Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate Cancer
Abstract
:1. Introduction
2. Results
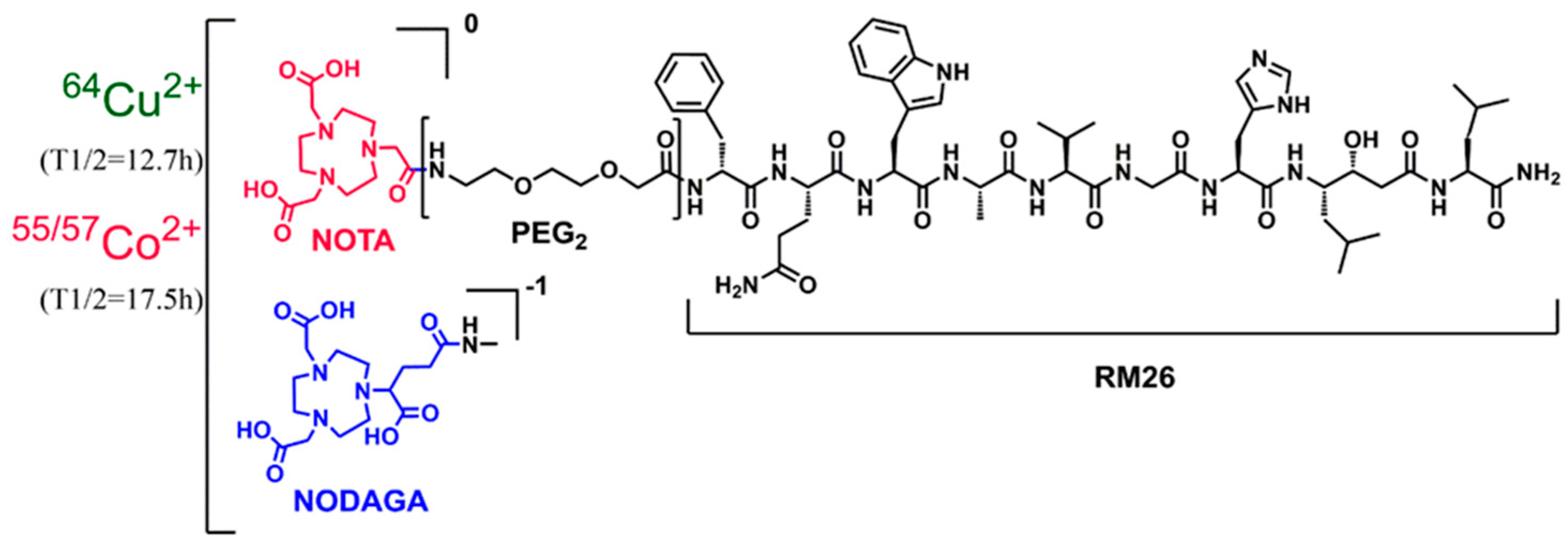
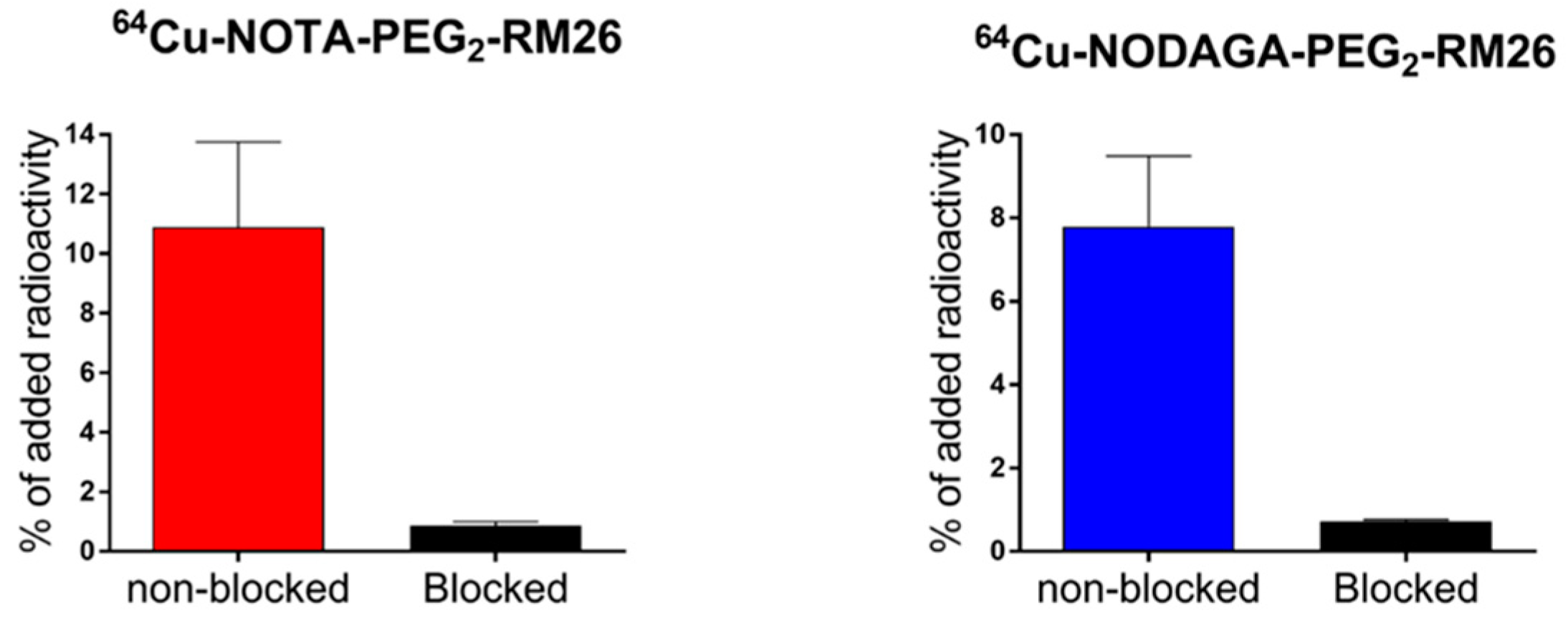
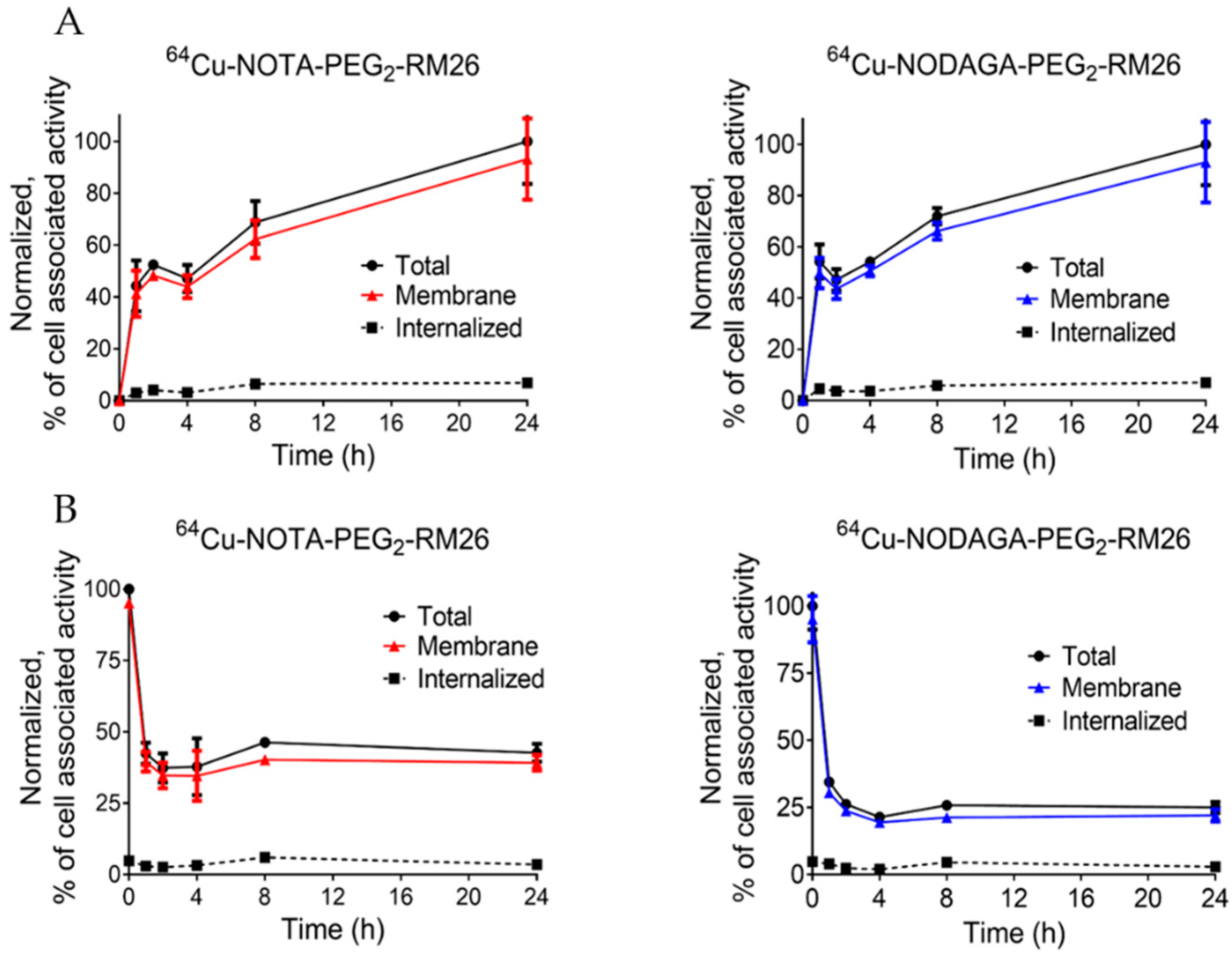
2.1. Labeling, Stability, and In Vitro Characterization of [64Cu]Cu-NOTA/NODAGA-PEG2-RM26
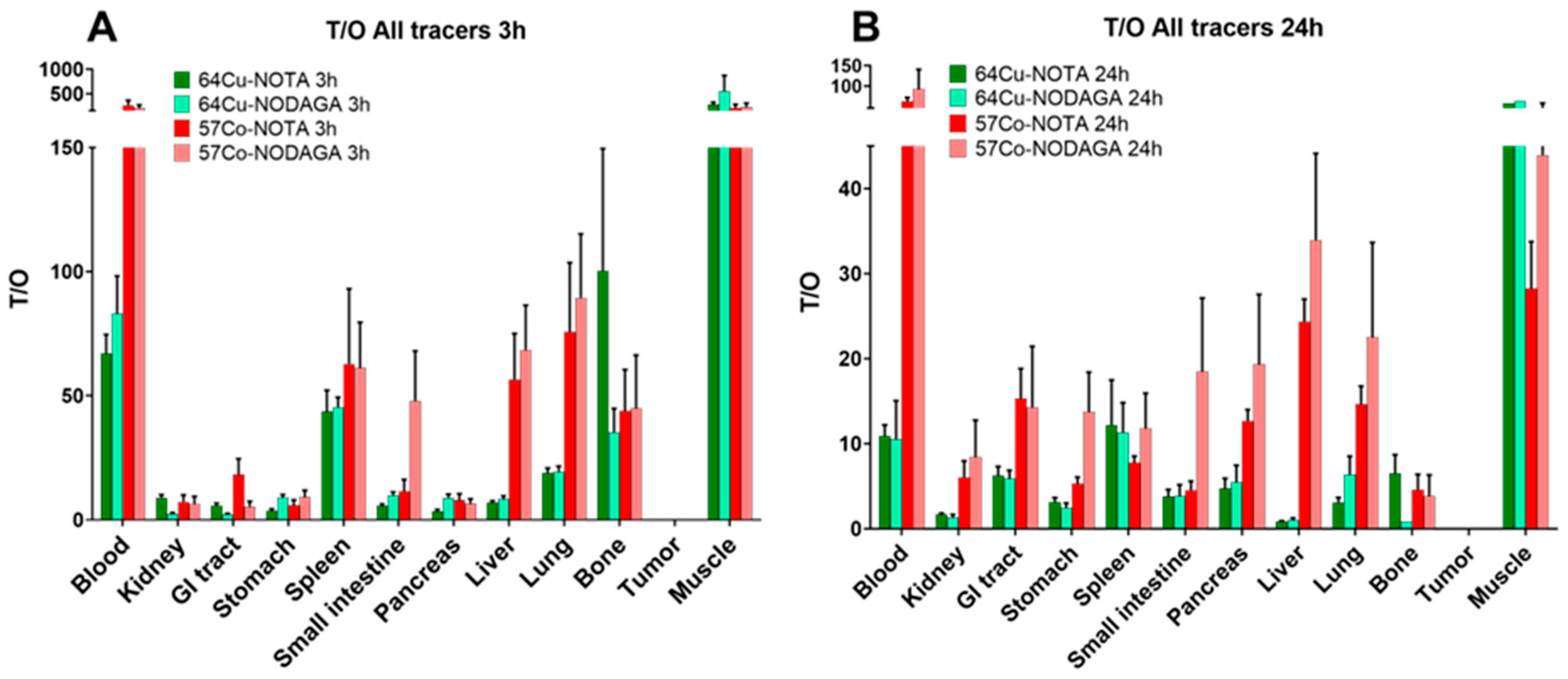
2.2. In Vivo Characterization of [64Cu]Cu-NOTA/NODAGA-PEG2-RM26 and Comparison with 55/57Co-Labeled Counterparts
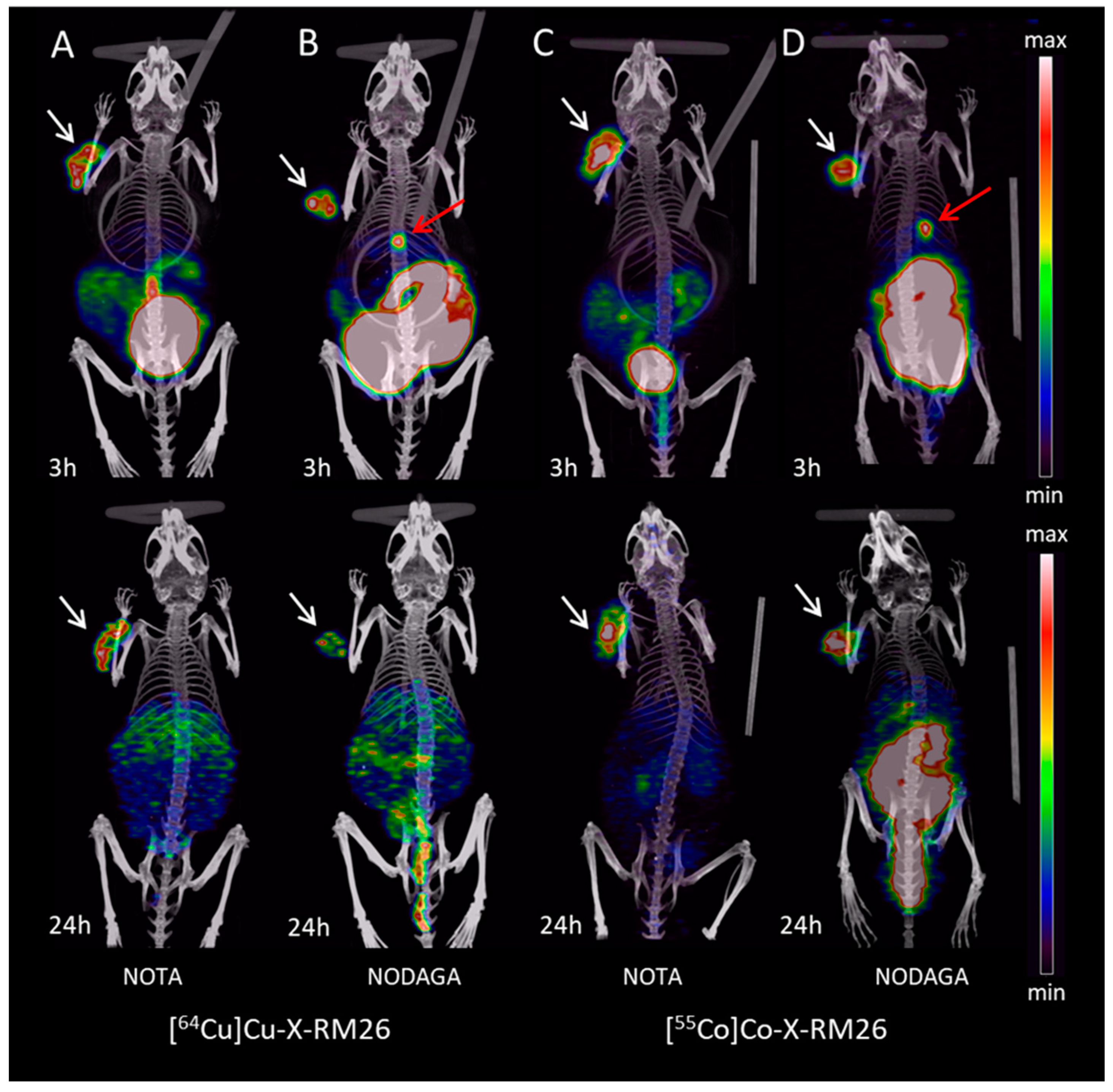
2.3. Imaging
3. Discussion
4. Materials and Methods
4.1. Labeling and Stability
4.2. In Vitro Studies
4.3. In Vivo Studies
4.4. Imaging
4.5. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global Cancer Statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Cornford, P.; Bellmunt, J.; Bolla, M.; Briers, E.; De Santis, M.; Gross, T.; Henry, A.M.; Joniau, S.; Lam, T.B.; Mason, M.D.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part II: Treatment of Relapsing, Metastatic, and Castration-Resistant Prostate Cancer. Eur. Urol. 2017, 71, 630–642. [Google Scholar] [CrossRef]
- Hövels, A.; Heesakkers, R.; Adang, E.; Jager, G.; Strum, S.; Hoogeveen, Y.; Severens, J.; Barentsz, J.O. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: A meta-analysis. Clin. Radiol. 2008, 63, 387–395. [Google Scholar] [CrossRef]
- Yu, C.Y.; Desai, B.; Ji, L.; Groshen, S.; Jadvar, H. Comparative performance of PET tracers in biochemical recurrence of prostate cancer: A critical analysis of literature. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 580–601. [Google Scholar]
- Schuster, D.M.; Savir-Baruch, B.; Nieh, P.T.; Master, V.A.; Halkar, R.K.; Rossi, P.J.; Lewis, M.M.; Nye, J.A.; Yu, W.; Bowman, F.D.; et al. Detection of Recurrent Prostate Carcinoma withanti-1-Amino-3-18F-Fluorocyclobutane-1-Carboxylic Acid PET/CT and111In–Capromab Pendetide SPECT/CT. Radiology 2011, 259, 852–861. [Google Scholar] [CrossRef] [Green Version]
- Schuster, D.M.; Taleghani, P.A.; Nieh, P.T.; Master, V.A.; Amzat, R.; Savir-Baruch, B.; Halkar, R.K.; Fox, T.; Osunkoya, A.O.; Moreno, C.S.; et al. Characterization of primary prostate carcinoma by anti-1-amino-2-[18F] -fluorocyclobutane-1-carboxylic acid (anti-3-[18F] FACBC) uptake. Am. J. Nucl. Med. Mol. Imaging 2013, 3, 85–96. [Google Scholar]
