Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of Volatile Substances in Citrus-Tea
2.2. Citrus-Tea Discrimination by Characteristic Volatile Fingerprints
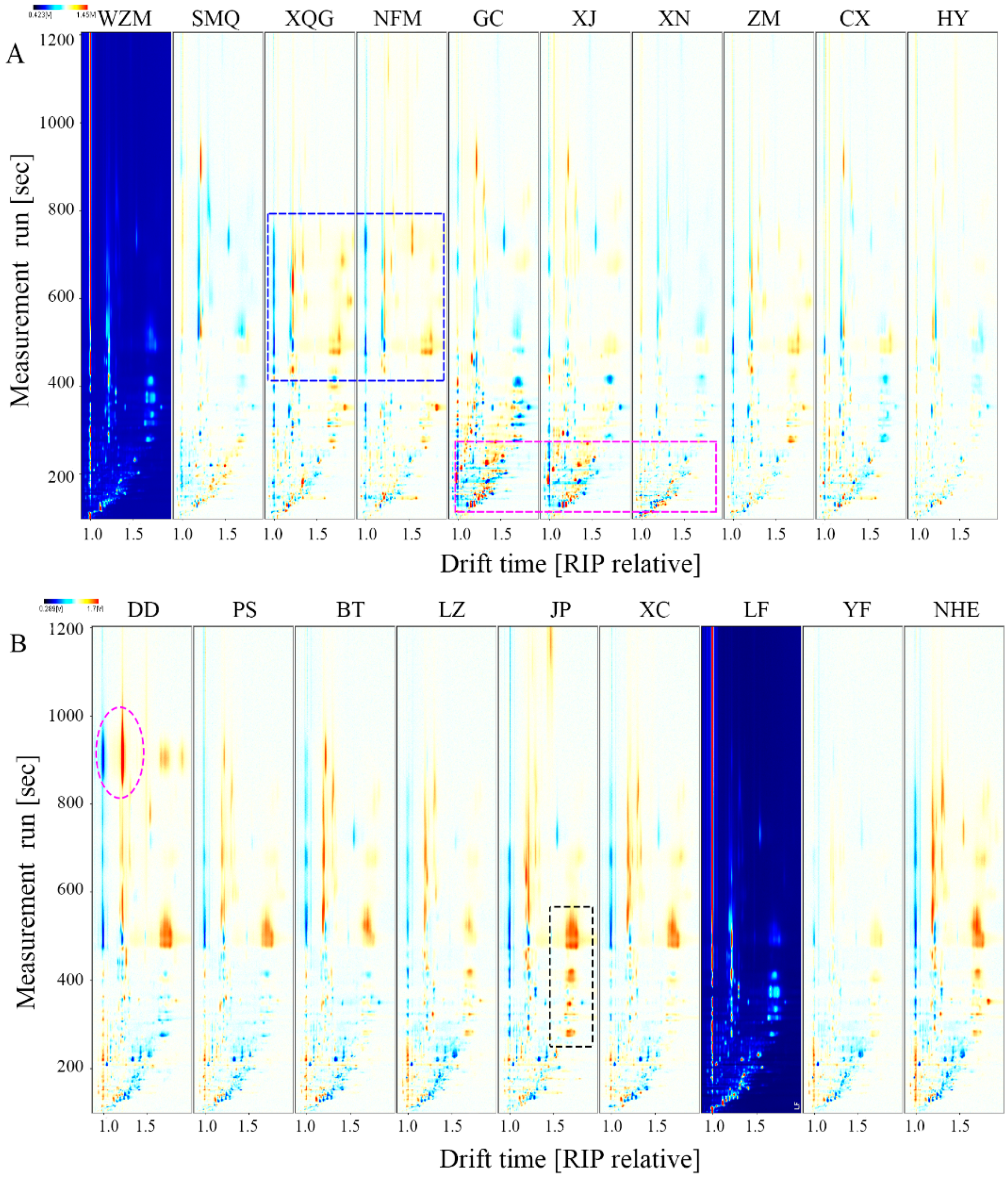
2.2.1. Differences in the Characteristic Volatile Fingerprints of Citrus-Tea
2.2.2. Establishment of Models for Citrus-Tea
2.2.3. Rapid Identification of Citrus-Tea by HS-GC-IMS
2.3. Key Aroma Compounds in Citrus-Tea by HS-SPME-GC-MS
2.3.1. Odor Activity Value of VOCs in Citrus-Tea
2.3.2. Distinctive Feature Analysis Based on Principal Component Analysis
2.3.3. Correlation Coefficient Analysis of the Key Aroma Compositions
3. Materials and Methods
3.1. Materials
3.2. Citrus-Tea Preparation
3.3. HS-GC-IMS Analysis Method
3.4. HS-SPME-GCMS Analysis Method
3.5. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HS-GC-IMS | Headspace gas chromatography-ion mobility spectrometry |
| HS-SPME-GC-MS | Headspace solid-phase microextraction-gas chromatography-mass spectrometry |
| VOCs | Volatile organic compounds |
| RI | Retention index |
| WZM | Citrus-tea made from Wenzhou Tangerine Dafen No. 4 and Tianjian tea |
| SMQ | Citrus-tea made from Shimeng orange and Tianjian tea |
| XQG | Citrus-tea made from Xiaoqing orange and Tianjian tea |
| NFM | Citrus-tea made from Nanfeng orange and Tianjian tea |
| GC | Citrus-tea made from Gongchuan orange and Tianjian tea |
| XJ | Citrus-tea made from Xinjing orange and Tianjian tea |
| XN | Citrus-tea made from Xinnv Ponkan and Tianjian tea |
| ZM | Citrus-tea made from Zaomi Ponkan and Tianjian tea |
| CX | Citrus-tea made from Chunxiang mixed Citrus and Tianjian tea |
| HY | Citrus-tea made from Huyou and Tianjian tea |
| DD | Citrus-tea made from Daidai lime and Tianjian tea |
| PS | Citrus-tea made from Pushi Cheng and Tianjian tea |
| BT | Citrus-tea made from Bingtang orange and Tianjian tea |
| LZ | Citrus-tea made from Luzai honey orange and Tianjian tea |
| JP | Citrus-tea made from Jinpenyou and Tianjian tea |
| XC | Citrus-tea made from Tarocco blood orange and Tianjian tea |
| LF | Citrus-tea made from Langfeng navel orange and Tianjian tea |
| YF | Citrus-tea made from Yuanfeng navel orange and Tianjian tea |
| NHE | Citrus-tea made from Newhall navel orange and Tianjian tea |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discrimination analysis |
| OAV | Odor activity value |
| ODT | Odor detection thresholds |
| VIP | Variable importance for the projection |
| PCC | Pearson correlation coefficients |
References
- Zheng, Y.; Zeng, X.; Chen, T.; Peng, W.; Su, W. Chemical Profile, Antioxidative, and Gut Microbiota Modulatory Properties of Ganpu Tea: A Derivative of Pu-erh Tea. Nutrients 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Du, W.-H.; Peng, S.-M.; Liu, Z.-H.; Shi, L.; Tan, L.-F.; Zou, X.-Q. Hypoglycemic effect of the water extract of Pu-erh tea. J. Agric. Food Chem. 2012, 60, 10126–10132. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.-L.; Weng, M.-S.; Chiang, C.-T.; Tsai, Y.-J.; Lin-Shiau, S.-Y.; Lin, J.-K. Comparative studies on the hypolipidemic and growth suppressive effects of oolong, black, pu-erh, and green tea leaves in rats. J. Agric. Food Chem. 2005, 53, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.J.; Xie, P.H.; Ren, F.; Lai, Z.J.; Ou, A.F. Flavor Analysis of Xinhui Citrus Pu’er Tea Based on Gas-phase Ion Migration Spectrum and Headspace Solid Phase Microextraction. Shipin Gongye Keji 2020, 41, 214–220. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, L.J.; Huang, X.B.; Li, J.H.; Peng, Z. Effect of Ultrasonic Treatment on the Volatile Components of Gan-pu Tea. Xiandai Shipin Keji 2017, 33, 250–256. [Google Scholar] [CrossRef]
- Cavanna, D.; Zanardi, S.; Dall’Asta, C.; Suman, M. Ion mobility spectrometry coupled to gas chromatography: A rapid tool to assess eggs freshness. Food Chem. 2019, 271, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Nölting, B. Ion Mobility Spectrometry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 177–197. [Google Scholar]
