Impact of Botrytis cinerea Contamination on the Characteristics and Foamability of Yeast Macromolecules Released during the Alcoholic Fermentation of a Model Grape Juice
Abstract
:1. Introduction
2. Results and Discussion
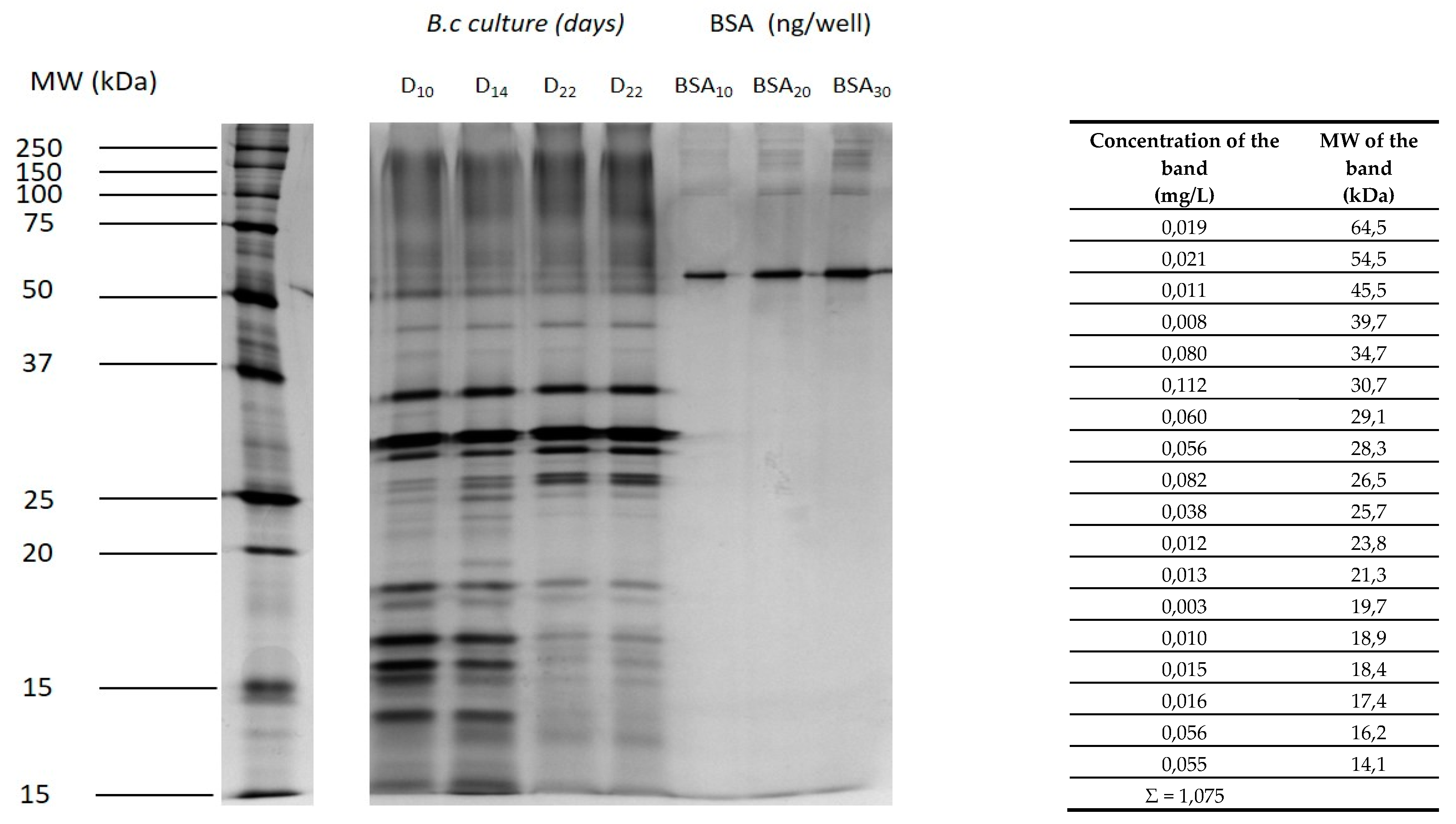
2.1. Proteins Secreted by Botrytis during the Culture of the Fungus
2.2. Protease Activity on BSA Followed by SDS-PAGE
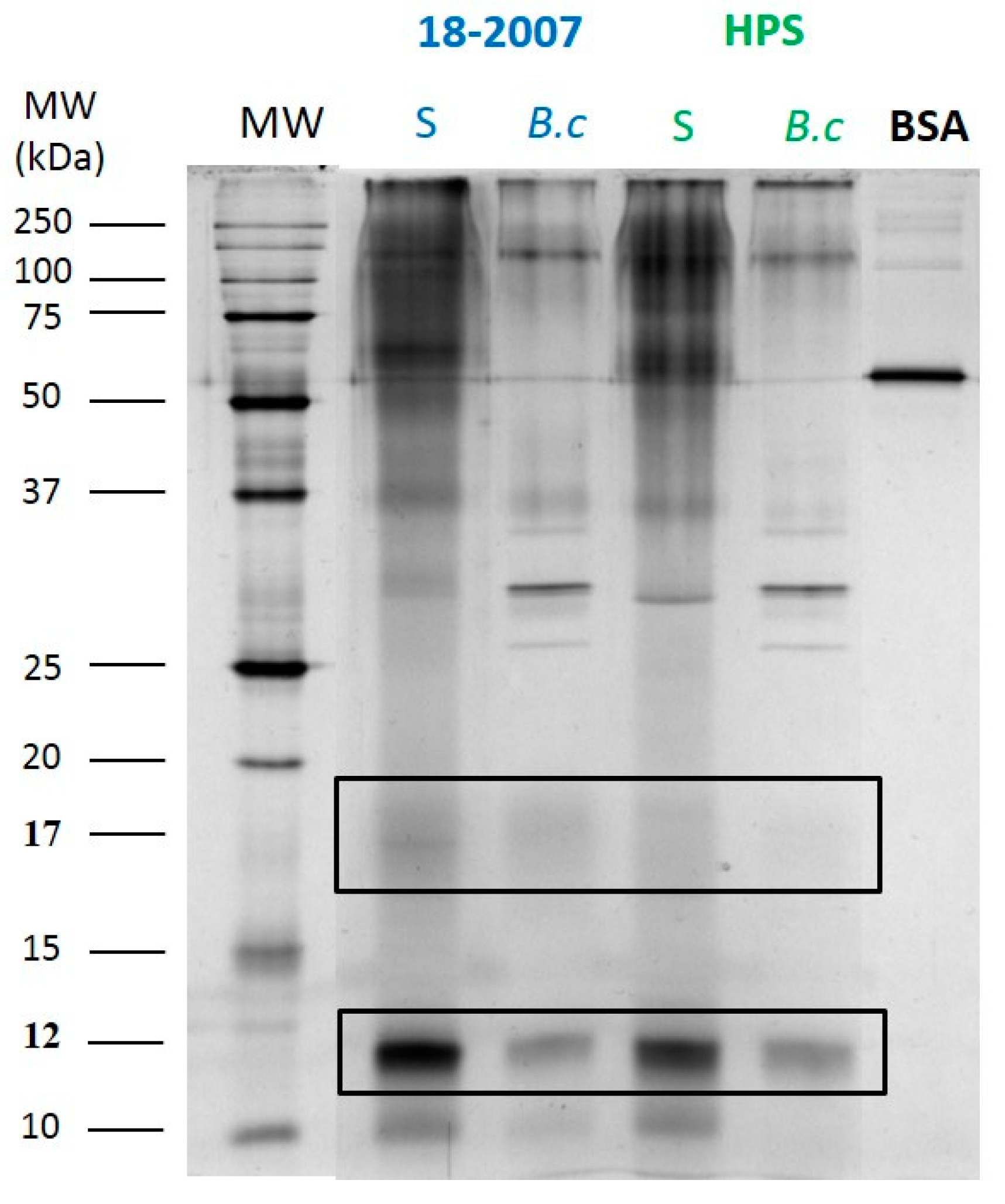
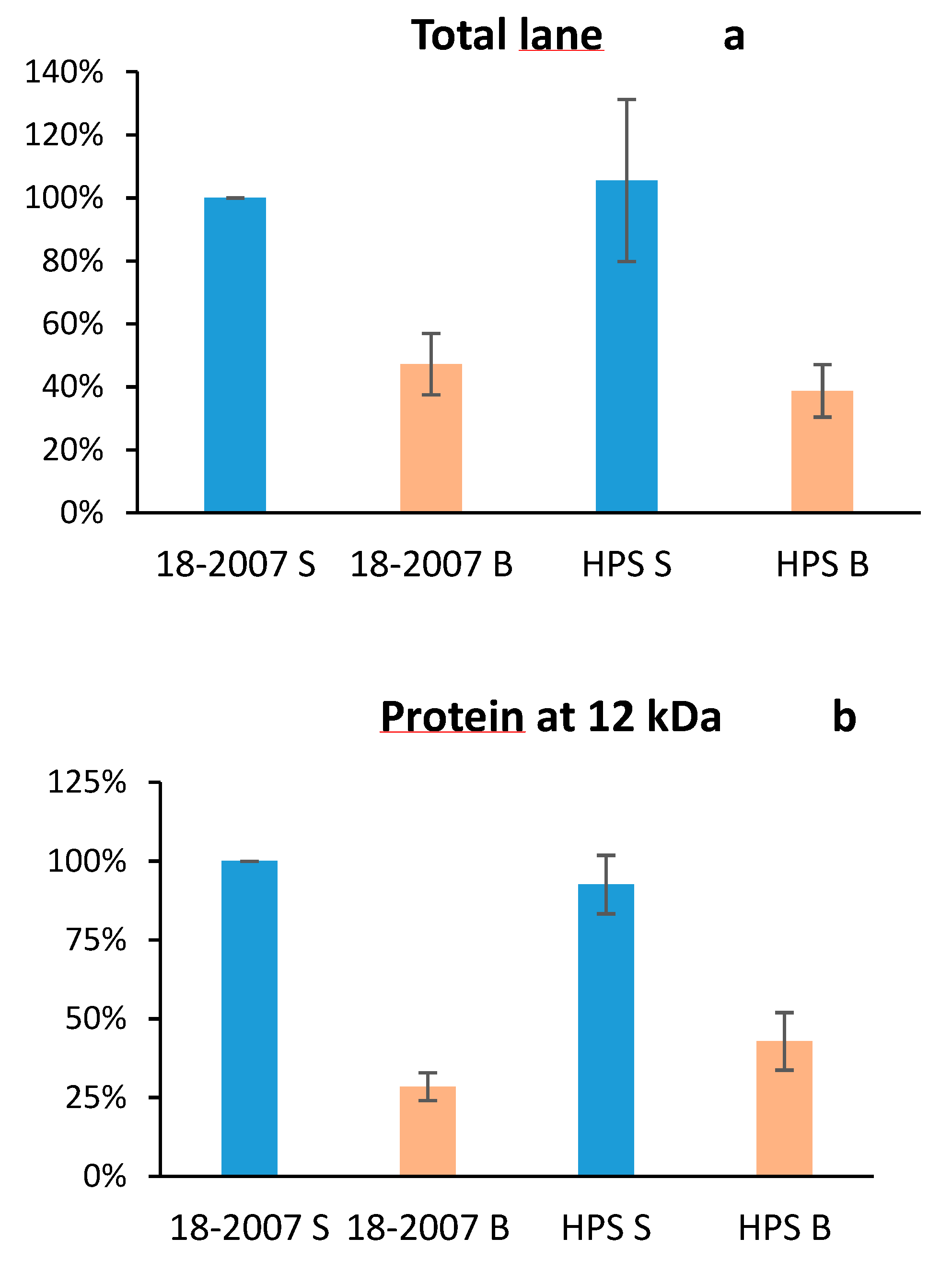
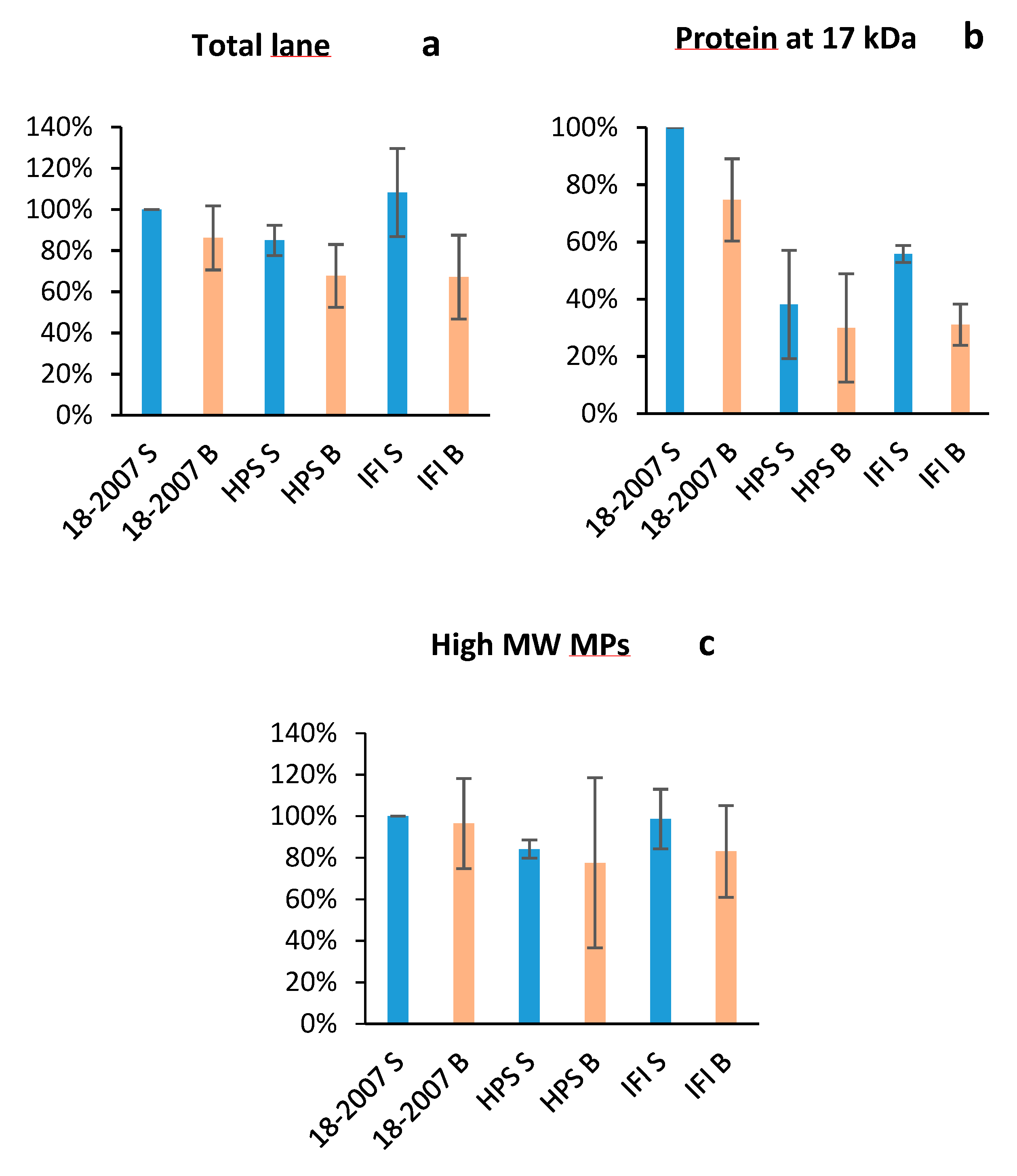