- Hohla, F.; Schally, A.V. Targeting gastrin releasing peptide receptors: New options for the therapy and diagnosis of cancer. Cell Cycle 2010, 9, 1738–1741. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, V.; Orlova, A. Radiolabeled GRPR Antagonists for Imaging of Disseminated Prostate Cancer Influence of Labeling Chemistry on Targeting Properties. Curr. Med. Chem. 2020, 27, 7090–7111. [Google Scholar] [CrossRef]
- Ananias, H.J.K.; Heuvel, M.C.V.D.; Helfrich, W.; De Jong, I.J. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Reile, H.; Armatis, P.E.; Schally, A.V. Characterization of high-affinity receptors for bombesin/gastrin releasing peptide on the human prostate cancer cell lines PC-3 and DU-145: Internalization of receptor bound 125I-(Tyr4) bombesin by tumor cells. Prostate 1994, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Körner, M.; Waser, B.; Rehmann, R.; Reubi, J.C. Early over-expression of GRP receptors in prostatic carcinogenesis. Prostate 2013, 74, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.; Montani, M.; Gerhardt, J.; Wild, P.J.; Hany, T.F.; Hermanns, T.; Müntener, M.; Kristiansen, G. Profiling gastrin-releasing peptide receptor in prostate tissues: Clinical implications and molecular correlates. Prostate 2011, 72, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Cornelio, D.; Roesler, R.; Schwartsmann, G. Gastrin-releasing peptide receptor as a molecular target in experimental anticancer therapy. Ann. Oncol. 2007, 18, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Pooja, D.; Gunukula, A.; Gupta, N.; Adams, D.; Kulhari, H. Bombesin receptors as potential targets for anticancer drug delivery and imaging. Int. J. Biochem. Cell Biol. 2019, 114, 105567. [Google Scholar] [CrossRef]
- Moreno, P.; Ramos-Álvarez, I.; Moody, T.W.; Jensen, R.T. Bombesin related peptides/receptors and their promising therapeutic roles in cancer imaging, targeting and treatment. Expert Opin. Ther. Targets 2016, 20, 1055–1073. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, C.D.A.; Fuscaldi, L.L.; Townsend, D.M.; Rubello, D.; De Barros, A.L.B. Radiolabeled bombesin derivatives for preclinical oncological imaging. Biomed. Pharmacother. 2017, 87, 58–72. [Google Scholar] [CrossRef] [Green Version]