- Cao, G.; Shou, Q.; Li, Q.; Jiang, J.; Chen, X. Static Headspace-multicapillary Column with Gas Chromatography Coupled to Ion Mobility Spectrometry as a Simple Approach for the Discrimination of Crude and Processed Traditional Chinese Medicines. J. Sep. Sci. 2014, 37, 3090–3093. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; García-Nicolás, M.; Castell, A.; Campillo, N.; Viñas, P.; López-García, I.; Hernández-Córdoba, M. Untargeted headspace gas chromatography—Ion mobility spectrometry analysis for detection of adulterated honey. Talanta 2019, 205, 120123. [Google Scholar] [CrossRef]
- Li, H.; Jiang, D.; Liu, W.; Yang, Y.; Zhang, Y.; Jin, C.; Sun, S. Comparison of fermentation behaviors and properties of raspberry wines by spontaneous and controlled alcoholic fermentations. Food Res. Int. 2020, 128, 108801. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, D.; Dong, Y.; Ju, H.; Wu, C.; Lin, S. Characteristic volatiles fingerprints and changes of volatile compounds in fresh and dried Tricholoma matsutake Singer by HS-GC-IMS and HS-SPME-GC-MS. J. Chromatogr. B 2018, 1099, 46–55. [Google Scholar] [CrossRef]
- Grimm, J.E.; Steinhaus, M. Characterization of the Major Odorants in Cempedak—Differences to Jackfruit. J. Agric. Food Chem. 2019, 68, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Flaig, M.; Qi, S.; Wei, G.; Yang, X.; Schieberle, P. Characterization of the Key Odorants in a High-Grade Chinese Green Tea Beverage (Camellia sinensis; Jingshan cha) by Means of the Sensomics Approach and Elucidation of Odorant Changes in Tea Leaves Caused by the Tea Manufacturing Process. J. Agric. Food Chem. 2020, 68, 5168–5179. [Google Scholar] [CrossRef] [PubMed]
- Mall, V.; Schieberle, P. Evaluation of Key Aroma Compounds in Processed Prawns (Whiteleg Shrimp) by Quantitation and Aroma Recombination Experiments. J. Agric. Food Chem. 2017, 65, 2776–2783. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Maecker, R.; Vyhmeister, E.; Meisen, S.; Martinez, A.R.; Kuklya, A.; Telgheder, U. Identification of terpenes and essential oils by means of static headspace gas chromatography-ion mobility spectrometry. Anal. Bioanal. Chem. 2017, 409, 6595–6603. [Google Scholar] [CrossRef]
- Lantsuzskaya, E.V.; Krisilov, A.V.; Levina, A.M. Structure of aldehyde cluster ions in the gas phase, according to data from ion mobility spectrometry and ab initio calculations. Russ. J. Phys. Chem. A 2015, 89, 1590–1594. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Liu, C.; Chen, S.; Hu, S.; Xie, Z.; Deng, X.; Xu, J. Comprehensive comparative analysis of volatile compounds in citrus fruits of different species. Food Chem. 2017, 230, 316–326. [Google Scholar] [CrossRef]
- Yang, Z.; Baldermann, S.; Watanabe, N. Recent studies of the volatile compounds in tea. Food Res. Int. 2013, 53, 585–599. [Google Scholar] [CrossRef]
- Nagegowda, D.A. Plant volatile terpenoid metabolism: Biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett. 2010, 584, 2965–2973. [Google Scholar] [CrossRef]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus Essential Oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes in the volatile composition of yuzu (Citrus junos Tanaka) cold-pressed oil during storage. J. Agric. Food Chem. 1996, 44, 550–556. [Google Scholar] [CrossRef]
- Pérez-Cacho, P.R.; Rouseff, R. Processing and Storage Effects on Orange Juice Aroma: A Review. J. Agric. Food Chem. 2008, 56, 9785–9796. [Google Scholar] [CrossRef] [PubMed]
- Delgado, F.J.; González-Crespo, J.; Cava, R.; Ramírez, R. Changes in the volatile profile of a raw goat milk cheese treated by hydrostatic high pressure at different stages of maturation. Int. Dairy J. 2011, 21, 135–141. [Google Scholar] [CrossRef]