2.3. Botrytis Protease Activity on Yeast Proteins Followed by SDS-PAGE
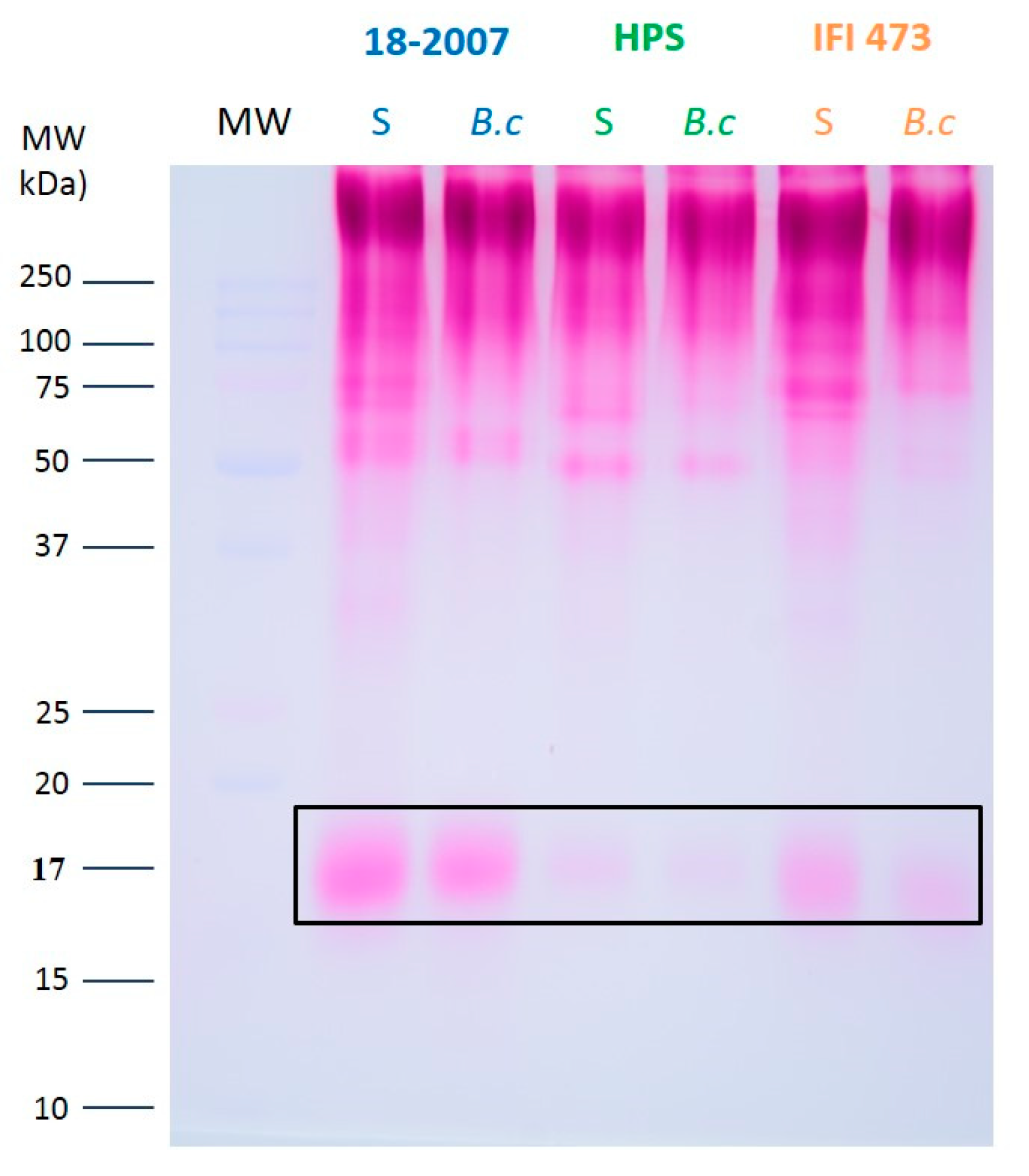
2.4. Botrytis Enzyme Activities on Yeast Macromolecules Followed by SEC-MALLS
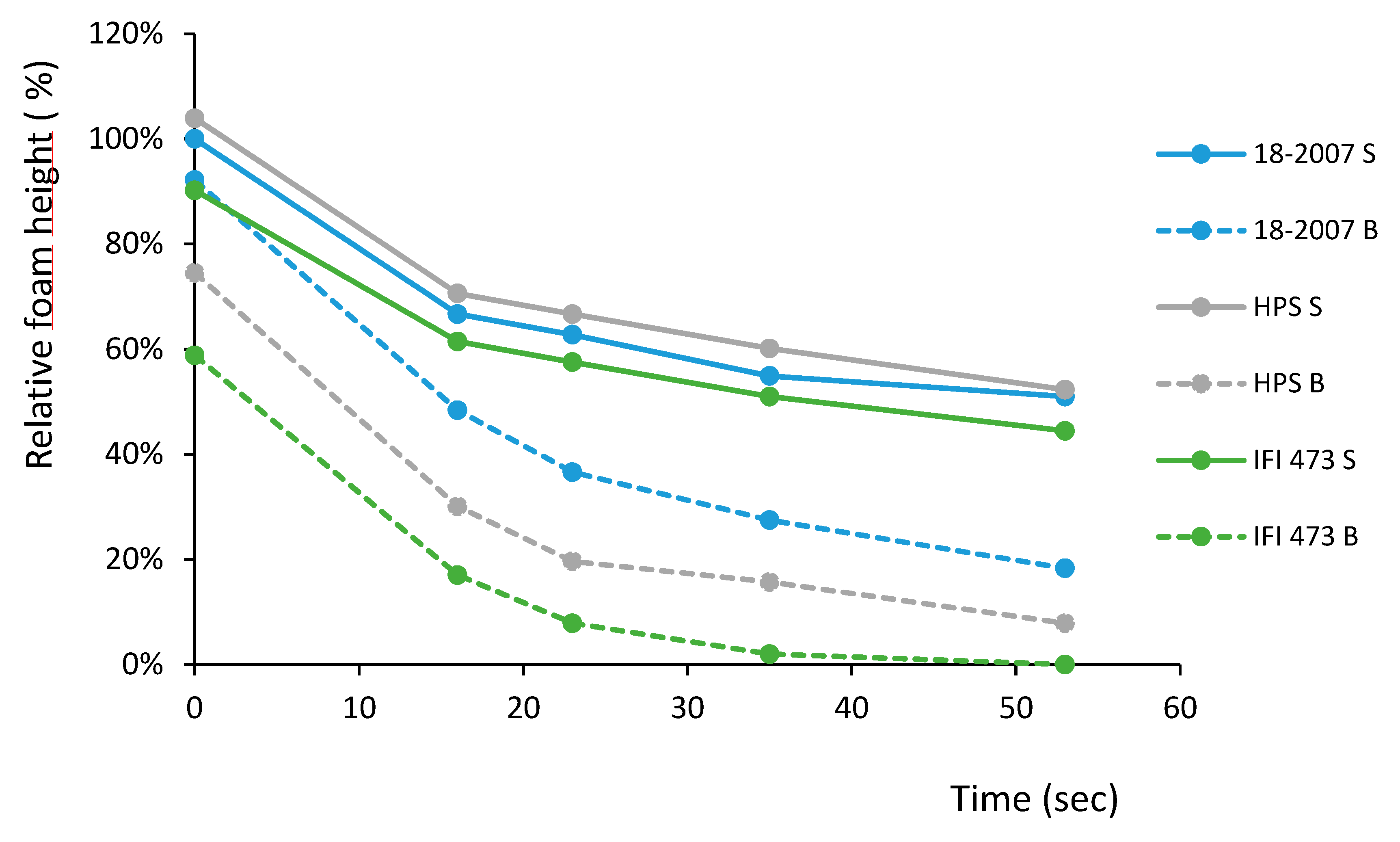
2.5. Wine Foam Characteristics
3. Materials and Methods
3.1. Botrytis Cinerea Culture Conditions
3.2. Isolation and Quantification of B. Cinerea Proteins
3.3. Evidence of Botrytis Proteasic Activity
3.4. Model Juice Alcoholic Fermentation
3.5. Yeast Protein Analysis by SDS-PAGE
3.6. Isolation of Polysaccharide Fractions