- Mansi, R.; Wang, X.; Forrer, F.; Kneifel, S.; Tamma, M.-L.; Waser, B.; Cescato, R.; Reubi, J.C.; Maecke, H.R. Evaluation of a 1,4,7,10-Tetraazacyclododecane-1,4,7,10-Tetraacetic Acid-Conjugated Bombesin-Based Radioantagonist for the Labeling with Single-Photon Emission Computed Tomography, Positron Emission Tomography, and Therapeutic Radionuclides. Clin. Cancer Res. 2009, 15, 5240–5249. [Google Scholar] [CrossRef] [Green Version]
- Maina, T.; Bergsma, H.; Kulkarni, H.R.; Mueller, D.; Charalambidis, D.; Krenning, E.P.; Nock, B.A.; De Jong, M.; Baum, R.P. Preclinical and first clinical experience with the gastrin-releasing peptide receptor-antagonist [68Ga]SB3 and PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2015, 43, 964–973. [Google Scholar] [CrossRef]
- Ramos-Álvarez, I.; Moreno, P.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moody, T.W.; Coy, D.H.; Jensen, R.T. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides 2015, 72, 128–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cescato, R.; Maina, T.; Nock, B.; Nikolopoulou, A.; Charalambidis, D.; Piccand, V.; Reubi, J.C. Bombesin Receptor Antagonists May Be Preferable to Agonists for Tumor Targeting. J. Nucl. Med. 2008, 49, 318–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varasteh, Z.; Velikyan, I.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Sandström, M.; Selvaraju, R.K.; Malmberg, J.; Tolmachev, V.; Orlova, A. Synthesis and Characterization of a High-Affinity NOTA-Conjugated Bombesin Antagonist for GRPR-Targeted Tumor Imaging. Bioconjugate Chem. 2013, 24, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Mitran, B.; Thisgaard, H.; Rosenström, U.; Dam, J.H.; Larhed, M.; Tolmachev, V.; Orlova, A. High Contrast PET Imaging of GRPR Expression in Prostate Cancer Using Cobalt-Labeled Bombesin Antagonist RM26. Contrast Media Mol. Imaging 2017, 2017, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mitran, B.; Varasteh, Z.; Selvaraju, R.K.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Tolmachev, V.; Rosenström, U.; Orlova, A. Selection of optimal chelator improves the contrast of GRPR imaging using bombesin analogue RM26. Int. J. Oncol. 2016, 48, 2124–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varasteh, Z.; Mitran, B.; Rosenström, U.; Velikyan, I.; Rosestedt, M.; Lindeberg, G.; Sörensen, J.; Larhed, M.; Tolmachev, V.; Orlova, A. The effect of macrocyclic chelators on the targeting properties of the 68 Ga-labeled gastrin releasing peptide receptor antagonist PEG 2 -RM26. Nucl. Med. Biol. 2015, 42, 446–454. [Google Scholar] [CrossRef]
