Visible-light Promoted Atom Transfer Radical Addition−Elimination (ATRE) Reaction for the Synthesis of Fluoroalkylated Alkenes Using DMA as Electron-donor
Abstract
:1. Introduction
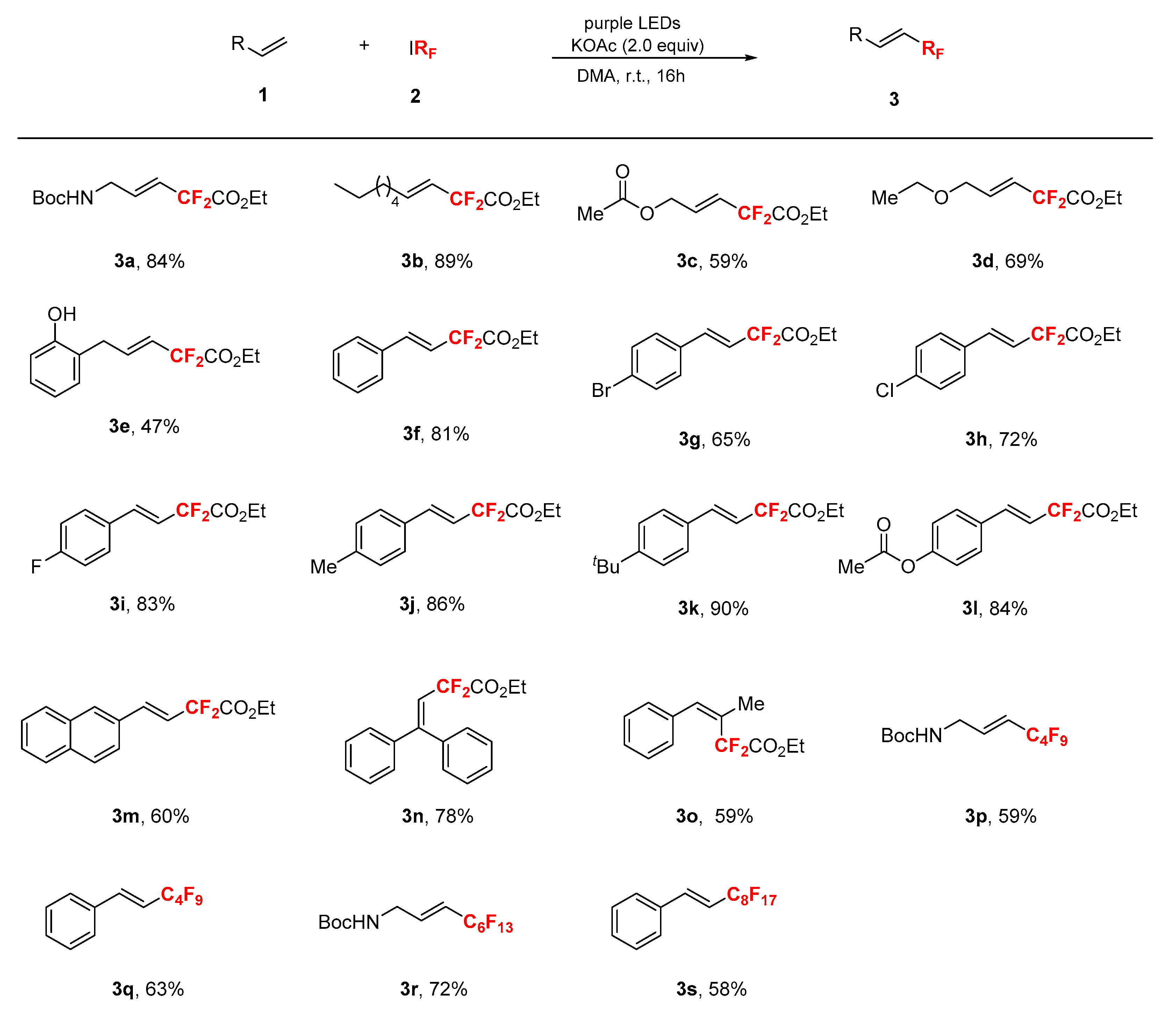
2. Results
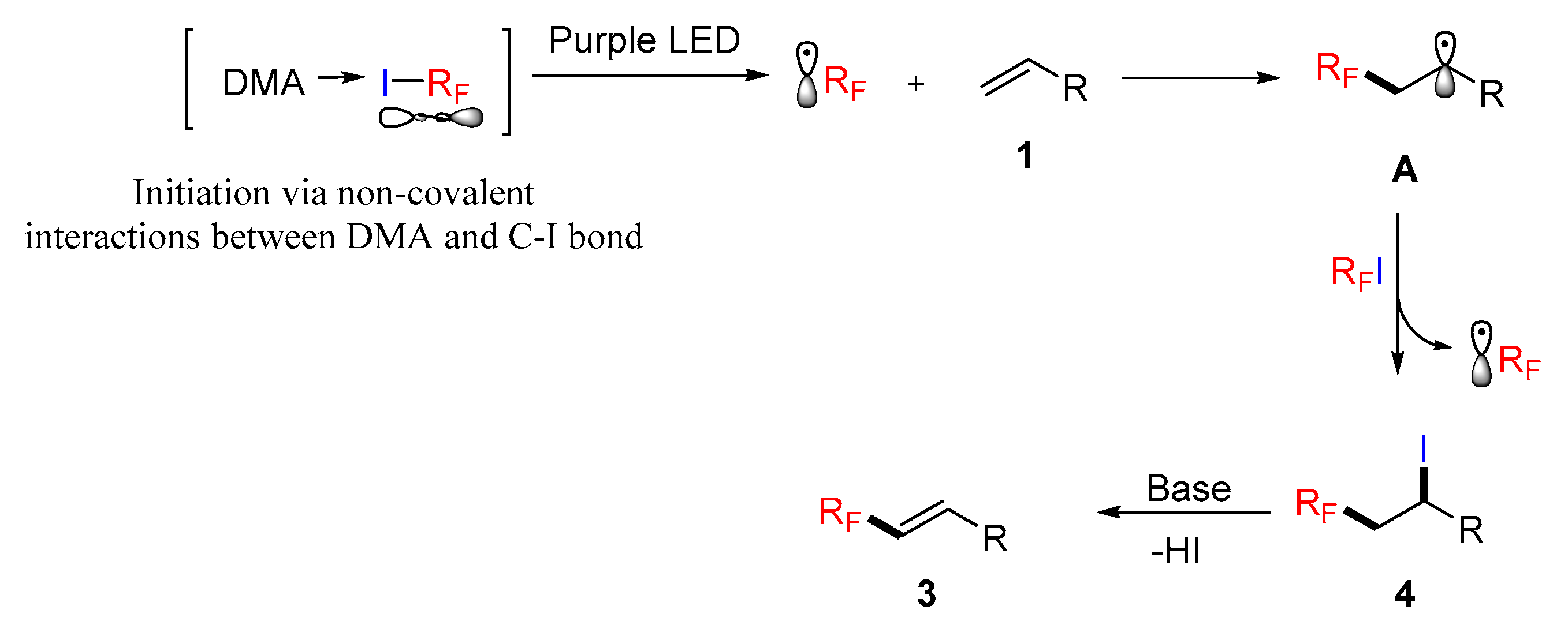
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef]
- Müller, K.; Faeh, C.; Diederich, F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881–1886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Sánchez-Roselló, M.; Aceña, J.L.; del Pozo, C.; Sorochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Preshlock, S.; Tredwell, M.; Gouverneur, V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719–766. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D. Understanding organofluorine chemistry. An Introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Burgey, C.S.; Robinson, K.A.; Lyle, T.A.; Sanderson, P.E.J.; Dale Lewis, S.; Lucas, B.J.; Krueger, J.A.; Singh, R.; Miller-Stein, C.; White, R.B.; et al. Metabolism-Directed Optimization of 3-Aminopyrazinone Acetamide Thrombin Inhibitors. Development of an Orally Bioavailable Series Containing P1 and P3 Pyridines. J. Med. Chem. 2003, 46, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, G.L.; Seim, M.R.; Lu, J.; Makboul, M.; Criscione, K.R. Application of the Goldilocks Effect to the Design of Potent and Selective Inhibitors of Phenylethanolamine N-Methyltransferase: Balancing pKa and Steric Effects in the Optimization of 3-Methyl-1,2,3,4-tetrahydroisoquinoline Inhibitors by β-Fluorination. J. Med. Chem. 2006, 49, 2939–2952. [Google Scholar] [CrossRef] [Green Version]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Charpentier, J.; Früh, N.; Togni, A. Electrophilic Trifluoromethylation by Use of Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650–682. [Google Scholar] [CrossRef]
- Alonso, C.; Marigorta, E.M.; Rubiales, G.; Palacios, F. Carbon Trifluoromethylation Reactions of Hydrocarbon Derivatives and Heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar] [CrossRef]
- Tomashenko, O.A.; Grushin, V.V. Aromatic Trifluoromethylation with Metal Complexes. Chem. Rev. 2011, 111, 4475–4521. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Qing, F.-L. Oxidative Trifluoromethylation and Trifluoromethylthiolation Reactions Using (Trifluoromethyl)trimethylsilane as a Nucleophilic CF3 Source. Acc. Chem. Res. 2014, 47, 1513–1522. [Google Scholar] [CrossRef]