- Wang, Z.; Xiao, Q.; Zhuang, J.; Feng, T.; Ho, C.-T.; Song, S. Characterization of Aroma-Active Compounds in Four Yeast Extracts Using Instrumental and Sensory Techniques. J. Agric. Food Chem. 2019, 68, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Bertolino, M.; Dolci, P.; Giordano, M.; Rolle, L.; Zeppa, G. Evolution of chemico-physical characteristics during manufacture and ripening of Castelmagno PDO cheese in wintertime. Food Chem. 2011, 129, 1001–1011. [Google Scholar] [CrossRef]
- Ou, X.Q.; Wang, J.; Li, P.; Huang, Q.W.; Yang, X.M.; Tan, M.L. Comparison among volatile oil compositions from tangerine peels and their kindreds. Zhongchengyao 2015, 2, 364–370. Available online: https://hnu-primo.hosted.exlibrisgroup.com/permalink/f/1hh8v3t/TN_cqvip663686742 (accessed on 14 December 2020).
- Mottram, D.S.; Mottram, H.R. An overview of the contribution of sulfur-containing compounds to the aroma in heated foods. ACS Symp. Ser. 2002, 826, 73–92. Available online: https://hnu-primo.hosted.exlibrisgroup.com/permalink/f/1hh8v3t/TN_wos000181054000004 (accessed on 14 December 2020).
- Du, X.; Song, M.; Baldwin, E.; Rouseff, R. Identification of sulphur volatiles and GC-olfactometry aroma profiling in two fresh tomato cultivars. Food Chem. 2015, 171, 306–314. [Google Scholar] [CrossRef]
- Schuh, C.; Schieberle, P. Characterization of the key aroma compounds in the beverage prepared from Darjeeling black tea: Quantitative differences between tea leaves and infusion. J. Agric. Food Chem. 2006, 54, 916–924. [Google Scholar] [CrossRef]
- Rizzi, G.P. The Strecker Degradation of Amino Acids: Newer Avenues for Flavor Formation. Food Rev. Int. 2008, 24, 416–435. [Google Scholar] [CrossRef]
- Vázquez-Araújo, L.; Enguix, L.; Verdú, A.; García-García, E.; Carbonell-Barrachina, A.A. Investigation of aromatic compounds in toasted almonds used for the manufacture of turrón. Eur. Food Res. Technol. 2007, 227, 243–254. [Google Scholar] [CrossRef]
- Njoroge, S.M.; Ukeda, H.; Sawamura, M. Changes of the volatile profile and artifact formation in Daidai (Citrus aurantium) cold-pressed peel oil on storage. J. Agric. Food Chem. 2003, 51, 4029–4035. [Google Scholar] [CrossRef] [PubMed]
- Klesk, K. Aroma Comparison of Marion (Rubus sp. L.) and Thornless Evergreen (Rubus laciniatus L.) Blackberries. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 2004. [Google Scholar]
- Boccard, J.; Rutledge, D.N. A consensus orthogonal partial least squares discriminant analysis (OPLS-DA) strategy for multiblock Omics data fusion. Anal. Chim. Acta 2013, 769, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Afanador, N.L.; Tran, T.N.; Buydens, L.M.C. Use of the bootstrap and permutation methods for a more robust variable importance in the projection metric for partial least squares regression. Anal. Chim. Acta. 2013, 768, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-H.; Wang, C.; Li, S.-X.; Su, Z.-Z.; Zhou, H.-N.; Mao, L.-J.; Feng, X.-X.; Liu, P.-P.; Chen, X.; Snyder, J.H.; et al. Friend or foe: Differential responses of rice to invasion by mutualistic or pathogenic fungi revealed by RNAseq and metabolite profiling. Sci. Rep. 2015, 5, 13624. [Google Scholar] [CrossRef]
- Phi, N.T.L.; Sawamura, M. Characteristic Aroma Composition Profile of Mature Stage Citrus junos (Yuzu) Peel Oil from Different Origins. Food Sci. Technol. Res. 2008, 14, 359–366. [Google Scholar] [CrossRef][Green Version]
- Gemert, L.J.V. Odour Thresholds. Compilations of Odour Threshold Values in Air, Water and Other Media; Oliemans Punter &Partners BV: Utrecht, The Netherlands, 2011. [Google Scholar]
- Miyazaki, T.; Plotto, A.; Baldwin, E.A.; Reyes-De-Corcuera, J.I.; Gmitter, F.G., Jr. Aroma characterization of tangerine hybrids by gas-chromatography–olfactometry and sensory evaluation. J. Sci. Food Agric. 2012, 92, 727–735. [Google Scholar] [CrossRef]