- Varasteh, Z.; Rosenström, U.; Velikyan, I.; Mitran, B.; Altai, M.; Honarvar, H.; Rosestedt, M.; Lindeberg, G.; Sörensen, J.; Larhed, M.; et al. The Effect of Mini-PEG-Based Spacer Length on Binding and Pharmacokinetic Properties of a 68Ga-Labeled NOTA-Conjugated Antagonistic Analog of Bombesin. Molecules 2014, 19, 10455–10472. [Google Scholar] [CrossRef] [Green Version]
- Mitran, B.; Thisgaard, H.; Rinne, S.; Dam, J.H.; Azami, F.; Tolmachev, V.; Orlova, A.; Rosenström, U. Selection of an optimal macrocyclic chelator improves the imaging of prostate cancer using cobalt-labeled GRPR antagonist RM26. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Sancho, V.; Di Florio, A.; Moody, T.W.; Jensen, R.T. Bombesin receptor-mediated imaging and cytotoxicity: Review and current status. Curr. Drug Deliv. 2011, 8, 79–134. [Google Scholar] [CrossRef] [Green Version]
- Nijsen, J.F.W.; Krijger, G.C.; Schip, A.D.V.H. The Bright Future of Radionuclides for Cancer Therapy. Anti-Cancer Agents Med. Chem. 2007, 7, 271–290. [Google Scholar] [CrossRef]
- Anderson, C.J.; Ferdani, R. Copper-64 Radiopharmaceuticals for PET Imaging of Cancer: Advances in Preclinical and Clinical Research. Cancer Biother. Radiopharm. 2009, 24, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Prasanphanich, A.F.; Retzloff, L.; Lane, S.R.; Nanda, P.K.; Sieckman, G.L.; Rold, T.L.; Ma, L.; Figueroa, S.D.; Sublett, S.V.; Hoffman, T.J.; et al. In vitro and in vivo analysis of [64Cu-NO2A-8-Aoc-BBN(7–14)NH2]: A site-directed radiopharmaceutical for positron-emission tomography imaging of T-47D human breast cancer tumors. Nucl. Med. Biol. 2009, 36, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, T.J.; Smith, C.J. True radiotracers: Cu-64 targeting vectors based upon bombesin peptide. Nucl. Med. Biol. 2009, 36, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Lopci, E.; Grassi, I.; Chiti, A.; Nanni, C.; Cicoria, G.; Toschi, L.; Fonti, C.; Lodi, F.; Mattioli, S.; Fanti, S. PET radiopharmaceuticals for imaging of tumor