3.7. Multi-Detector High Performance Size Exclusion Chromatography (HPSEC)
3.8. Sugar and Ethanol Contents
3.9. Foamability
3.10. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Depond Didier. What generates the fine mousse in a Champagne? Ask Decanter. Available online: https://www.decanter.com/learn/advice/generates-fine-champagne-mousse-ask-decanter-354038/ (accessed on 5 December 2019).
- France Agrimer, Synthèse filière vins. Le marché mondial des vins effervescents (Avril 2016). Available online: http://www.franceagrimer.fr/content/download/44021/419586/file/SYN-VIN-2016-march%C3%A9%20mondial%20effervescents.pdf (accessed on 5 December 2019).
- Technavio’s report. Global Sparkling Wine Market—Size, Projections, Drivers, Trends, Vendors, and Analysis Through 2021. Available online: http://www.businesswire.com/news/home/20170614006068/en/Global-Sparkling-Wine-Market---Size-Projections (accessed on 5 December 2019).
- Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z. Influence of Wine Chemical Compounds on the Foaming Properties of Sparkling Wines. In Grapes and Wines-Advances in Production, Processing, Analysis and Valorization; IntechOpen: London, UK, 2018. [Google Scholar]
- Slinkard, S. Best Champagne and Sparkling Wines for a Celebration. Available online: https://www.thespruceeats.com/champagne-and-sparkling-wine-reviews-3510906 (accessed on 5 December 2019).
- WINE.NET. Sparkling Wine for Life’s Celebrations. Available online: https://www.wine.net/sparkling-wine-lifes-celebrations (accessed on 5 December 2019).