- Vilanova, M.; Campo, E.; Escudero, A.; Graña, M.; Masa, A.; Cacho, J. Volatile composition and sensory properties of Vitis vinifera red cultivars from North West Spain: Correlation between sensory and instrumental analysis. Anal. Chim. Acta 2012, 720, 104–111. [Google Scholar] [CrossRef]
- Bicchi, C. CITRUS OILS: COMPOSITION, ADVANCED ANALYTICAL TECHNIQUES, CONTAMINANTS AND BIOLOGICAL ACTIVITY, edited by Giovanni Dugo and Duigi Mondello. Volume 49 of the book series Medicinal and Aromatic Plants—Industrial Profiles, Series Editor: Roland Hardman. CRC Press, Boca Raton, Fl, 33487–2742 (USA), 2011, pp. 561, ISBN 978-1-4398-0028-7. Flavour Fragr. J. 2012, 27, 260–261. [Google Scholar] [CrossRef]
- Bengtsson, M.; Bäckman, A.C.; Liblikas, I.; Ramirez, M.I.; Borg-Karlson, A.K.; Ansebo, L.; Anderson, P.; Löfqvist, J.; Witzgall, P. Plant odor analysis of apple: Antennal response of codling moth females to apple volatiles during phenological development. J. Agric. Food Chem. 2001, 49, 3736–3741. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.; Konings, M.C.J.M.; Gershenzon, J.; Karp, F.; Croteau, R. Cytochrome P-450 dependent (+)-limonene-6-hydroxylation in fruits of caraway (Carum carvi). Phytochemistry 1999, 50, 243–248. [Google Scholar] [CrossRef]
- Xu, X.; Yan, M.; Zhu, Y. Influence of Fungal Fermentation on the Development of Volatile Compounds in the Puer Tea Manufacturing Process. Eng. Life Sci. 2005, 5, 382–386. [Google Scholar] [CrossRef]
- Kawakami, M.; Shibamoto, T. The Volatile Constituents of Piled Tea: Toyama Kurocha. Agric. Biol. Chem. 1991, 55, 1839–1847. [Google Scholar] [CrossRef][Green Version]
- Wan, X.C. Tea Biochemistry, 3rd ed.; China Agriculture Press: Beijing, China, 2003; pp. 20–22. [Google Scholar]
- Nie, C.-N.; Zhong, X.-X.; He, L.; Gao, Y.; Zhang, X.; Wang, C.-M.; Du, X. Comparison of different aroma-active compounds of Sichuan Dark brick tea (Camellia sinensis) and Sichuan Fuzhuan brick tea using gas chromatography-mass spectrometry (GC-MS) and aroma descriptive profile tests. Eur. Food Res. Technol. 2019, 245, 1963–1979. [Google Scholar] [CrossRef]
- Cheng, S.T. The Study of Purification of Soybean Lipoxygenase and Its Catalysis of Polyunsaturated Fatty Acids. Master’s Thesis, Jiangnan University, Wuxi, China, 2011. [Google Scholar]
- Wei, X.; Song, M.; Chen, C.; Tong, H.; Liang, G.; Gmitter, F.G., Jr. Juice volatile composition differences between Valencia orange and its mutant Rohde Red Valencia are associated with carotenoid profile differences. Food Chem. 2018, 245, 223–232. [Google Scholar] [CrossRef]
- Kesen, S.; Kelebek, H.; Selli, S. Characterization of the key aroma compounds in Turkish olive oils from different geographic origins by application of aroma extract dilution analysis (AEDA). J. Agric. Food Chem. 2014, 62, 391–401. [Google Scholar] [CrossRef]
- Huang, X.; Lin, X.; Zeng, J.; Wang, L.; Yin, P.; Zhou, L.; Hu, C.; Yao, W. A Computational Method of Defining Potential Biomarkers based on Differential Sub-Networks. Sci. Rep. 2017, 7, 14339. [Google Scholar] [CrossRef]
- Chao, T.; Ji, Z.; Hou, L.; Wang, J.; Zhang, C.; Wang, G.; Wang, J. Sheep skeletal muscle transcriptome analysis reveals muscle growth regulatory lncRNAs. PeerJ 2018, 6, e4619. [Google Scholar] [CrossRef]
- Cao, X. Investigation and Analysis on the Quality and Industrial Development of Anhua Dark Tea in YiYang. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2015. [Google Scholar]
- Denawaka, C.J.; Fowlis, I.A.; Dean, J.R. Evaluation and application of static headspace–multicapillary column-gas chromatography–ion mobility spectrometry for complex sample analysis. J. Chromatogr. A 2014, 1338, 136–148. [Google Scholar] [CrossRef]
- Lv, H.P.; Yang, T.; Zhu, Y.; Zhang, Y.; Lin, Z. Analysis of aroma fingerprint of Xihu Longjing tea using HS-SPME/GC-MS. Xiandai Shipin Keji 2015, 11, 339–347. [Google Scholar] [CrossRef]
