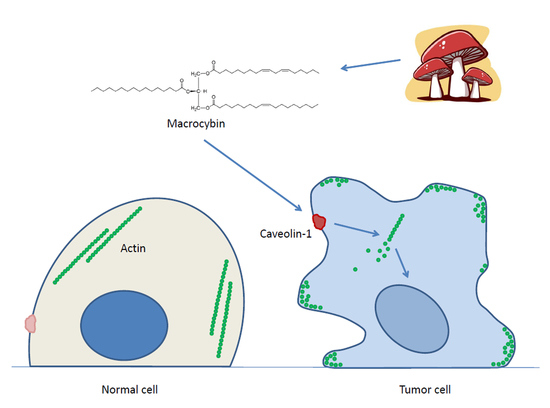
Macrocybin, a Natural Mushroom Triglyceride, Reduces Tumor Growth In Vitro and In Vivo through Caveolin-Mediated Interference with the Actin Cytoskeleton
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Mushroom Collection and Extract Preparation
4.2. Anticancer Screening Strategy (Toxicity Assays)
4.3. Fractionation Strategies
4.4. Elucidation of the Compound’s Chemical Structure
4.5. Triglyceride Synthesis
4.6. Anticancer Activity in a Xenograft Model
4.7. Cytoskeleton Staining and Confocal Microscopy
4.8. Gene Expression
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Y.; Zhao, T.; Zhang, N.; Zhang, Y.; Cheng, L. A Review of Recent Advances and Research on Drug Target Identification Methods. Curr. Drug Metab. 2019, 20, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Rates, S. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Last 25 Years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Guéritte-Voegelein, F.; Guenard, D.; Potier, P. Taxol and Derivatives: A Biogenetic Hypothesis. J. Nat. Prod. 1987, 50, 9–18. [Google Scholar] [CrossRef]
- Barbuti, A.M.; Chen, Z.-S. Paclitaxel Through the Ages of Anticancer Therapy: Exploring Its Role in Chemoresistance and Radiation Therapy. Cancers 2015, 7, 2360–2371. [Google Scholar] [CrossRef]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Wall, M.E.; Wani, M.C.; Cook, C.E.; Palmer, K.H.; McPhail, A.T.; Sim, G.A. Plant Antitumor Agents. I. The Isolation and Structure of Camptothecin, a Novel Alkaloidal Leukemia and Tumor Inhibitor from Camptotheca acuminata1,2. J. Am. Chem. Soc. 2005, 88, 3888–3890. [Google Scholar] [CrossRef]
- Jimenez, P.; Wilke, D.V.; Branco, P.C.; Bauermeister, A.; Rezende-Teixeira, P.; Susana, G.; Costa-Lotufo, L.V. Enriching cancer pharmacology with drugs of marine origin. Br. J. Pharmacol. 2020, 177, 3–27. [Google Scholar] [CrossRef]
- Hamburger, M.; Hostettmann, K. 7. Bioactivity in plants: The link between phytochemistry and medicine. Phytochemistry 1991, 30, 3864–3874. [Google Scholar] [CrossRef]
- Matson, D.R.; Stukenberg, P.T. Spindle Poisons and Cell Fate: A Tale of Two Pathways. Mol. Interv. 2011, 11, 141–150. [Google Scholar] [CrossRef]
- Guggenheim, A.G.; Wright, K.M.; Zwickey, H.L. Immune Modulation From Five Major Mushrooms: Application to Integrative Oncology. Integr. Med. 2014, 13, 32–44. [Google Scholar]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The mycelium of the Trametes versicolor (Turkey tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro. BMC Complement. Altern. Med. 2019, 19, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Blagodatski, A.; Yatsunskaya, M.; Mikhailova, V.; Tiasto, V.; Kagansky, A.; Katanaev, V.L. Medicinal mushrooms as an attractive new source of natural compounds for future cancer therapy. Oncotarget 2018, 9, 29259–29274. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Smith, J.E.; Rowan, N.J. Medicinal Mushrooms and Cancer Therapy: Translating a traditional practice into Western medicine. Perspect. Biol. Med. 2006, 49, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Sohretoglu, D. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anti-Cancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ina, K.; Kataoka, T.; Ando, T. The Use of Lentinan for Treating Gastric Cancer. Anti-Cancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-glucans from Grifola frondosa and Ganoderma lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Pohleven, J.; Kos, J.; Sabotic, J. Medicinal Properties of the Genus Clitocybe and of Lectins from the Clouded Funnel Cap Mushroom, C. nebularis (Agaricomycetes): A Review. Int. J. Med. Mushrooms 2016, 18, 965–975. [Google Scholar] [CrossRef]
- Baek, J.; Roh, H.-S.; Baek, K.; Lee, S.; Lee, S.; Song, S.-S.; Kim, K.H. Bioactivity-based analysis and chemical characterization of cytotoxic constituents from Chaga mushroom (Inonotus obliquus) that induce apoptosis in human lung adenocarcinoma cells. J. Ethnopharmacol. 2018, 224, 63–75. [Google Scholar] [CrossRef]
- Mueller, G.M.; Schmit, J.P. Fungal biodiversity: What do we know? What can we predict? Biodivers. Conserv. 2007, 16, 1–5. [Google Scholar] [CrossRef]
- Pegler, D.N.; Lodge, D.J.; Nakasone, K.K. The Pantropical Genus Macrocybe Gen. nov. Mycologia 1998, 90, 494. [Google Scholar] [CrossRef]
- Lev, S. Non-vesicular lipid transport by lipid-transfer proteins and beyond. Nat. Rev. Mol. Cell Biol. 2010, 11, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N.A.; Niveiro, N.; Michlig, A.; Popoff, O. First record of Macrocybe titans (Tricholomataceae, Basidiomycota) in Argentina. Check List. 2017, 13, 153–158. [Google Scholar] [CrossRef]
- Razaq, A.; Nawaz, R.; Khalid, A.N. An Asian edible mushroom, Macrocybe gigantea: Its distribution and ITS-rDNA based phylogeny. Mycosphere 2016, 7, 525–530. [Google Scholar] [CrossRef]
- Milhorini, S.D.S.; Smiderle, F.R.; Biscaia, S.M.P.; Rosado, F.R.; Trindade, E.S.; Iacomini, M. Fucogalactan from the giant mushroom Macrocybe titans inhibits melanoma cells migration. Carbohydr. Polym. 2018, 190, 50–56. [Google Scholar] [CrossRef]
- Gaur, T.; Rao, P.B. Analysis of Antibacterial Activity and Bioactive Compounds of the Giant Mushroom, Macrocybe gigantea (Agaricomycetes), from India. Int. J. Med. Mushrooms 2017, 19, 1083–1092. [Google Scholar] [CrossRef]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar]
- Ulmer, H.; Borena, W.; Rapp, K.; Klenk, J.; Strasak, A.; Diem, G.; Concin, H.; Nagel, G. Serum triglyceride concentrations and cancer risk in a large cohort study in Austria. Br. J. Cancer 2009, 101, 1202–1206. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, C.; Ren, Y.; Ye, J. A comprehensive look at the role of hyperlipidemia in promoting colorectal cancer liver metastasis. J. Cancer 2018, 9, 2981–2986. [Google Scholar] [CrossRef]
- Ni, H.; Liu, H.; Gao, R. Serum Lipids and Breast Cancer Risk: A Meta-Analysis of Prospective Cohort Studies. PLoS ONE 2015, 10, e0142669. [Google Scholar] [CrossRef]
- Cannella, C.; Giusti, A. Conjugated linoleic acid: A natural anticarcinogenic substance from animal food. Ital. J. Food Sci. 2000, 12, 123–127. [Google Scholar]