- Abdallah, Z.; Aguié-Béghin, V.; Abou-Saleh, K.; Douillard, R.; Bliard, C. Isolation and analysis of macromolecular fractions responsible for the surface properties in native Champagne wines. Food Res. Int. 2010, 43, 982–987. [Google Scholar] [CrossRef]
- Maujean, A.; Poinsaut, P.; Dantan, H.; Brissonnet, F.; Cossiez, E. Study of the performance and quality of the foam in sparkling wines. 2. Perfecting of a measuring technique for foaming ability, performance and stability of the foam in sparkling wines (measurement of foam properties, bentonite, vegetable black carbon). Bulletin de l’OIV 1990, 63, 405–427. [Google Scholar]
- Malvy, J.; Robillard, B.; Duteurtre, B. Influence des protéines sur le comportement de la mousse des vins de Champagne. (Influence of proteins on Champagne wine foaming behavior). Sci. Aliment. 1994, 14, 88–98. [Google Scholar]
- Coelho, E.; Reis, A.; Domingues, M.R.M.; Rocha, S.M.; Coimbra, M.A. Synergistic effect of high and low molecular weight molecules in the foamability and foam stability of sparkling wines. J. Agric. Food Chem. 2011, 59, 3168–3179. [Google Scholar] [CrossRef] [PubMed]
- Dambrouck, T.; Marchal, R.; Cilindre, C.; Parmentier, M.; Jeandet, P. Determination of the grape invertase content (using PTA-ELISA) following various fining treatments vs. changes in the total protein content of wine. Relationships with wine foamability. J. Agric. Food Chem. 2005, 53, 8782–8789. [Google Scholar] [CrossRef] [PubMed]
- Dambrouck, T.; Marchal, R.; Marchal-Delahaut, L.; Parmentier, M.; Maujean, A.; Jeandet, P. Immunodetection of proteins from grapes and yeast in a white wine. J. Agric. Food Chem. 2003, 51, 2727–2732. [Google Scholar] [CrossRef]
- Brissonnet, F.; Maujean, A. Characterization of foaming proteins in a champagne base wine. Am. J. Enol. Vitic. 1993, 44, 297–301. [Google Scholar]
- Marchal, R.; Tabary, I.; Valade, M.; Moncomble, D.; Viaux, L.; Robillard, B.; Jeandet, P. Effects of Botrytis cinerea infection on Champagne wine foaming properties. J. Sci. Food Agric. 2001, 81, 1371–1378. [Google Scholar] [CrossRef]
- Cilindre, C.; Castro, A.J.; Clément, C.; Jeandet, P.; Marchal, R. Influence of Botrytis cinerea infection on Champagne wine proteins characterized by 2D-electrophoresis and immunodetection. Relationship with foaming properties. Food Chem. 2007, 103, 139–149. [Google Scholar] [CrossRef]
- Marchal, R.; Berthier, L.; Legendre, L.; Marchal-Delahaut, L.; Jeandet, P.; Maujean, A. Effects of Botrytis cinerea infection on the must protein electrophoretic characteristics. J. Agric. Food Chem. 1998, 12, 4945–4949. [Google Scholar] [CrossRef]
- Ten Have, A.; Dekkers, E.; Kay, J.; Phylip, L.H.; Van Kan, J.A. An aspartic proteinase gene family in the filamentous fungus Botrytis cinerea contains members with novel features. Microbiology 2004, 150, 2475–2489. [Google Scholar] [CrossRef] [PubMed]
- Marchal, R.; Warchol, M.; Cilindre, C.; Jeandet, P. Evidence for protein degradation by Botrytis cinerea and relationships with alteration of synthetic wine foaming properties. J. Agric. Food Chem. 2006, 54, 5157–5165. [Google Scholar] [CrossRef] [PubMed]
- Culbert, J.; McRae, J.M.; Condé, B.; Schmidtke, L.M.; Nicholson, E.; Smith, P.A.; Howell, K.; Boss, P.K.; Wilkinson, K.L. Influence of production method on the chemical composition, foaming properties and quality of Australian carbonated and sparkling white wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, Y.P.; Carrascosa, A.V.; Gonzalez, R.; Polo, M.C.; Martinez-Rodriguez, A.J. Effect of accelerated autolysis of yeast on the composition and foaming properties of sparkling wines elaborated by a Champenoise method. J. Agric. Food Chem. 2005, 53, 7232–7237. [Google Scholar] [CrossRef]
- Blasco, L.; Viñas, M.; Villa, T.G. Proteins influencing foam formation in wine and beer: The role of yeast. Int. Microbiol. 2011, 14, 61–71. [Google Scholar]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Barcenilla, J.M.; Pozo-Bayón, M.A.; Polo, M.C. Autolytic capacity and foam analysis as additional criteria for the selection of yeast strains for sparkling wine production. Food Microbiol. 2001, 18, 183–191. [Google Scholar] [CrossRef]
- Shah, P.; Gutierrez-Sanchez, G.; Orlando, R.; Bergmann, C. A proteomic study of pectin-degrading enzymes secreted by Botrytis cinerea grown in liquid culture. Proteomics 2009, 9, 3126–3135. [Google Scholar] [CrossRef]