hypoxia: A review of the evidence. Am. J. Nucl. Med. Mol. Imaging 2014, 4, 365–384. [Google Scholar]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjær, A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2016, 58, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Follacchio, G.A.; De Feo, M.S.; Monteleone, F.; De Vincentis, G.; Liberatore, M. Radiopharmaceuticals Labelled with Copper Radionuclides: Clinical Results in Human Beings. Curr. Radiopharm. 2017, 11, 22–33. [Google Scholar] [CrossRef]
- Baratto, L.; Duan, H.; Mäcke, H.; Iagaru, A.; Maecke, H.R. Imaging the Distribution of Gastrin-Releasing Peptide Receptors in Cancer. J. Nucl. Med. 2020, 61, 792–798. [Google Scholar] [CrossRef]
- Baratto, L.; Jadvar, H.; Iagaru, A. Prostate Cancer Theranostics Targeting Gastrin-Releasing Peptide Receptors. Mol. Imaging Biol. 2018, 20, 501–509. [Google Scholar] [CrossRef]
- Wieser, G.; Mansi, R.; Grosu, A.L.; Schultze-Seemann, W.; Dumont-Walter, R.A.; Meyer, P.T.; Maecke, H.R.; Reubi, J.C.; Weber, W.A. Positron Emission Tomography (PET) Imaging of Prostate Cancer with a Gastrin Releasing Peptide Receptor Antagonist-from Mice to Men. Theranostics 2014, 4, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Maina, T.; Nock, B.A. From Bench to Bed. PET Clin. 2017, 12, 205–217. [Google Scholar] [CrossRef]
- Anderson, C.J.; Wadas, T.J.; Wong, E.H.; Weisman, G.R. Cross-bridged macrocyclic chelators for stable complexation of copper radionuclides for PET imaging. Q. J. Nucl. Med. Mol. Imaging 2007, 52, 185–192. [Google Scholar] [PubMed]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.-F.; Thisgaard, H. In Vivo Evaluation of a Bombesin Analogue Labeled with Ga-68 and Co-55/57. Mol. Imaging Biol. 2015, 18, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.P.J.; Müller, C.; Reneman, S.; Melis, M.L.; Breeman, W.A.P.; De Blois, E.; Bangma, C.H.; Krenning, E.P.; Van Weerden, W.M.; De Jong, M. A standardised study to compare prostate cancer targeting efficacy of five radiolabelled bombesin analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1386–1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.L.; Baun, C.; Olsen, B.B.; Dam, J.H.; Thisgaard, H. Improving Contrast and Detectability: Imaging with [55Co]Co-DOTATATE in Comparison with [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE. J. Nucl. Med. 2019, 61, 228–233. [Google Scholar] [CrossRef] [Green Version]
