.H.; Kahn, C.R. Developmental origin of fat: Tracking obesity to its source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seale, P. Transcriptional Regulatory Circuits Controlling Brown Fat Development and Activation. Diabetes 2015, 64, 2369–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koutsari, C.; Jensen, M.D. Thematic review series: Patient-oriented research. Free fatty acid metabolism in human obesity. J. Lipid Res. 2006, 47, 1643–1650. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Dellsperger, K.C.; Zhang, C. The link between metabolic abnormalities and endothelial dysfunction in type 2 diabetes: An update. Basic Res. Cardiol. 2012, 107, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, G.F.; Carpentier, A.; Adeli, K.; Giacca, A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr. Rev. 2002, 23, 201–229. [Google Scholar] [CrossRef]
- Peirce, V.; Carobbio, S.; Vidal-Puig, A. The different shades of fat. Nature 2014, 510, 76–83. [Google Scholar] [CrossRef]
- Wang, W.; Seale, P. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 2016, 17, 691–702. [Google Scholar] [CrossRef]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Medina-Gómez, G.; López, M. Hypothalamus and thermogenesis: Heating the BAT, browning the WAT. Mol. Cell. Endocrinol. 2016, 438, 107–115. [Google Scholar] [CrossRef]
- Harms, M.; Seale, P. Brown and beige fat: Development, function and therapeutic potential. Nat. Med. 2013, 19, 1252–1263. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wei, Y.; Ning, C.; Zhang, M.; Fan, P.; Lei, D.; Du, J.; Gale, M.; Ma, Y.; Yang, Y. Ellagic acid promotes browning of white adipose tissues in high-fat diet-induced obesity in rats through suppressing white adipocyte maintaining genes. Endocr. J. 2019, 66, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Park, W.Y.; Choe, S.K.; Park, J.; Um, J.Y. Black Raspberry (Rubus coreanus Miquel) Promotes Browning of Preadipocytes and Inguinal White Adipose Tissue in Cold-Induced Mice. Nutrients 2019, 11, 2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.; Sanyal, A.J. The metabolic abnormalities associated with non-alcoholic fatty liver disease. Best Pract. Res. Clin. Gastroenterol. 2002, 16, 709–731. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Rao, M.S. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G852–G858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, A.; Wang, Y.; Keshaw, H.; Xu, L.Y.; Lam, K.S.; Cooper, G.J. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J. Clin. Investig. 2003, 112, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Milagro, F.I.; Campión, J.; Martínez, J.A. Weight gain induced by high-fat feeding involves increased liver oxidative stress. Obesity 2006, 14, 1118–1123. [Google Scholar] [CrossRef]
- Radi, Z.A.; Koza-Taylor, P.H.; Bell, R.R.; Obert, L.A.; Runnels, H.A.; Beebe, J.S.; Lawton, M.P.; Sadis, S. Increased serum enzyme levels associated with kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am. J. Pathol. 2011, 179, 240–247. [Google Scholar] [CrossRef]
- Cuccioloni, M.; Mozzicafreddo, M.; Bonfili, L.; Cecarini, V.; Eleuteri, A.M.; Angeletti, M. Natural occurring polyphenols as template for drug design. Focus on serine proteases. Chem. Biol. Drug Des. 2009, 74, 1–15. [Google Scholar] [CrossRef]
