Selenium Attenuates Chronic Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in Mouse Granulosa Cells
Abstract
:1. Introduction
2. Results
2.1. Heat Stress Induces Cell Apoptosis via the Activation of ER Stress in Mouse Granulosa Cells
2.2. Sodium Selenite Attenuates the Heat Stress-Induced Apoptosis and ER Stress in Mouse Granulosa Cells
2.3. 4-Phenylbutyrate (4-PBA) Attenuates the Heat Stress-Induced Apoptosis and ER Stress in Mouse Granulosa Cells
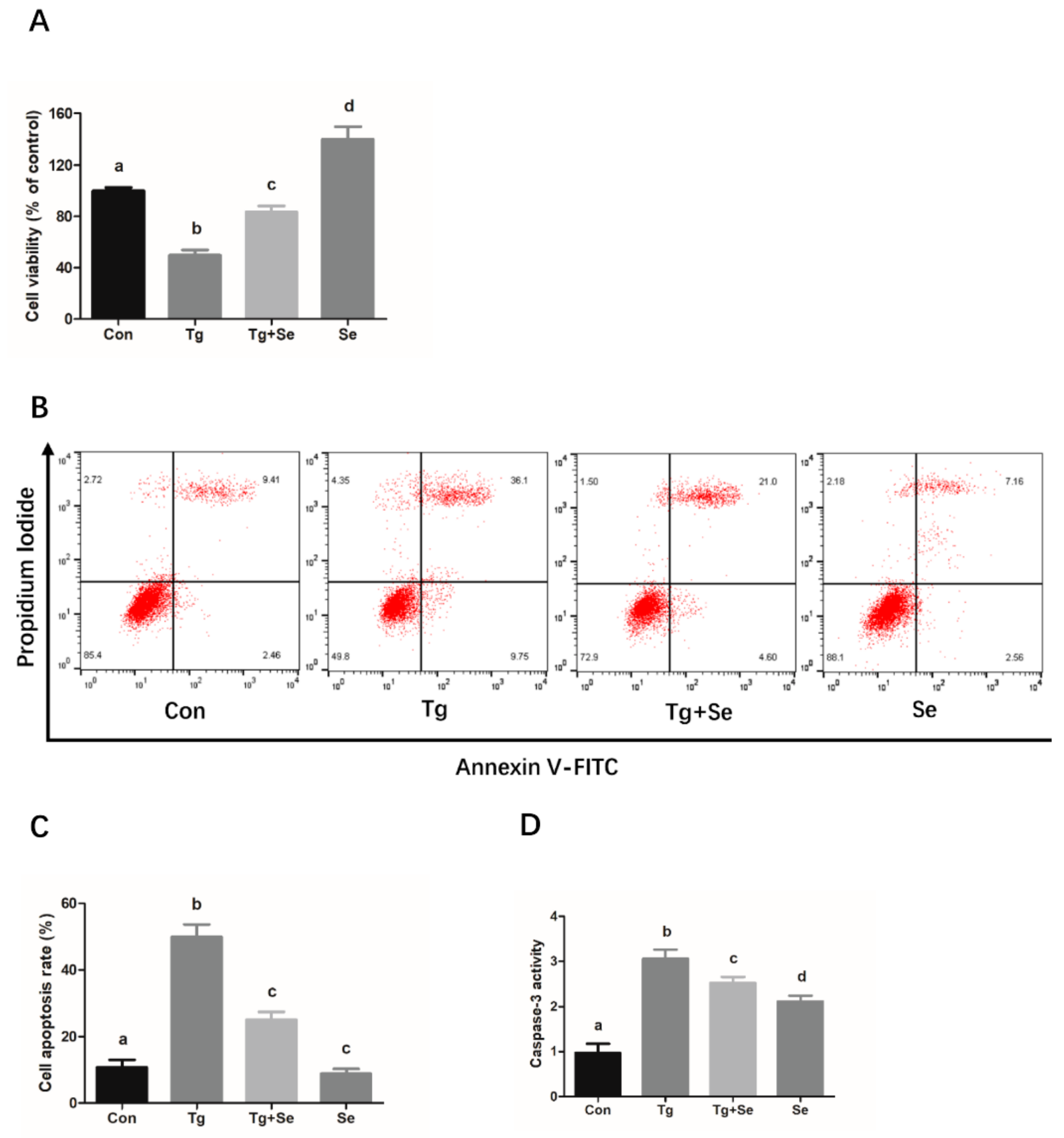
2.4. Sodium Selenite Protects the Cells Against Thapsigargin (Tg)-Induced Cytotoxicity, Apoptosis, and ER Stress in Mouse Granulosa Cells
2.5. Sodium Selenite Ameliorates Heat Stress-Induced Reduction of Estradiol Production in Mouse Granulosa Cells
3. Discussion
4. Materials and Methods
4.1. Animal Care and Treatment
4.2. Collection, Culture, and Heat Treatment of Mouse Granulosa Cells
4.3. Analysis of Cell Viability
4.4. Analysis of Cell Apoptotic Rate
4.5. Analysis of Caspase 3 Activity
4.6. Western Blotting
4.7. Analysis of Estradiol Levels
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HS | Heat stress |
| ER | Endoplasmic reticulum |
| GRP78 | Glucose-regulated protein 78 |
| CHOP | CCAAT/enhancer-binding protein (C/EBP) homologous protein |
| CYP19A1 | Cytochrome P450 family 19 subfamily A member 1 |
| Se | Selenium |
| HSP70 | Heat Shock Protein 70 |
| PMSG | Pregnant Mare Serum Gonadotropin |
| 4-PBA | 4-Phenylbutyrate |
| Tg | Thapsigargin |
References
- Belhadj, S.I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, N.W.; Rowland, K.; Schmidt, C.J.; Lamont, S.J.; Rothschild, M.F.; Ashwell, C.M.; Persia, M.E. Effects of acute and chronic heat stress on the performance, egg quality, body temperature, and blood gas parameters of laying hens. Poult. Sci. 2019, 98, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Akbarinejad, V.; Gharagozlou, F.; Vojgani, M. Temporal effect of maternal heat stress during gestation on the fertility and anti-Mullerian hormone concentration of offspring in bovine. Theriogenology 2017, 99, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hao, Y.; Li, J.; Bao, W.; Li, G.; Gao, Y.; Gu, X. Chronic heat stress induces immune response, oxidative stress response, and apoptosis of finishing pig liver: A proteomic approach. Int. J. Mol. Sci. 2016, 17, E393. [Google Scholar] [CrossRef] [Green Version]
- Manabe, N.; Goto, Y.; Matsuda-Minehata, F.; Inoue, N.; Maeda, A.; Sakamaki, K.; Miyano, T. Regulation mechanism of selective atresia in porcine follicles: Regulation of granulosa cell apoptosis during atresia. J. Reprod. Dev. 2004, 50, 493–514. [Google Scholar] [CrossRef] [Green Version]
- Hale, B.J.; Hager, C.L.; Seibert, J.T.; Selsby, J.T.; Baumgard, L.H.; Keating, A.F.; Ross, J.W. Heat stress induces autophagy in pig ovaries during follicular development. Biol. Reprod. 2017, 97, 426–437. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Elsheikh, N.; Li, C.; Yang, F.; Wang, G.; Li, L. HO-1 reduces heat stress-induced apoptosis in bovine granulosa cells by suppressing oxidative stress. Aging 2019, 11, 5535–5547. [Google Scholar] [CrossRef]