- Lane, S.R.; Nanda, P.; Rold, T.L.; Sieckman, G.L.; Figueroa, S.D.; Hoffman, T.J.; Jurisson, S.S.; Smith, C.J. Optimization, biological evaluation and microPET imaging of copper-64-labeled bombesin agonists, [64Cu-NO2A-(X)-BBN(7–14)NH2], in a prostate tumor xenografted mouse model. Nucl. Med. Biol. 2010, 37, 751–761. [Google Scholar] [CrossRef]
- Mansour, N.; Dumulon-Perreault, V.; Ait-Mohand, S.; Paquette, M.; LeComte, R.; Guérin, B. Impact of dianionic and dicationic linkers on tumor uptake and biodistribution of [64Cu]Cu/NOTA peptide-based gastrin-releasing peptide receptors antagonists. J. Label. Compd. Radiopharm. 2017, 60, 200–212. [Google Scholar] [CrossRef]
- Mitran, B.; Rinne, S.S.; Konijnenberg, M.W.; Maina, T.; Nock, B.A.; Altai, M.; Vorobyeva, A.; Larhed, M.; Tolmachev, V.; De Jong, M.; et al. Trastuzumab cotreatment improves survival of mice with PC-3 prostate cancer xenografts treated with the GRPR antagonist 177Lu-DOTAGA-PEG2-RM26. Int. J. Cancer 2019, 145, 3347–3358. [Google Scholar] [CrossRef] [Green Version]
- Gourni, E.; Mansi, R.; Jamous, M.; Waser, B.; Smerling, C.; Burian, A.; Buchegger, F.; Reubi, J.C.; Maecke, H.R. N-Terminal Modifications Improve the Receptor Affinity and Pharmacokinetics of Radiolabeled Peptidic Gastrin-Releasing Peptide Receptor Antagonists: Examples of 68Ga- and 64Cu-Labeled Peptides for PET Imaging. J. Nucl. Med. 2014, 55, 1719–1725. [Google Scholar] [CrossRef] [Green Version]
- Tolmachev, V.; Yim, C.-B.; Rajander, J.; Perols, A.; Karlström, A.E.; Haaparanta-Solin, M.; Grönroos, T.J.; Solin, O.; Orlova, A. Comparative Evaluation of Anti-HER2 Affibody Molecules Labeled with 64Cu Using NOTA and NODAGA. Contrast Media Mol. Imaging 2017, 2017, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gynther, M.; Peura, L.; Vernerová, M.; Leppänen, J.; Kärkkäinen, J.; Lehtonen, M.; Rautio, J.; Huttunen, K.M. Amino Acid Promoieties Alter Valproic Acid Pharmacokinetics and Enable Extended Brain Exposure. Neurochem. Res. 2016, 41, 2797–2809. [Google Scholar] [CrossRef]