- Waters, E.J.; Wallace, W.; Williams, P.J. Identification of heat-unstable wine proteins and their resistance to peptidases. J. Agric. Food Chem. 1992, 40, 1514–1519. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Carrascosa, A.V.; Martin-Álvarez, P.J.; Moreno-Arribas, V.; Polo, M.C. Influence of the yeast strain on the changes of the amino acids, peptides and proteins during sparkling wine production by the traditional method. J. Microbiol. Biotechnol. 2002, 29, 314–322. [Google Scholar] [CrossRef]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of Native Yeast Strains for In-Bottle Fermentation to Face the Uniformity in Sparkling Wine Production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef]
- Nishiyama, T.; Kimura, N.; Jitsuhara, Y.; Uchida, M.; Ochi, F.; Yamaguchi, H. N-glycans protect proteins from protease digestion through their binding affinities for aromatic amino acid residues. J. Biochem. 2000, 127, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Cilindre, C.; Jégou, S.; Hovasse, A.; Schaeffer, C.; Castro, A.J.; Clément, C.; Van Dorsselaer, A.; Jeandet, P.; Marchal, R. Proteomic approach to identify Champagne wine proteins as modified by Botrytis cinerea infection. J. Proteome Res. 2008, 7, 1199–1208. [Google Scholar] [CrossRef] [PubMed]
- Dunaevskii, Y.E.; Matveeva, A.R.; Fatkhullina, G.N.; Belyakova, G.A.; Kolomiets, T.M.; Kovalenko, E.D.; Belozersky, M.A. Extracellular proteases of mycelial fungi as participants of pathogenic process. Russ. J. Bioorg. Chem. 2008, 34, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Waters, E.J.; Pellerin, P.; Brillouet, J.M. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Vidal, S.; Williams, P.; Doco, T.; Moutounet, M.; Pellerin, P. The polysaccharides of red wine: Total fractionation and characterization. Carbohydr. Polym. 2003, 54, 439–447. [Google Scholar] [CrossRef]
- Riebe, D.; Thorn, W. Influence of carbohydrate moieties of human serotransferrin on the determination of its molecular mass by polyacrylamide gradient gel electrophoresis and staining with PAS reagent. Electrophoresis 1991, 12, 287–293. [Google Scholar] [CrossRef]
- Llaubères, R.M.; Dubourdieu, D.; Villetaz, J.C. Exocellular polysaccharides from Saccharomyces in wine. J. Sci. Food Agric. 1988, 41, 277–286. [Google Scholar] [CrossRef]
- Gonçalves, F.; Heyraud, A.; De Pinho, M.N.; Rinaudo, M. Characterization of White Wine Mannoproteins. J. Agric. Food Chem. 2002, 50, 6097–6101. [Google Scholar] [CrossRef]
- Urbanek, H.; Zalewska-Sobczak, J. Multiplicity of cell wall degrading glycosidic hydrolases produced by apple infecting Botrytis cinerea. Phytopathol. Z. 1984, 110, 261–271. [Google Scholar] [CrossRef]
- Péron, N.; Cagna, A.; Valade, M.; Marchal, R.; Maujean, A.; Robillard, B.; Aguié-Béghin, V.; Douillard, R. Characterization by drop tensiometry and by ellipsometry of the adsorption layer formed at the air/Champagne wine interface. Adv. Colloid Interface Sci. 2000, 88, 19–36. [Google Scholar] [CrossRef]
- González-Ramos, D.; Gonzalez, R. Genetic Determinants of the release of mannoproteins of enological interest by Saccharomyces cerevisiae. J. Agric. Food Chem. 2006, 54, 9411–9416. [Google Scholar] [CrossRef] [PubMed]
- Jégou, S.; Hoang, D.A.; Salmon, T.; Williams, P.; Oluwa, S.; Vrigneau, C.; Doco, T.; Marchal, R. Effect of grape juice press fractioning on polysaccharide and oligosaccharide compositions of Pinot meunier and Chardonnay Champagne base wines. Food Chem. 2017, 232, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Péron, N.; Cagna, A.; Valade, M.; Bliard, C.; Aguié-Béghin, V.; Douillard, R. Layers of macromolecules at the Champagne/air interface and the stability of Champagne bubbles. Langmuir 2001, 17, 791–797. [Google Scholar] [CrossRef]
- Andrés-Lacueva, C.; Lamuela-Raventós, R.; Buxaderas, S.; De la Torre-Boronat, M.C. Influence of variety and aging on foaming properties of cava (sparkling wine). J. Agric. Food Chem. 1997, 45, 2520–2525. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Ortega-Heras, M.; Bueno-Herrer, M.; Martἱnez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Use of commercial dry yeast products rich in mannoproteins for white and rosé sparkling wine elaboration. J. Agric. Food Chem. 2015, 63, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Rabilloud, T.; Vuillard, L.; Gilly, C.; Lawrence, J.J. Silver-staining of proteins in polyacrylamide gels: A general overview. Cell. Mol. Biol. 1994, 40, 57–75. [Google Scholar]