- Bi, S.; Sun, S.; Lao, F.; Liao, X.; Wu, J. Gas chromatography–mass spectrometry combined with multivariate data analysis as a tool for differentiating between processed orange juice samples on the basis of their volatile markers. Food Chem. 2020, 311, 125913. [Google Scholar] [CrossRef]
- Ren, J.-N.; Tai, Y.-N.; Dong, M.; Shao, J.-H.; Yang, S.-Z.; Pan, S.-Y.; Fan, G. Characterisation of free and bound volatile compounds from six different varieties of citrus fruits. Food Chem. 2015, 185, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Guo, X.; Qin, Z.; Yao, Y.; Hu, X.; Wu, J. Identification of aroma-active compounds in Jiashi muskmelon juice by GC-O-MS and OAV calculation. J. Agric. Food Chem. 2012, 60, 4179–4185. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds used are available from the authors. |








| No | Compound | Odor Quality | ODT a (μg /kg) | OAV b | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WZM | SMQ | XQG | NFM | GC | XJ | XN | ZM | CX | HY | DD | PS | LZ | BT | JP | XC | LF | YF | NHE | ||||
| Phenolic acids | ||||||||||||||||||||||
| A1 | Acetic acid | Vinegar | 99,000 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 | <0.2 |
| A2 | Tridecanoic acid | Vinegar | 10,000 | – | 0.3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| A3 | n-Decanoic acid | Oily | 10,000 | – | – | – | – | – | – | – | <0.2 | – | – | – | – | – | – | – | – | – | – | – |
| A4 | Thymol | Thyme, spiced | 1700 | – | – | – | – | 2 | – | – | – | 1 | – | – | – | 3 | – | 1 | – | – | – | – |
| A5 | Carvacrol | Herbal | 2290 | 16 | 7 | 4 | 2 | 10 | 19 | 5 | 3 | 12 | 11 | 7 | 8 | 2 | 10 | 1 | 27 | 3 | 1 | 11 |
| Esters | ||||||||||||||||||||||
| B1 | Linalyl acetate | Petitgrain | 1000 | 49 | 35 | – | – | – | – | 37 | – | – | – | 240 | – | – | 26 | – | 41.16 | – | 44.914 | – |
| B2 | Methyl benzoate | Floral, fruity | 73 | – | – | – | – | – | 304 | – | – | – | – | – | – | 394 | – | – | – | – | – | – |
| B3 | Ethyl caprate | Coconut | 5 | – | – | – | – | – | – | – | 202 | – | – | – | – | – | – | – | – | – | – | – |
| B4 | Octyl acetate | Wax | 47 | 137 | 63 | – | 100 | – | – | 81 | 66 | 93 | 66 | 308 | 67 | – | – | – | 98 | 54 | 95 | – |
| B5 | Geranyl acetate | Rose, floral | 150 | 117 | 101 | 127 | 154 | 413 | 48 | 97 | 55 | 100 | 87 | 625 | 71 | 273 | 94 | 98 | 104 | 67 | 130 | 195 |
| B6 | Decyl acetate | Wax, honey | 5900 | – | 0.8 | – | – | 0.8 | – | – | – | – | – | – | – | – | – | <0.2 | – | – | – | – |
| B7 | 1-Decanol acetate | Orange, rose, ananas | 225 | – | – | – | – | – | – | – | 3 | 7 | 4 | 45 | 5 | – | – | – | – | – | – | – |
| B8 | Methyl caprate | Nicotian | 4.3 | – | – | – | – | – | – | – | 113 | – | – | – | – | – | – | – | – | – | – | – |
| Alcohols | ||||||||||||||||||||||
| C1 | Terpinen-4-ol | Woody | 1200 | 18 | – | – | – | – | 16 | 24 | – | 14 | 10 | 26 | 10 | 40 | – | 30 | 15 | – | – | 16 |
| C2 | 1-Octanol | Citrus, sweet, oily | 125.8 | – | 35 | 157 | 86 | – | 52 | 65 | 37 | – | – | – | – | – | – | – | – | 38 | 57 | – |
| C3 | Linalool | Floral, fruity, sweet | 0.22 | 658,386 | 2,103,090 | 674,931 | 1,225,683 | 836,653 | 850,477 | 780,538 | 5,380,078 | 598,228 | 1,326,022 | 426,628 | 488,540 | 1,315,688 | 791,603 | 415,056 | 988,041 | 1,755,751 | 492,007 | 672,381 |
| C4 | Nerolidolcistrans | Malic, rose, woody | 2250 | – | – | – | – | – | – | – | – | – | – | – | – | 1 | – | – | – | – | – | – |
| C5 | p-Menth-1-en-8-ol | Lemon, minty, piney | 1200 | 12 | 33 | 713 | 46 | – | 19 | 26 | 23 | 12 | 8 | 41 | 9 | 43 | 9 | 29 | 13 | – | 19 | – |
| C6 | Nerol | Citrus, lemon, minty | 680 | – | – | – | 41 | – | – | 38 | – | 25 | – | – | 20 | – | – | – | – | 38 | 48 | – |
| C7 | (Z)-Carveol | Citrus, spearmint | 250 | 159 | 73 | 375 | 149 | 519 | 232 | 148 | 62 | 80 | 106 | 315 | 98 | 535 | 171 | 121 | 178 | 46 | 189 | 236 |