- Makino-Wakagi, Y.; Yoshimura, Y.; Uzawa, Y.; Zaima, N.; Moriyama, T.; Kawamura, Y. Ellagic acid in pomegranate suppresses resistin secretion by a novel regulatory mechanism involving the degradation of intracellular resistin protein in adipocytes. Biochem. Biophys. Res. Commun. 2012, 417, 880–885. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, H.; Kim, S.; Ha, T. Resveratrol inhibits TNF-α-induced changes of adipokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 364, 972–977. [Google Scholar] [CrossRef]
- Lee, K.H.; Kim, S. Comparison of ellagic acid contents in Korean and Chinese cultivated species of unripe black raspberries. Korean J. Food Preserv. 2018, 25, 549–556. [Google Scholar] [CrossRef]
- Bertin, E.; Nguyen, P.; Guenounou, M.; Durlach, V.; Potron, G.; Leutenegger, M. Plasma levels of tumor necrosis factor-α (TNF-α) are essentially dependent on visceral fat amount in type 2 diabetic patients. Diabetes Metab. 2000, 26, 178–182. [Google Scholar] [PubMed]
- Fain, J.N.; Madan, A.K.; Hiler, M.L.; Cheema, P.; Bahouth, S.W. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004, 145, 2273–2282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotamisligil, G.S.; Shargill, N.S.; Spiegelman, B.M. Adipose expression of tumor necrosis factor-α: Direct role in obesity-linked insulin resistance. Science 1993, 259, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Barnes, G.T.; Yang, Q.; Tan, G.; Yang, D.; Chou, C.J.; Sole, J.; Nichols, A.; Ross, J.S.; Tartaglia, L.A.; et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Investig. 2003, 112, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Maachi, M.; Piéroni, L.; Bruckert, E.; Jardel, C.; Fellahi, S.; Hainque, B.; Capeau, J.; Bastard, J.-P. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 993–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Choi, Y.; Choi, Y.H.; Park, T. Obesity activates toll-like receptor-mediated proinflammatory signaling cascades in the adipose tissue of mice. J. Nutr. Biochem. 2012, 23, 113–122. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.H.; Kang, W.S.; Kim, J.S.; Kim, S. Endothelium-dependent and endothelium-independent vasorelaxant effects of unripe Rubus coreanus Miq. and Dendropanax morbiferus H. Lév. extracts on rat aortic rings. BMC Complement. Med. Ther. 2020, 20, 190. [Google Scholar] [CrossRef]
- The European Parliament and the Council of the European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the Protection of Animals Used for Scientific Purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Biomarker | NFD | HFD | 5-uRCK 30 | 5-uRCK 50 | 5-uRCK 100 |
|---|---|---|---|---|---|
| TG (mg/dL) | 117.50 ± 6.72 | 160.50 ± 5.55 *** | 161.00 ± 3.27 | 152.10 ± 7.85 | 143.90 ± 3.94 # |
| TC (mg/dL) | 115.80 ± 3.52 | 210.30 ± 7.22 *** | 205.30 ± 4.10 | 197.50 ± 3.92 | 187.70 ± 4.40 # |
| HDL-C (mg/dL) | 48.59 ± 1.52 | 23.25 ± 1.14 *** | 42.62 ± 1.77 ### | 47.33 ± 0.72 ### | 44.86 ± 4.29 ### |
| LDL-C (mg/dL) | 67.51 ± 4.17 | 139.00 ± 6.26 *** | 121.00 ± 6.01 | 119.90 ± 1.95 # | 109.20 ± 5.02 ## |
| AST (U/I) | 127.10 ± 6.18 | 235.50 ± 24.62 *** | 143.60 ± 8.44 ## | 119.90 ± 3.88 ### | 123.90 ± 10.26 ## |