- Xu, X.-H.; Matsuzaki, K.; Shibata, N. Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731–764. [Google Scholar] [CrossRef]
- Feng, Z.; Xiao, Y.-L.; Zhang, X. Transition-Metal (Cu, Pd, Ni)-Catalyzed Difluoroalkylation via Cross-Coupling with Difluoroalkyl Halides. Acc. Chem. Res. 2018, 51, 2264–2278. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, T.; Iqbal, N.; You, Y.; Cho, E.J. Controlled Fluoroalkylation Reactions by Visible-Light Photoredox Catalysis. Acc. Chem. Res. 2016, 49, 2284–2294. [Google Scholar] [CrossRef] [PubMed]
- Yerien, D.E.; Barata-Vallejo, S.; Postigo, A. Difluoromethylation Reactions of Organic Compounds. Chem. Eur. J. 2017, 23, 14676–14701. [Google Scholar] [CrossRef] [PubMed]
- Moschner, J.; Stulberg, V.; Fernandes, R.; Huhmann, S.; Leppkes, J.; Koksch, B. Approaches to Obtaining Fluorinated α-Amino Acids. Chem. Rev. 2019, 119, 10718–10801. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Xu, C.; Lu, L.; Shen, Q. Shelf-Stable Electrophilic Reagents for Trifluoromethylthiolation. Acc. Chem. Res. 2015, 48, 1227–1236. [Google Scholar] [CrossRef]
- Furuya, T.; Kamlet, A.S.; Ritter, T. Catalysis for Fluorination and Trifluoromethylation. Nature 2011, 473, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Yajima, T.; Ikegami, M. Metal-Free Visible-Light Radical Iodoperfluoroalkylation of Terminal Alkenes and Alkynes. Eur. J. Org. Chem. 2017, 2017, 2126–2129. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Iqbal, N.; Park, S.; Cho, E.J. Selective Difluoroalkylation of Alkenes by using Visible Light Photoredox Catalysis. Chem. Commun. 2014, 50, 12884–12887. [Google Scholar] [CrossRef]
- Tang, X.-J.; Dolbier, W.R. Efficient Cu-catalyzed Atom Transfer Radical Addition Reactions of Fluoroalkylsulfonyl Chlorides with Electron-deficient Alkenes Induced by Visible Light. Angew. Chem. Int. Ed. 2015, 54, 4246–4249. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Cheung, C.W.; Hu, X. Iron-Catalyzed 1,2-Addition of Perfluoroalkyl Iodides to Alkynes and Alkene. Angew. Chem. Int. Ed. 2014, 53, 4910–4914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrends, I.; Baehr, S.; Czekelius, C. Perfluoroalkylation of Alkenes by Frustrated Lewis Pairs. Chem. Eur. J. 2016, 22, 17177–17181. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.D.; Tucker, J.W.; Konieczynska, M.D.; Stephenson, C.R.J. Intermolecular Atom Transfer Radical Addition to Olefins Mediated by Oxidative Quenching of Photoredox Catalysts. J. Am. Chem. Soc. 2011, 133, 4160–4163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G.; von Wangelin, J.A. Stereoselective Cobalt-catalyzed Halofluoroalkylation of Alkynes. Chem. Sci. 2018, 9, 1795–1802. [Google Scholar] [CrossRef] [Green Version]
- Beniazza, R.; Remisse, L.; Jardel, D.; Lastecoueres, D.; Vincent, J.-M. Light-mediated Iodoperfluoroalkylation of Alkenes/Alkynes Catalyzed by Chloride Ions: Role of Halogen Bonding. Chem. Commun. 2018, 54, 7451–7454. [Google Scholar] [CrossRef]
- Rawner, T.; Lutsker, E.; Kaiser, C.A.; Reiser, O. The Different Faces of Photoredox Catalysts: Visible-Light-Mediated Atom Transfer Radical Addition (ATRA) Reactions of Perfluoroalkyl Iodides with Styrenes and Phenylacetylenes. ACS Catal. 2018, 8, 3950–3956. [Google Scholar] [CrossRef]
- Magagnano, G.; Gualandi, A.; Marchini, M.; Mengozzi, L.; Ceroni, P.; Cozzi, P.G. Photocatalytic ATRA Reaction Promoted by Iodo-Bodipy and Sodium Ascorbate. Chem. Commun. 2017, 53, 1591–1594. [Google Scholar] [CrossRef]
- Long, Z.-Y.; Chen, Q.-Y. The Activation of Carbon−Chlorine Bonds in Per- and Polyfluoroalkyl Chlorides: DMSO-Induced Hydroperfluoroalkylation of Alkenes and Alkynes with Sodium Dithionite. J. Org. Chem. 1999, 64, 4775–4782. [Google Scholar] [CrossRef]
- Murakami, S.; Ishii, H.; Fuchigami, T. Electrosynthesis of Ethyl a,a-difluoro-a-(phenylseleno)acetate and its Photochemical Synthetic Application. J. Fluorine. Chem. 2004, 125, 609–614. [Google Scholar] [CrossRef]
- Ghattas, W.; Hess, C.R.; Iacazio, G.; Hardre, R.; Klinman, J.P.; Reglier, M. Pathway for the Stereocontrolled Z and E Production of α,α-Difluorine-Substituted Phenyl Butenoates. J. Org. Chem. 2006, 71, 8618–8621. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.P.; Dabral, S.; Wen, J.; Wiesenthal, J.; Terhorst, S.; Bolm, C. Organic Dye-Catalyzed Atom Transfer Radical Addition–Elimination (ATRE) Reaction for the Synthesis of Perfluoroalkylated Alkenes. Org. Lett. 2017, 19, 4295–4298. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, S.; Liu, J.; Zhu, D.; Guo, M.; Tang, X.; Wang, G. Copper-Catalyzed C–H Difluoroalkylations and Perfluoroalkylations of Alkenes and (Hetero)arenes. Org. Lett. 2017, 19, 4187–4190. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Min, Q.-Q.; Zhao, H.-Y.; Gu, J.-W.; Zhang, X. A General Synthesis of Fluoroalkylated Alkenes by Palladium-Catalyzed Heck-Type Reaction of Fluoroalkyl Bromides. Angew. Chem. Int. Ed. 2015, 54, 1270–1274. [Google Scholar] [CrossRef]
- Xie, J.; Li, J.; Weingand, V.; Rudolph, M.; Hashmi, A.S.K. Intermolecular Photocatalyzed Heck-like Coupling of Unactivated Alkyl Bromides by a Dinuclear Gold Complex. Chem. Eur. J. 2016, 22, 12646–12650. [Google Scholar] [CrossRef]
- Nappi, M.; Bergonzini, G.; Melchiorre, P. Metal-Free Photochemical Aromatic Perfluoroalkylation of α-Cyano Arylacetates. Angew. Chem. Int. Ed. 2014, 53, 4921–4925. [Google Scholar] [CrossRef]
- Fernández-Alvarez, V.M.; Nappi, M.; Melchiorre, P.; Maseras, F. Computational Study with DFT and Kinetic Models on the Mechanism of Photoinitiated Aromatic Perfluoroalkylations. Org. Lett. 2015, 17, 2676–2679. [Google Scholar] [CrossRef]
- Kandukuri, S.R.; Bahamonde, A.; Chatterjee, I.; Jurberg, I.D.; Escudero-Adán, E.C.; Melchiorre, P. X-Ray Characterization of an Electron Donor–Acceptor Complex that Drives the Photochemical Alkylation of Indoles. Angew. Chem. Int. Ed. 2015, 54, 1485–1489. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, M.; Liu, H.; Wang, R.; Xu, Z. Visible-Light-Promoted Dearomative Fluoroalkylation of β-Naphthols through Intermolecular Charge Transfer. Angew. Chem. Int. Ed. 2018, 57, 4747–4751. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Li, G.-X.; He, G.; Chen, G. Halogen-Bond-Promoted Photoactivation of Perfluoroalkyl Iodides: A Photochemical Protocol for Perfluoroalkylation Reactions. Org. Lett. 2017, 19, 1442–1445. [Google Scholar] [CrossRef] [PubMed]
- Helmecke, L.; Spittler, L.; Baumgarten, K.; Czekelius, C. Metal-Free Activation of C–I Bonds and Perfluoroalkylation of Alkenes with Visible Light Using Phosphine Catalysts. Org. Lett. 2019, 21, 7823–7827. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.-L.; Sun, K.; Li, X.-Y.; Zeng, F.-L.; Liu, X.-C.; Qu, L.-B.; Zhao, Y.