- Doco, T.; Quellec, N.; Moutounet, M.; Pellerin, P. Polysaccharide patterns during the aging of Carignan noir red wines. Am. J. Enol. Vitic. 1999, 50, 25–32. [Google Scholar]
- Ayestarán, B.; Guadalupe, Z.; Leon, D. Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process. Anal. Chim. Act. 2004, 513, 29–39. [Google Scholar] [CrossRef]
- Doco, T.; Williams, P. Purification and Structural Characterization of a Type II Arabinogalactan-Protein from Champagne Wine. Am. J. Enol. Vitic. 2013, 64, 364–369. [Google Scholar] [CrossRef]
- Esteruelas, M.; González-Royo, E.; Kontoudakis, N.; Orte, A.; Cantos, A.; Canals, J.M.; Zamora, F. Influence of grape maturity on the foaming properties of base wines and sparkling wines (Cava). J. Sci. Food Agric. 2015, 15, 2071–2080. [Google Scholar] [CrossRef] [PubMed]
- OIV. Compendium of International Methods of Wine and Must Analysis; International Organization of Vine and Wine (O.I.V.): Paris, France, 2017; Volume 1. [Google Scholar]
Sample Availability: Samples are not available from the authors. |

| Fexp | F* | ||
|---|---|---|---|
| 18-2007 S vs. 18-2007 B | S*** | 227.02 | 25.41 (P < 0.001) |
| HPS S vs. HPS B | S*** | 46.95 | 25.41 (P < 0.001) |
| 18-2007 S vs. HPS S | NS | 0.36 | 5.32 (P < 0.05) |
| 18-2007 B vs. HPS B | NS | 3.38 | 5.32 (P < 0.05) |
| S. c. 18-2007 | S. c. 18-2007 + B. Cinerea | S. c. HPS | S. c. HPS + B. Cinerea | S. c. IFI 473 | S. c. IFI 473 + B. Cinerea | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Means | S.D | Means | S.D | p-Value | Means | S.D | Means | S.D | p-Value | Means | S.D | Means | S.D | p-Value | |
| Mn (g/mol) | 207 833 | ±27 775 | 143 850 | ±8 980 | 0.057 | 207 350 | ±6 250 | 170 433 | ±23 481 | 0.058 | 214 466 | ±15 315 | 161 100 | ±12 162 | 0.026 |
| Mw (g/mol) | 423 033 | ±52 465 | 313 050 | ±37 547 | 0.086 | 361 550 | ±129 40 | 24 666 | ±24 808 | 0.158 | 478 766 | ±41 101 | 412 050 | ±23 263 | 0.136 |
| Mw/Mn | 2.03 | ±0.07 | 2.17 | ±0.12 | 0.232 | 1.74 | ±0.00 | 1.91 | ±0.12 | 0.174 | 2.23 | ±0.10 | 2.55 | ±0.04 | 0.029 |
| Rz | 19.83 | ±1.81 | 24.15 | ±6.43 | 0.322 | 17.44 | ±0.50 | 16.76 | ±0.65 | 0.311 | 23.03 | ±0.90 | 24.75 | ±1.48 | 0.194 |
| [ŋ] (mL/g) | 40.71 | ±0.62 | 35.16 | ±3.91 | 0.078 | 38.68 | ±0.18 | 43.27 | ±1.12 | 0.012 | 45.22 | ±2.85 | 42.58 | ±2.92 | 0.389 |
| Rh | 12.93 | ±0.52 | 11.225 | ±0.75 | 0.054 | 12.26 | ±0.16 | 12.24 | ±0.20 | 0.910 | 3.57 | ±0.39 | 12.52 | ±0.52 | 0.078 |
| mass (µg)c | 51.4 | ±10.1 | 56.7 | ±4.8 | 0.455 | 47.6 | ±1.02 | 38.1 | ±4.3 | 0.062 | 50.8 | ±0.4 | 43.7 | ±3.0 | 0.052 |
| Range 1 | 77.2 | ±2.8 | 83.2 | ±4.0 | 0.099 | 80.1 | ±5.3 | 89.4 | ±5.2 | 0.098 | 80.8 | ±1.5 | 87.5 | ±1.9 | 0.009 |
| Range 2 | 18.2 | ±1.8 | 13.7 | ±2.4 | 0.066 | 14.3 | ±4.1 | 7.9 | ±4.9 | 0.158 | 14.7 | ±1.0 | 9.9 | ±1.0 | 0.004 |
| Range 3 | 4.6 | ±1.4 | 2.8 | ±1.3 | 0.188 | 5.0 | ±1.2 | 2.4 | ±0.6 | 0.031 | 4.16 | ±0.9 | 2.2 | ±0.6 | 0.042 |
| Time (s) | T16 | T23 | T35 | T53 |
|---|---|---|---|---|
| 18-2007 S vs. 18-2007 B | S * | S * | S | S |
| HPS S vs. HPS B | S * | S | S | S * |
| IFI S vs. IFI B | S *** | S *** | S *** | S *** |
| 18-2007 S vs. HPS S | NS | NS | NS | NS |
| 18-2007 S vs. IFI S | NS | NS | NS | NS |
| HPS S vs. IFI S | NS | NS | NS | NS |
| 18-2007 B vs. HPS B | NS | NS | NS | NS |
| 18-2007 B vs. IFI B | S* | S | S | S |
| HPS B vs. IFI B | NS | NS | NS | NS |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchal, R.; Salmon, T.; Gonzalez, R.; Kemp, B.; Vrigneau, C.; Williams, P.; Doco, T. Impact of Botrytis cinerea Contamination on the Characteristics and Foamability of Yeast Macromolecules Released during the Alcoholic Fermentation of a Model Grape Juice. Molecules 2020, 25, 472. https://doi.org/10.3390/molecules25030472
Marchal R, Salmon T, Gonzalez R, Kemp B, Vrigneau C, Williams P, Doco T. Impact of Botrytis cinerea Contamination on the Characteristics and Foamability of Yeast Macromolecules Released during the Alcoholic Fermentation of a Model Grape Juice. Molecules. 2020; 25(3):472. https://doi.org/10.3390/molecules25030472
Chicago/Turabian StyleMarchal, Richard, Thomas Salmon, Ramon Gonzalez, Belinda Kemp, Céline Vrigneau, Pascale Williams, and Thierry Doco. 2020. "Impact of Botrytis cinerea Contamination on the Characteristics and Foamability of Yeast Macromolecules Released during the Alcoholic Fermentation of a Model Grape Juice" Molecules 25, no. 3: 472. https://doi.org/10.3390/molecules25030472