- Strand, J.; Honarvar, H.; Perols, A.; Orlova, A.; Selvaraju, R.K.; Karlström, A.E.; Tolmachev, V. Influence of Macrocyclic Chelators on the Targeting Properties of 68Ga-Labeled Synthetic Affibody Molecules: Comparison with 111In-Labeled Counterparts. PLoS ONE 2013, 8, e70028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansour, N.; Paquette, M.; Aït-Mohand, S.; Dumulon-Perreault, V.; Guérin, B. Evaluation of a novel GRPR antagonist for prostate cancer PET imaging: [64Cu]-DOTHA2-PEG-RM26. Nucl. Med. Biol. 2018, 56, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Gourni, E.; Del Pozzo, L.; Kheirallah, E.; Smerling, C.; Waser, B.; Reubi, J.-C.; Paterson, B.M.; Donnelly, P.S.; Meyer, P.T.; Maecke, H.R. Copper-64 Labeled Macrobicyclic Sarcophagine Coupled to a GRP Receptor Antagonist Shows Great Promise for PET Imaging of Prostate Cancer. Mol. Pharm. 2015, 12, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Tolmachev, V.; Stone-Elander, S. Radiolabelled proteins for positron emission tomography: Pros and cons of labelling methods. Biochim. Biophys. Acta Gen. Subj. 2010, 1800, 487–510. [Google Scholar] [CrossRef] [PubMed]
- Thisgaard, H.; Olesen, M.L.; Dam, J.H. Radiosynthesis of 55Co- and 58mCo-labelled DOTATOC for positron emission tomography imaging and targeted radionuclide therapy. J. Label. Compd. Radiopharm. 2011, 54, 758–762. [Google Scholar] [CrossRef]

| [64Cu]Cu-X-RM26 | NOTA | NODAGA |
|---|---|---|
| Labeling yield (4 MBq/nmol), % | 98.93 ± 0.09 | 98.9 ± 0.2 |
| Release in serum (1 h, 37 °C), % | 0.2 ± 0.2 | 0.10 ± 0.06 |
| Release in the presence of excess EDTA (1 h, RT), % | 0.8 ± 0.2 | 0.96 ± 1.07 |
| Organ | [64Cu]Cu-NOTA | [64Cu]Cu-NODAGA | [57Co]Co-NOTA | [57Co]Co-NODAGA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | 3 h | 24 h | |||||||||
| Blood | 0.06 | ±0.006 a,b,c | 0.04 | ±0.001 a,b,c | 0.03 | ±0.002 a | 0.02 | ±0.01 a | 0.02 | ±0.002 b | 0.01 | ±0.001 b | 0.03 | ±0.01 c | 0.01 | ±0.001 c |
| Kidney | 0.46 | ±0.04 a | 0.25 | ±0.008 | 0.90 | ±0.09 a,d | 0.12 | ±0.01 | 0.51 | ±0.08 d,f | 0.06 | ±0.02 | 0.84 | ±0.13 f | 0.07 | ±0.01 |
| GI tract | 0.76 | ±0.15 b | 0.07 | ±0.02 | 1.02 | ±0.15 d | 0.03 | ±0.01 | 0.26 | ±0.08 b,d,f | 0.02 | ±0.004 | 0.99 | ±0.16 f | 0.24 | ±0.22 |
| Stomach | 1.19 | ±0.16 a,b,c | 0.14 | ±0.03 | 0.27 | ±0.05 a,d | 0.08 | ±0.03 | 0.59 | ±0.04 b,d | 0.06 | ±0.01 | 0.48 | ±0.04 c | 0.04 | ±0.01 |