-F.; Yu, B. Visible-Light Induced Radical Perfluoroalkylation/Cyclization Strategy To Access 2-Perfluoroalkylbenzothiazoles/Benzoselenazoles by EDA Complex. Org. Lett. 2019, 21, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.-Y.; Zhu, S.; Qing, F.-L.; Chu, L. A Four-component Radical Cascade Trifluoromethylation Reaction of Alkenes Enabled by an Electron-donor–acceptor Complex. Chem. Commun. 2018, 54, 12710–12713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, G.-R.; Choi, Y.; Choi, M.G.; Chang, S.-K.; Cho, E.J. Metal-Free Visible-Light-Induced Trifluoromethylation Reactions. Asian J. Org. Chem. 2017, 6, 436–440. [Google Scholar] [CrossRef]
- Cheng, Y.; Yuan, X.; Ma, J.; Yu, S. Direct Aromatic C-H Trifluoromethylation via an Electron-Donor–Acceptor Complex. Chem. Eur. J. 2015, 21, 8355–8359. [Google Scholar] [CrossRef]
- Postigo, A. Electron Donor-Acceptor Complexes in Perfluoroalkylation Reactions. Eur. J. Org. Chem. 2018, 46, 6391–6404. [Google Scholar] [CrossRef]
- Zhao, L.; Huang, Y.; Wang, Z.; Zhu, E.; Mao, T.; Jia, J.; Gu, J.; Li, X.-F.; He, C.-Y. Organophosphine-Catalyzed Difluoroalkylation of Alkenes. Org. Lett. 2019, 21, 6705–6709. [Google Scholar] [CrossRef]
- Zhu, E.; Liu, X.-X.; Wang, A.-J.; Mao, T.; Zhao, L.; Zhang, X.; He, C.-Y. Visible Light Promoted Fluoroalkylation of Alkenes and Alkynes using 2-Bromophenol as a Catalyst. Chem. Commun. 2019, 55, 12259–12262. [Google Scholar] [CrossRef]
- Huang, Y.; Lei, Y.-Y.; Zhao, L.; Gu, J.; Yao, Q.; Wang, Z.; Li, X.-F.; Zhang, X.; He, C.-Y. Catalyst-free and Visible Light Promoted Trifluoromethylation and Perfluoroalkylation of Uracils and Cytosines. Chem. Commun. 2018, 54, 13662–13665. [Google Scholar] [CrossRef]
- Mao, T.; Ma, M.-J.; Zhao, L.; Xue, D.-P.; Yu, Y.; Gu, J.; He, C.-Y. A General and Green Fluoroalkylation Reaction Promoted via Noncovalent Interactions between Acetone and Fluoroalkyl Iodides. Chem. Commun. 2020, 56. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, X.; Chen, J.; Gao, Y.; Yang, C.; Zhang, K.; Zhou, Y.; Fan, B. Blue Light Induced Difluoroalkylation of Alkynes and Alkenes. Org. Lett. 2019, 21, 9914–9918. [Google Scholar] [CrossRef] [PubMed]