| C8 | (E)-Carveol | Citrus, spicy | 250 | – | 43 | – | – | 197 | 71 | – | – | – | – | – | – | – | 33 | 52 | – | – | – | – |
| C9 | L(−)-Perillyl alcohol | Grassy, woody, floral | 7000 | – | – | 4 | 2 | – | 1 | – | – | – | – | – | – | 6 | – | – | – | – | – | – |
| C10 | Elemol | Grassy, floral | 100 | 5 | 43 | 16 | – | 7 | 6 | 4 | – | 24 | 4 | – | 4 | – | – | 22 | – | – | – | 7 |
| C11 | Phytol | Vegetal | 640 | – | – | – | 0.5 | – | – | 0.3 | 0.6 | – | – | – | – | – | – | – | – | – | – | 0.5 |
| C12 | trans-Nerolidol | Malic, rose | 250 | – | – | – | – | – | – | – | – | 8 | – | 126 | – | – | – | – | – | – | – | – |
| C13 | (+)-β-Citronellol | Rose | 40 | – | – | 1138 | – | – | – | – | 576 | – | – | – | – | – | – | – | – | – | – | – |
| C14 | L-α-Terpineol | Lilac | 9180 | – | – | – | – | 5 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| C15 | L(−)-Menthol | Minty | 2280 | – | – | 3 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| C16 | 2-heptanol | Potato, cheese, milk, powder | 65 | – | – | – | – | – | – | – | – | – | – | – | 2 | – | – | – | – | – | – | – |
| C17 | (±)-trans-4-Thujanol | Minty, green | 55,000 | – | – | – | – | – | – | – | <0.2 | – | – | – | – | – | – | – | – | – | – | – |
| C18 | cis-Linaloloxide | Woody, floral | 100 | – | – | – | – | – | – | – | – | – | – | 25 | – | – | – | – | – | – | – | – |
| Aldehydes | ||||||||||||||||||||||
| D1 | Dodecanal | Lilac, violet | 10 | – | – | – | – | – | – | – | – | – | – | – | – | – | 70 | – | – | 60 | – | – |
| D2 | Benzeneacetaldehyde | Malic | 6.3 | – | – | – | – | – | 647 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D3 | Nonanal | Citrus, green, fruity | 1.1 | – | – | – | 92,371 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D4 | Citronellal | Floral, rose, sweet | 6 | – | 158 | – | – | – | – | – | 375 | – | – | – | – | – | – | – | – | – | – | – |
| D5 | Citral | citric | 32 | – | – | – | – | – | – | – | – | – | – | – | – | – | 65 | – | – | – | – | – |
| D6 | Undecanal | Wax, floral | 12.5 | – | – | – | – | – | – | – | – | 904 | 41 | – | 31 | – | – | – | – | – | 139 | 145 |
| D7 | Octanal | citrusy, soapy | 0.587 | – | – | 7234 | 25,880 | – | – | – | 8074 | – | – | – | – | – | – | – | – | – | – | – |
| D8 | Decanal | Citrus, fatty, green | 3 | 2088 | 2420 | 6087 | 9097 | 7563 | 1102 | 3487 | 8960 | 2202 | 1829 | 6956 | 1448 | 4375 | 2412 | 1893 | 2336 | 2365 | 6635 | 4346 |
| D9 | (S)-(−)-Perillaldehyde | Green, oily, fatty, cherry | 30 | – | 233 | 578 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D10 | (E)-2-Decenal | Floral, sweet | 17 | – | – | 129 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D11 | L-Perillaldehyde | Perilla-like, spicy | 30 | 136 | – | – | 550 | 498 | 176 | 329 | 205 | 164.741 | 76 | 589 | 94 | 580 | 138 | 162 | 172 | 125 | 376 | 318 |
| D12 | α-Sinensal | orange | 220 | – | – | 50 | – | – | – | 3 | 3 | – | – | – | – | – | – | – | – | – | – | – |
| D13 | Tridecanal | Citrus, wax, oily | 10 | – | – | 189 | – | 988 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| D14 | Benzaldehyde | Fruity, almond | 750.89 | – | – | – | – | – | – | – | – | – | – | 0.5 | – | – | – | – | – | – | – | – |
| D15 | (+)-Citronellal | Lemon, citronella, rose | 30 | – | – | – | 91 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Ketones | ||||||||||||||||||||||
| E1 | (+)-Dihydrocarvone | Herbs | 3250 | 1.822 | – | 2.291 | – | – | – | – | – | 0.7 | – | – | – | – | – | – | 2 | – | – | – |
| E2 | Hydroxyacetone | Tobacco-like | 80,000 | – | – | – | – | – | – | – | <0.2 | – | – | – | – | – | – | – | – | – | – | – |
| E3 | L(−)-Carvone | Carvi | 7 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 36 | – | – |
| E4 | D(+)-Carvone | Minty, minty, licorice | 160 | 144 | 979 | 295 | 158 | 614 | 208 | 1511 | 91 | 145 | 82 | 383 | 117 | 578 | 153 | 143 | 209 | – | 195 | 186 |