- Hanafy, N.A.N.; El-Kemary, M.; Leporatti, S. Micelles structure development as a strategy to improve smart cancer therapy. Cancers 2018, 10, 238. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Couvreur, P. Polymer nanoparticles for the delivery of anticancer drug. Med. Sci. 2017, 33, 11–17. [Google Scholar]
- Joshi, S.; Durden, D.L. Combinatorial Approach to Improve Cancer Immunotherapy: Rational Drug Design Strategy to Simultaneously Hit Multiple Targets to Kill Tumor Cells and to Activate the Immune System. J. Oncol. 2019, 2019, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Meshulam, T.; Simard, J.R.; Wharton, J.; Hamilton, J.A.; Pilch, P.F. Role of Caveolin-1 and Cholesterol in Transmembrane Fatty Acid Movement. Biochemistry 2006, 45, 2882–2893. [Google Scholar] [CrossRef] [PubMed]
- Siddiqi, S.; Sheth, A.; Patel, F.; Barnes, M.; Mansbach, C.M. Intestinal caveolin-1 is important for dietary fatty acid absorption. Biochim. Biophys. Acta 2013, 1831, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Gerbod-Giannone, M.-C.; Dallet, L.; Naudin, G.; Sahin, A.; Decossas, M.; Poussard, S.; Lambert, O. Involvement of caveolin-1 and CD36 in native LDL endocytosis by endothelial cells. Biochim. Biophys. Acta 2019, 1863, 830–838. [Google Scholar] [CrossRef]
- Chen, D.; Shen, C.; Du, H.; Zhou, Y.; Che, G.-W. Duplex value of caveolin-1 in non-small cell lung cancer: A meta analysis. Fam. Cancer 2014, 13, 449–457. [Google Scholar] [CrossRef]
- Shi, Y.-B.; Li, J.; Lai, X.-N.; Jiang, R.; Zhao, R.-C.; Xiong, L.-X. Multifaceted Roles of Caveolin-1 in Lung Cancer: A New Investigation Focused on Tumor Occurrence, Development and Therapy. Cancers 2020, 12, 291. [Google Scholar] [CrossRef]
- Alonso, E.N.; Ferronato, M.J.; Fermento, M.E.; Gandini, N.A.; Romero, A.L.; Guevara, J.A.; Facchinetti, M.M.; Curino, A.C. Antitumoral and antimetastatic activity of Maitake D-Fraction in triple-negative breast cancer cells. Oncotarget 2018, 9, 23396–23412. [Google Scholar] [CrossRef]
- Guo, H.; Kuang, S.; Song, Q.-L.; Liu, M.; Sun, X.-X.; Yu, Q. Cucurbitacin I inhibits STAT3, but enhances STAT1 signaling in human cancer cells in vitro through disrupting actin filaments. Acta Pharmacol. Sin. 2018, 39, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Chikara, S.; Lindsey, K.; Borowicz, P.; Christofidou-Solomidou, M.; Reindl, K. Enterolactone alters FAK-Src signaling and suppresses migration and invasion of lung cancer cell lines. BMC Complement. Altern. Med. 2017, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Goietsenoven, G.; Mathieu, V.; Lefranc, F.; Kornienko, A.; Evidente, A.; Kiss, R. Narciclasine as well as other Amaryllidaceae Isocarbostyrils are Promising GTP-ase Targeting Agents against Brain Cancers. Med. Res. Rev. 2013, 33, 439–455. [Google Scholar] [CrossRef] [PubMed]
- Iwai, N.; Martinez, A.; Miller, M.-J.; Vos, M.; Mulshine, J.L.; Treston, A.M. Autocrine growth loops dependent on peptidyl α-amidating enzyme as targets for novel tumor cell growth inhibitors. Lung Cancer 1999, 23, 209–222. [Google Scholar] [CrossRef]
- Zitvogel, L.; Pitt, J.M.; Daillère, L.Z.J.M.P.R.; Smyth, M.J.; Kroemer, G. Mouse models in oncoimmunology. Nat. Rev. Cancer 2016, 16, 759–773. [Google Scholar] [CrossRef]