- Cismesia, M.; Yoon, T. Characterizing Chain Processes in Visible Light Photoredox Catalysis. Chem. Sci. 2015, 6, 5426–5434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buzzetti, L.; Crisenza, G.E.; Melchiorre, P. Mechanistic Studies in Photocatalysis. Angew. Chem. Int. Ed. 2019, 58, 3730–3747. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Not available. |



| Entry | Light Source | Base (equiv) | Sovent | 3a, yield (%) | 4a, yield (%) |
|---|---|---|---|---|---|
| 1 | Blue LEDs | K3PO4 (2) | MeCN | ---- | 29 |
| 2 | Blue LEDs | K3PO4 (2) | DCE | ---- | 55 |
| 3 | Blue LEDs | K3PO4 (2) | THF | ---- | ---- |
| 4 | Blue LEDs | K3PO4 (2) | Toluene | ---- | 7 |
| 5 | Blue LEDs | K3PO4 (2) | Dioxane | ---- | trace |
| 6 | Blue LEDs | K3PO4 (2) | DMSO | 10 | ---- |
| 7 | Blue LEDs | K3PO4 (2) | DMF | 21 | 51 |
| 8 | Blue LEDs | K3PO4 (2) | DMA | 45 | 36 |
| 9 | Blue LEDs | K2CO3 (2) | DMA | 43 | 26 |
| 10 | Blue LEDs | Cs2CO3 (2) | DMA | 59 | 5 |
| 11 | Blue LEDs | KOAc (2) | DMA | 64 | ---- |
| 12 | Green LEDs | KOAc (2) | DMA | 50 | ---- |
| 13 | Purple LEDs | KOAc (2) | DMA | 95(84) | ---- |
| 14 | Purple LEDs | None | DMA | ---- | ---- |
| 15 c | none | KOAc (2) | DMA | ---- | ---- |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.-W.; Wang, L.; Mao, T.; Gu, J.; Li, X.-F.; He, C.-Y. Visible-light Promoted Atom Transfer Radical Addition−Elimination (ATRE) Reaction for the Synthesis of Fluoroalkylated Alkenes Using DMA as Electron-donor. Molecules 2020, 25, 508. https://doi.org/10.3390/molecules25030508
Xu W-W, Wang L, Mao T, Gu J, Li X-F, He C-Y. Visible-light Promoted Atom Transfer Radical Addition−Elimination (ATRE) Reaction for the Synthesis of Fluoroalkylated Alkenes Using DMA as Electron-donor. Molecules. 2020; 25(3):508. https://doi.org/10.3390/molecules25030508
Chicago/Turabian StyleXu, Wen-Wen, Le Wang, Ting Mao, Jiwei Gu, Xiao-Fei Li, and Chun-Yang He. 2020. "Visible-light Promoted Atom Transfer Radical Addition−Elimination (ATRE) Reaction for the Synthesis of Fluoroalkylated Alkenes Using DMA as Electron-donor" Molecules 25, no. 3: 508. https://doi.org/10.3390/molecules25030508
APA StyleXu, W.-W., Wang, L., Mao, T., Gu, J., Li, X.-F., & He, C.-Y. (2020). Visible-light Promoted Atom Transfer Radical Addition−Elimination (ATRE) Reaction for the Synthesis of Fluoroalkylated Alkenes Using DMA as Electron-donor. Molecules, 25(3), 508. https://doi.org/10.3390/molecules25030508