| Spleen | 0.10 | ±0.01 a | 0.05 | ±0.01 | 0.05 | ±0.01 a | 0.02 | ±0.01 | 0.07 | ±0.01 | 0.04 | ±0.003 | 0.08 | ±0.01 | 0.04 | ±0.003 |
| Small int. | 0.72 | ±0.10 a,b,c | 0.12 | ±0.01 | 0.24 | ±0.04 a | 0.05 | ±0.01 | 0.34 | ±0.06 b,f | 0.08 | ±0.02 | 0.12 | ±0.03 c,f | 0.04 | ±0.02 |
| Pancreas | 1.31 | ±0.33 a,b | 0.10 | ±0.02 | 0.27 | ±0.04 a | 0.04 | ±0.02 | 0.44 | ±0.05 b | 0.02 | ±0.001 | 0.76 | ±0.20 | 0.03 | ±0.001 |
| Liver | 0.59 | ±0.07 a,b,c | 0.49 | ±0.01 a,b,c | 0.28 | ±0.04 a,d,e | 0.16 | ±0.02 a,d,e | 0.06 | ±0.001 b,d | 0.01 | ±0.002 b,d | 0.07 | ±0.01 c,e | 0.01 | ±0.002 c,e |
| Lung | 0.21 | ±0.02 a,b,c | 0.14 | ±0.01 a,b,c | 0.12 | ±0.01 a,d,e | 0.04 | ±0.01 a | 0.05 | ±0.004 b,d | 0.02 | ±0.001 b | 0.05 | ±0.01 c,e | 0.02 | ±0.002 c |
| Bone | 0.07 | ±0.02 | 0.15 | ±0.09 | 0.08 | ±0.02 | 0.13 | ±0.00 | 0.11 | ±0.04 | 0.09 | ±0.03 | 0.13 | ±0.03 | 0.14 | ±0.01 |
| Tumor | 3.94 | ±0.36 | 0.42 | ±0.04 | 2.31 | ±0.41 | 0.16 | ±0.04 | 3.27 | ±1.07 | 0.31 | ±0.04 | 4.58 | ±1.56 | 0.50 | ±0.21 |
| Muscle | 0.02 | ±0.003 | 0.01 | ±0.0000 | 0.02 | ±0.01 | 0.00 | ±0.0000 | 0.02 | ±0.002 | 0.01 | ±0.002 | 0.02 | ±0.01 | 0.01 | ±0.002 |
Sample Availability: Samples of the compound NOTA/NODAGA-PEG2-RM26 is available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baun, C.; Mitran, B.; Rinne, S.S.; Dam, J.H.; Olsen, B.B.; Tolmachev, V.; Orlova, A.; Thisgaard, H. Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate Cancer. Molecules 2020, 25, 5993. https://doi.org/10.3390/molecules25245993
Baun C, Mitran B, Rinne SS, Dam JH, Olsen BB, Tolmachev V, Orlova A, Thisgaard H. Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate Cancer. Molecules. 2020; 25(24):5993. https://doi.org/10.3390/molecules25245993
Chicago/Turabian StyleBaun, Christina, Bogdan Mitran, Sara S. Rinne, Johan H. Dam, Birgitte B. Olsen, Vladimir Tolmachev, Anna Orlova, and Helge Thisgaard. 2020. "Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate Cancer" Molecules 25, no. 24: 5993. https://doi.org/10.3390/molecules25245993
APA StyleBaun, C., Mitran, B., Rinne, S. S., Dam, J. H., Olsen, B. B., Tolmachev, V., Orlova, A., & Thisgaard, H. (2020). Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate Cancer. Molecules, 25(24), 5993. https://doi.org/10.3390/molecules25245993