- Monga, S.P.S.; Wadleigh, R.; Sharma, A.; Adib, H.; Strader, D.; Singh, G.; Harmon, J.W.; Berlin, M.; Monga, D.K.; Mishra, L. Intratumoral Therapy of Cisplatin/Epinephrine Injectable Gel for Palliation in Patients With Obstructive Esophageal Cancer. Am. J. Clin. Oncol. 2000, 23, 386–392. [Google Scholar] [CrossRef]
- Ochoa-Callejero, L.; Garcia-Sanmartin, J.; Martínez-Herrero, S.; Rubio-Mediavilla, S.; Narro-Íñiguez, J.; Martinez, A. Small molecules related to adrenomedullin reduce tumor burden in a mouse model of colitis-associated colon cancer. Sci. Rep. 2017, 7, 17488. [Google Scholar] [CrossRef]








| Internal Code | Chemical Formula |
|---|---|
| TG1 | TG (C16:0; C18:1, 9z; C20:2, 11z,14z) |
| TG2 | TG (C16:0; C20:2, 11z,14z; C18:1, 9z) |
| TG3 | TG (C16:0; C18:1, 9z; C18:1, 9z) |
| TG4 | TG (C18:0; C18:1, 9z; C18:2, 9z,12z) |
| TG5 | 2S-TG (C20:2, 11z,14z; C18:1, 9z; C16:0) |
| TG6 | 2R-TG (C20:2, 11z,14z; C18:1, 9z; C16:0) |
| TG7 | 2S-TG (C20:2, 11z,14z; C16:0; C18:1, 9z) |
| TG8 | 2R-TG (C20:2, 11z,14z; C16:0; C18:1, 9z) |
| TG9 | 2S-TG (C18:2, 9z,12z; C16:0; C18:1, 9z) |
| TG10 (Macrocybin) | 2R-TG (C18:2, 9z,12z; C16:0; C18:1, 9z) |
| Target Gene | Forward Primer | Reverse Primer | Amplicon Size |
|---|---|---|---|
| CAV1 | GCGACCCTAAACACCTCAAC | CAGCAAGCGGTAAAACCAGT | 149 |
| hGOT2_F (=FABPpm) | TGGTGCCTACCGGGATGATA | GGCAGAAAGACATCTCGGCT | 153 |
| hSLC27A1_F (=FATP1) | GCCAAATCGGGGAGTTCTAC | TTGAAACCACAGGAGCCGA | 85 |
| hSLC27A4_F (=FATP4) | CAAGACCATCAGGCGCGATA | CCGAACGGTAGAGGCAAACA | 118 |
| GAPDH | AAATCCCATCACCATCTTCC | GACTCCACGACGTACTCAGC | 81 |
Sample Availability: Samples of Macrocybin are available from the authors, while supplies last. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilariño, M.; García-Sanmartín, J.; Ochoa-Callejero, L.; López-Rodríguez, A.; Blanco-Urgoiti, J.; Martínez, A. Macrocybin, a Natural Mushroom Triglyceride, Reduces Tumor Growth In Vitro and In Vivo through Caveolin-Mediated Interference with the Actin Cytoskeleton. Molecules 2020, 25, 6010. https://doi.org/10.3390/molecules25246010
Vilariño M, García-Sanmartín J, Ochoa-Callejero L, López-Rodríguez A, Blanco-Urgoiti J, Martínez A. Macrocybin, a Natural Mushroom Triglyceride, Reduces Tumor Growth In Vitro and In Vivo through Caveolin-Mediated Interference with the Actin Cytoskeleton. Molecules. 2020; 25(24):6010. https://doi.org/10.3390/molecules25246010
Chicago/Turabian StyleVilariño, Marcos, Josune García-Sanmartín, Laura Ochoa-Callejero, Alberto López-Rodríguez, Jaime Blanco-Urgoiti, and Alfredo Martínez. 2020. "Macrocybin, a Natural Mushroom Triglyceride, Reduces Tumor Growth In Vitro and In Vivo through Caveolin-Mediated Interference with the Actin Cytoskeleton" Molecules 25, no. 24: 6010. https://doi.org/10.3390/molecules25246010
APA StyleVilariño, M., García-Sanmartín, J., Ochoa-Callejero, L., López-Rodríguez, A., Blanco-Urgoiti, J., & Martínez, A. (2020). Macrocybin, a Natural Mushroom Triglyceride, Reduces Tumor Growth In Vitro and In Vivo through Caveolin-Mediated Interference with the Actin Cytoskeleton. Molecules, 25(24), 6010. https://doi.org/10.3390/molecules25246010