| E5 | Piperiton | Minty | 680 | – | – | 2 | 1 | – | – | 1 | 1 | – | – | – | – | – | 1 | 2 | – | – | 2 | – |
| E6 | β-Lonone | Violet | 0.007 | – | – | – | – | – | – | – | 183,529 | – | – | – | 233,159 | 504,179 | – | – | 202,171 | – | 457,431 | – |
| E7 | β-Ionone | Woody, violet | 3.5 | – | – | 559,318 | 1145 | 899,822 | – | 798 | – | 572 | – | – | – | – | 463 | – | – | 347 | – | 534,380 |
| E8 | α-Ionone | Woody, violet | 3.78 | – | 223 | – | 129 | – | – | – | – | – | – | – | 66 | – | – | – | – | – | – | – |
| E9 | 4-Methylacetophenone | Hawthorn-like | 21 | – | – | – | – | 686 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| E10 | (E,E)-3,5-Octadien-2-one | Floral | 100 | – | – | – | – | – | – | – | – | 29 | – | – | – | – | – | 26 | – | – | – | – |
| E11 | 6-Methylhept-5-en-one | Citronnelle | 68 | 32 | – | – | – | – | – | 11 | – | – | – | – | 1 | – | 2 | – | – | – | 21 | – |
| E12 | 3-Hydroxy-2,3-dihydromaltol | Floral | 35,000 | – | – | – | – | – | – | – | <0.2 | – | – | – | – | – | – | – | – | – | – | – |
| Hydrocarbons | ||||||||||||||||||||||
| F1 | 1r-.α.-Pinene | Pine-like | 2.2 | – | – | – | – | – | – | – | 3350 | – | – | – | 646 | – | – | 3318 | 2866 | – | – | 390 |
| F2 | 1s-.α.-Pinene | Pine-like | 100 | – | – | – | – | – | – | – | – | – | – | 15 | – | – | 14 | – | – | – | – | – |
| F3 | Sabinene | woody | 980 | – | – | 9 | – | – | 8 | 7 | – | – | – | – | 2 | 1 | 3 | – | 8 | – | – | 12 |
| F4 | (1S)-(−)-β-Pinene | Resinous, pine-like | 4160 | – | 2 | – | 4 | 0.5 | – | – | – | – | – | 0.5 | – | – | – | – | – | – | – | – |
| F5 | α-Pinene | Resin, pine, ethereal | 14 | 218 | 235 | 628 | 557 | 465 | 237 | 22 | 916 | 414 | 257 | 72 | 62 | 160 | 46 | 72 | 132 | 234 | 198 | 48 |
| F6 | β-Pinene | Resinous, pungent, green, pine-like | 140 | 64 | – | – | – | – | – | – | – | 35 | – | – | – | – | – | 56 | – | – | – | – |
| F7 | β-Myrcene | Tea, Ethereal, oily | 1.2 | 24,346 | 19,068 | 14,497 | 29,658 | 21,805 | 16,356 | 14,657 | 14,412 | 20,180 | 14,436 | 46,253 | 9689 | 26,693 | 10,799 | 21,372 | 24,909 | 11,581 | 19,112 | 25,413 |
| F8 | D-Limonene | Citrus, lemon, minty | 34 | 23,633 | 22,203 | 22,919 | 26,510 | 46,910 | 24,795 | 17,967 | 14,990 | 19,839 | 12,935 | 39,124 | 10,875 | 59,930 | 14,492 | 27,900 | 28,664 | 12,074 | 25,152 | 31,450 |
| F9 | (Z)-β-Ocimene | Green, floral, neroli | 34 | 164 | 93 | 263 | – | 230 | – | 100 | 85 | – | 91 | 496 | 58 | 187 | – | – | 122 | 118 | 93 | 35 |
| F10 | γ-Terpinene | Sweet, citrus | 1000 | 62 | 109 | 34 | 76 | 68 | 21 | 45 | 61 | 65 | 36 | 20 | 12 | 38 | 17 | 97 | 25 | 8 | 28 | 28 |
| F11 | Terpinolene | Resinous, pine | 200 | – | 34 | – | – | – | – | – | – | 3 | – | – | – | – | – | – | – | – | – | – |
| F12 | α-Humulene | Woody, spicy | 160 | – | – | – | – | – | – | – | – | – | – | 134 | – | – | – | – | – | 9 | – | – |
| F13 | β-Caryophyllene | Spicy, citrus | 64 | 111 | 203 | 140 | 132 | 235 | 44 | 79 | 68 | 179 | – | 683 | 117 | 219 | 65 | 370 | 51 | 42 | 101 | 148 |
| F14 | α-Terpinene | Citrus | 80 | – | – | – | – | – | – | – | – | – | 3 | – | – | – | – | 7 | – | – | – | – |
| F15 | 7,11-Dimethyl-3-methylene-1,6,10-dodecatriene | Rose, sweet | 87 | – | – | – | – | 120 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| F16 | β-Phellandrene | Citrus, pepper | 500 | – | – | – | – | – | – | – | 22 | – | 12 | – | – | – | – | – | – | – | – | – |
| F17 | Limonene-1,2-epoxide | Lemon-like | 100 | – | – | – | – | – | 1 | – | – | – | – | – | – | – | – | – | – | – | – | – |
| Others | ||||||||||||||||||||||
| G1 | 4-Ethylguaiacol | Sweet, spicy, herbs | 89.25 | – | – | – | – | 130 | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| G2 | 2-Methoxy-4-vinylphenol | Strong spicy | 12.02 | – | – | 186 | – | 16,135 | 2131 | – | 256 | 204 | – | – | 123 | 9466 | – | – | – | – | – | – |