| ALT (U/I) | 23.00 ± 1.09 | 58.50 ± 2.85 *** | 53.63 ± 5.93 | 44.90 ± 7.33 | 41.90 ± 2.73 ### |
| CRE (mg/dL) | 0.21 ± 0.03 | 0.56 ± 0.04 *** | 0.25 ± 0.01 ### | 0.28 ± 0.01 ### | 0.28 ± 0.02 ### |
| Amylase (U/I) | 839.90 ± 25.31 | 1241.00 ± 47.45 *** | 1045.00 ± 16.18 ## | 1017.00 ± 45.18 ## | 906.10 ± 19.38 ### |
| UA (U/I) | 1.55 ± 0.12 | 3.30 ± 0.46 ** | 3.06 ± 0.11 | 1.46 ± 0.04 ### | 1.44 ± 0.04 ### |
| LDH (U/I) | 213.60 ± 13.49 | 334.60 ± 23.44 ** | 235.90 ± 21.26 ## | 223.30 ± 13.91 ### | 196.40 ± 25.00 ### |
| Glucose (mg/dL) | 189.30 ± 13.08 | 268.20 ± 33.67 * | 255.50 ± 8.30 | 235.20 ± 10.57 | 236.10 ± 19.21 |
| Biomarker | NFD | HFD | EA 1 | EA 2 | EA 4 |
|---|---|---|---|---|---|
| TG (mg/dL) | 101.31 ± 6.19 | 233.71 ± 22.01 *** | 112.11 ± 7.30 ### | 96.91 ± 4.26 ### | 112.01 ± 7.57 ### |
| TC (mg/dL) | 112.72 ± 5.45 | 175.01 ± 5.08 *** | 161.61 ± 11.91 | 126.41 ± 6.42 ### | 126.11 ± 3.28 ### |
| HDL-C (mg/dL) | 46.22 ± 1.39 | 24.00 ± 2.39 *** | 35.00 ± 1.53 ## | 39.92 ± 3.45 ## | 46.18 ± 3.78 ### |
| LDL-C (mg/dL) | 69.31 ± 4.83 | 99.28 ± 5.50 ** | 88.43 ± 11.88 | 67.53 ± 6.99 ## | 65.17 ± 3.91 ### |
| AST (U/I) | 150.60 ± 15.79 | 272.41 ± 20.51 *** | 154.01 ± 13.47 ### | 144.40 ± 14.81 ### | 149.31 ± 7.31 ### |
| ALT (U/I) | 27.57 ± 2.28 | 90.45 ± 10.37 *** | 56.29 ± 5.19 # | 39.71 ± 4.32 ## | 34.71 ± 1.36 ### |
| CRE (mg/dL) | 0.11 ± 0.01 | 0.38 ± 0.04 *** | 0.22 ± 0.05 # | 0.19 ± 0.01 ## | 0.19 ± 0.02 ## |
| Amylase (U/I) | 603.01 ± 29.08 | 1059.01 ± 47.63 *** | 690.70 ± 32.48 ### | 680.31 ± 29.71 ### | 615.61 ± 39.97 ### |
| UA (U/I) | 1.59 ± 0.19 | 3.26 ± 0.27 *** | 3.16 ± 0.12 | 3.16 ± 0.22 | 3.30 ± 0.05 |
| LDH (U/I) | 162.71 ± 23.92 | 402.91 ± 60.70 ** | 335.71 ± 25.31 | 321.61 ± 22.52 | 357.01 ± 26.22 |
| Glucose (mg/dL) | 225.62 ± 5.50 | 333.01 ± 25.20 ** | 210.71 ± 3.89 ### | 190.91 ± 7.68 ### | 118.71 ± 9.02 ### |
| Biomarker | NFD | HFD | 5-uRCK 30 | 5-uRCK 50 | 5-uRCK 100 |
|---|---|---|---|---|---|
| TG (mg/dL) | 138.90 ± 7.57 | 215.50 ± 14.64 *** | 154.90 ± 4.32 ## | 147.90 ± 4.42 ## | 148.60 ± 11.08 ## |
| TC (mg/dL) | 103.00 ± 9.01 | 193.30 ± 3.98 *** | 194. 30 ± 4.71 | 154.90 ± 13.15 # | 153.80 ± 3.06 ### |
| HDL-C (mg/dL) | 45.17 ± 1.09 | 23.64 ± 2.05 *** | 37.44 ± 3.53 ## | 38.75 ± 0.59 ### | 46.86 ± 2.09 ### |
| LDL-C (mg/dL) | 80.89 ± 6.26 | 145.60 ± 3.80 *** | 145.80 ± 2.35 | 121.20 ± 8.92 # | 111.90 ± 2.26 ### |
| AST (U/I) | 118.00 ± 3.33 | 316.00 ± 7.97 *** | 141.20 ± 11.14 ### | 121.30 ± 3.94 ### | 114.8 ± 12.92 ### |
| ALT (U/I) | 26.38 ± 0.91 | 79.25 ± 3.14 *** | 35.63 ± 1.00 ### | 26.13 ± 2.11 ### | 24.38 ± 1.31 ### |
| CRE (mg/dL) | 0.20 ± 0.03 | 0.44 ± 0.04 *** | 0.31 ± 0.01 ## | 0.26 ± 0.02 ### | 0.26 ± 0.02 ### |
| Amylase (U/I) | 708.00 ± 32.70 | 982.10 ± 35.35 *** | 1012.01 ± 22.67 | 914.50 ± 25.03 | 845.60 ± 31.84 # |
| UA (U/I) | 2.32 ± 0.10 | 4.62 ± 0.17 *** | 2.21 ± 0.24 ### | 1.81 ± 0.19 ### | 2.11 ± 0.23 ### |
| LDH (U/I) | 165.40 ± 12.20 | 429.00 ± 25.45 *** | 249.60 ± 22.34 ### | 217.90 ± 17.70 ### | 178.70 ± 10.73 ### |
| Glucose (mg/dL) | 173.80 ± 5.15 | 220.90 ± 7.93 *** | 202.60 ± 5.82 | 208.80 ± 7.44 | 203.80 ± 9.14 |
| Biomarker | NFD | HFD | EA 1 | EA 2 | EA 4 |
|---|---|---|---|---|---|
| TG (mg/dL) | 133.40 ± 11.53 | 190.01 ± 10.00 ** | 174.10 ± 4.38 | 138.40 ± 6.20 ### | 129.61 ± 4.08 ### |