| G3 | 1-Furfurylpyrrole | Filbert, coffee-like | 100 | – | – | – | – | – | 90 | – | – | – | – | – | – | 181 | – | – | – | – | – | – |
| G4 | 2-Propanamine | Ammonia | 5000 | – | – | – | – | – | – | – | – | <0.2 | – | – | – | – | – | – | – | – | – | – |
| Abbreviation of Citrus-Tea | Common Name of Citrus | Plant Species of Citrus | Source of Citrus | GPS Coordinate | Plucking Time | Dark Tea | Category |
|---|---|---|---|---|---|---|---|
| WZM | Wenzhou Tangerine Dafen No. 4 | Citrus unshiu Marc | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 15 July 2019 | Tianjian tea | Mandarin orange |
| SMQ | Shimeng orange | Citrus reticulata cv. “Shimeng” | Changde City | 25°27′ N, 121°58′ E | 8 July 2019 | ||
| XQG | Xiaoqing orange | Citrus reticulata cv. “Xiaoqing” | Xiangxi Autonomous Prefecture | 28°24′ N, 110°00′ E | 15 August 2019 | ||
| NFM | Nanfeng orange | Citrus reticulata Blanco “Kinokuni” | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 15 July 2019 | ||
| GC | Gongchuan orange | Citrus unshiu “Miyagawa Wase” | Hunan Horticultural Research Institute, Changsha | 28°21′ N, 113°10′ E | 18 July 2019 | ||
| XJ | Xinjing orange | Citrus unshiu “Xinjing” | Hunan Horticultural Research Institute, Changsha | 28°21′ N, 113°10′ E | 18 July 2019 | ||
| XN | Xinnv Ponkan | Citrus reticulata Blanco cv. Ponkan | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 20 July 2019 | Ponkan | |
| ZM | Zaomi Ponkan | Citrus reticulata Blanco cv. Ponkan | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 20 July 2019 | ||
| CX | Chunxiang mixed Citrus | Citrus poonensis Hort. ex Tanaka | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 18 July 2019 | Hybrid citrus | |
| HY | Huyou | Citrus maxima (Burm) Merr. | Yueyang City | 29°36′ N, 113°14′ E | 1 August 2019 | Grapefruits | |
| DD | Daidai lime | Citrus aurantium L. “Daidai” | Lianyuan city | 27°69′ N, 111°67′ E | 15 August 2019 | Sour orange | |
| PS | Pushi Cheng | Citrus sinensis (L.) Osbeck “Pushi Cheng” | Xiangxi Autonomous Prefecture | 28°16′ N, 109°99′ E | 18 August 2019 | Sweet orange | |
| BT | Bingtang orange | Citrus sinensis (L.) Osbeck “Bingtang” | Xiangxi Autonomous Prefecture | 28°16′ N, 109°99′ E | 18 August 2019 | ||
| LZ | Luzai honey orange | Citrus sinensis (L.) Osbeck “Luzai” | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 18 August 2019 | ||
| JP | Jinpenyou | Citrus junos Sieb. ex Tanaka “yuzu” | Yueyang City | 29°15′ N, 113°12′ E | 17 August 2019 | Yuzu | |
| XC | Tarocco blood orange | Citrus sinensis (L.) Osbeck “Tarocco” | Xiangxi Autonomous Prefecture | 28°27′ N, 110°17′ E | 18 August 2019 | Navel orange | |
| LF | Langfeng navel orange | Citrus sinensis Osbeck “Langfeng” | Shaoyang City | 39°90′ N, 116°52′ E | 15 September 2019 | ||
| YF | Yuanfeng navel orange | Citrus sinensis Osbeck “Yuanfeng” | Hunan Horticultural Research Institute, Changsha | 28°19′ N, 113°14′ E | 17 August 2019 | ||
| NHE | Newhall navel orange | Citrus sinensis Osbeck “Newhall” | Hunan Horticultural Research Institute, Changsha | 28°21′ N, 113°10′ E | 17 August 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, H.; Ding, S.; Pan, Z.; Li, X.; Fu, F. Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules 2020, 25, 6027. https://doi.org/10.3390/molecules25246027
Qi H, Ding S, Pan Z, Li X, Fu F. Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules. 2020; 25(24):6027. https://doi.org/10.3390/molecules25246027
Chicago/Turabian StyleQi, Heting, Shenghua Ding, Zhaoping Pan, Xiang Li, and Fuhua Fu. 2020. "Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS" Molecules 25, no. 24: 6027. https://doi.org/10.3390/molecules25246027
APA StyleQi, H., Ding, S., Pan, Z., Li, X., & Fu, F. (2020). Characteristic Volatile Fingerprints and Odor Activity Values in Different Citrus-Tea by HS-GC-IMS and HS-SPME-GC-MS. Molecules, 25(24), 6027. https://doi.org/10.3390/molecules25246027