| TC (mg/dL) | 122.30 ± 10.61 | 216.31 ± 4.21 *** | 202.71 ± 3.18 # | 180.31 ± 4.63 ### | 175.30 ± 3.44 ### |
| HDL-C (mg/dL) | 50.10 ± 3.30 | 31.86 ± 1.90 *** | 48.17 ± 1.47 ### | 53.43 ± 1.20 ### | 54.77 ± 1.84 ### |
| LDL-C (mg/dL) | 60.46 ± 12.14 | 137.01 ± 3.96 *** | 108.01 ± 3.51 ### | 96.03 ± 5.07 ### | 89.51 ± 4.74 ### |
| AST (U/I) | 156.31 ± 3.44 | 249.01 ± 6.91 *** | 191.40 ± 9.07 ### | 189.41 ± 13.21 ### | 184.30 ± 16.80 ### |
| ALT (U/I) | 25.14 ± 2.15 | 58.86 ± 4.43 *** | 49.71 ± 2.12 | 35.57 ± 2.10 ### | 24.86 ± 2.09 ### |
| CRE (mg/dL) | 0.21 ± 0.03 | 0.47 ± 0.02 *** | 0.43 ± 0.03 | 0.35 ± 0.02 ## | 0.25 ± 0.03 ### |
| Amylase (U/I) | 716.70 ± 30.43 | 1198.01 ± 45.59 *** | 921.30 ± 22.32 ### | 911.41 ± 22.72 ### | 899.00 ± 28.02 ### |
| UA (U/I) | 2.40 ± 0.19 | 5.89 ± 0.40 *** | 5.41 ± 0.50 | 4.41 ± 0.36 # | 2.87 ± 0.20 ### |
| LDH (U/I) | 112.70 ± 15.12 | 474.70 ± 34.67 *** | 407.20 ± 20.99 | 369.50 ± 19.53 # | 261.90 ± 16.81 ### |
| Glucose (mg/dL) | 169.10 ± 6.99 | 255.40 ± 16.56 *** | 203.30 ± 6.92 # | 198.60 ± 18.29 # | 179.40 ± 5.13 ### |
| Test 1 (Post-Treatment) | Test 2 (Post-Treatment) | Test 3 (Post-Treatment) | Test 4 (Pretreatment) | Test 5 (Pretreatment) | |
|---|---|---|---|---|---|
| Experimental Groups | Normal fat diet group (NFD) n = 9 | Normal fat diet group (NFD) n = 8 | Normal fat diet group (NFD) n = 8 | Normal fat diet group (NFD) n = 8 | Normal fat diet group (NFD) n = 8 |
| High-fat diet group (HFD)/16-weeks n = 9 | High-fat diet group (HFD)/8-weeks n = 8 | High-fat diet group (HFD)/8-weeks n = 8 | High-fat diet group (HFD)/6-weeks n = 8 | High-fat diet group (HFD)/6-weeks n = 8 | |
| NFD + 5-uRCK 100 mg/kg/day group, n = 9 | HFD + 5-uRCK 30 mg/kg/day group, n = 8 | HFD + EA 1 mg/kg/day group, n = 8 | HFD + 5-uRCK 30 mg/kg/day group, n = 8 | HFD + EA 1 mg/kg/day group, n = 8 | |
| HFD + 5-uRCK 30 mg/kg/day group, n = 9 | HFD + 5-uRCK 50 mg/kg/day group, n = 8 | HFD + EA 2 mg/kg/day group, n = 8 | HFD + 5-uRCK 50 mg/kg/day group, n = 8 | HFD + EA 2 mg/kg/day group, n = 8 | |
| HFD + 5-uRCK 50 mg/kg/day group, n = 9 | HFD + 5-uRCK 100 mg/kg/day group, n = 8 | HFD + EA 4 mg/kg/day group, n = 8 | HFD + 5-uRCK 100 mg/kg/day group, n = 8 | HFD + EA 4 mg/kg/day group, n = 8 | |
| HFD + 5-uRCK 100 mg/kg/day group, n = 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.J.; Jeong, E.-S.; Lee, K.H.; Na, J.-R.; Park, S.; Kim, J.S.; Na, C.-S.; Kim, Y.R.; Kim, S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Promotes Lipolysis and Thermogenesis In Vitro and In Vivo. Molecules 2020, 25, 5954. https://doi.org/10.3390/molecules25245954
Kim KJ, Jeong E-S, Lee KH, Na J-R, Park S, Kim JS, Na C-S, Kim YR, Kim S. Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Promotes Lipolysis and Thermogenesis In Vitro and In Vivo. Molecules. 2020; 25(24):5954. https://doi.org/10.3390/molecules25245954
Chicago/Turabian StyleKim, Kyeong Jo, Eui-Seon Jeong, Ki Hoon Lee, Ju-Ryun Na, Soyi Park, Jin Seok Kim, Chang-Su Na, Young Ran Kim, and Sunoh Kim. 2020. "Unripe Rubus coreanus Miquel Extract Containing Ellagic Acid Promotes Lipolysis and Thermogenesis In Vitro and In Vivo" Molecules 25, no. 24: 5954. https://doi.org/10.3390/molecules25245954