- Naseer, Z.; Ahmad, E.; Epikmen, E.T.; Ucan, U.; Boyacioglu, M.; Ipek, E.; Akosy, M. Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 2017, 96, 136–141. [Google Scholar] [CrossRef]
- Walter, P.; Ron, D. The unfolded protein response: From stress pathway to homeostatic regulation. Science 2011, 334, 1081–1086. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.L. Attenuation of endoplasmic reticulum stress as a treatment strategy against ischemia/reperfusion injury. Neural. Regen. Res. 2015, 10, 1930–1931. [Google Scholar] [CrossRef]
- Gao, L.; Chen, H.; Li, C.; Xiao, Y.; Yang, D.; Zhang, M.; Zhou, D.; Liu, W.; Wang, A.; Jin, Y. ER stress activation impairs the expression of circadian clock and clock-controlled genes in NIH3T3 cells via an ATF4-dependent mechanism. Cell Signal 2019, 57, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Iurlaro, R.; Munoz-Pinedo, C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, P.; Yang, Y.; Li, X.; Chen, F.; Cui, C.; Hu, L.; Li, Q.; Liu, W.; Jin, Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol. Reprod. Dev. 2012, 79, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Gupta, S.; Hu, W.; McGrath, B.C.; Cavener, D.R. Hyperthermia induces the ER stress pathway. PLoS ONE 2011, 6, e23740. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Park, S.J.; Kim, T.S.; Park, H.J.; Park, J.; Kim, B.K.; Kim, G.R.; Kim, J.M.; Huang, S.M.; Chae, J.I.; et al. Testicular hyperthermia induces Unfolded Protein Response signaling activation in spermatocyte. Biochem. Biophys. Res. Commun. 2013, 434, 861–866. [Google Scholar] [CrossRef]
- Lipinski, B. Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011, 2011, 809696. [Google Scholar] [CrossRef] [Green Version]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef]
- Spears, W.; Weiss, W.P. Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet. J. 2008, 176, 70–76. [Google Scholar] [CrossRef]
- Chen, J.; Pan, T.; Wan, N.; Sun, Z.; Zhang, Z.; Li, S. Cadmium-induced endoplasmic reticulum stress in chicken neutrophils is alleviated by selenium. J. Inorg. Biochem. 2017, 170, 169–177. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Qazi, I.; Angel, C.; Yang, H.; Pan, B.; Zoidis, E.; Zeng, C.; Han, H.; Zhou, G. Selenium, Selenoproteins, and Female Reproduction: A Review. Molecules 2018, 23, 3053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehdi, Y.; Hornick, J.L.; Istasse, L.; Dufrasne, I. Selenium in the environment, metabolism and involvement in body functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef] [Green Version]
- Reyes, L.; Bishop, D.P.; Hawkins, C.L.; Rayner, B.S. Assessing the efficacy of dietary selenomethionine supplementation in the setting of cardiac ischemia/reperfusion Injur. Antioxidants 2019, 8, E546. [Google Scholar] [CrossRef] [Green Version]
- Luo, M.; Li, L.; Xiao, C.; Sun, Y.; Wang, G.L. Heat stress impairs mice granulosa cell function by diminishing steroids production and inducing apoptosis. Mol. Cell Biochem. 2016, 412, 81–90. [Google Scholar] [CrossRef]
- Glamoclija, V.; Vilovic, K.; Saraga-Babic, M.; Baranovic, A.; Sapunar, D. Apoptosis and active caspase-3 expression in human granulosa cells. Fertil. Steril. 2005, 83, 426–431. [Google Scholar] [CrossRef]
- Liu, Y.; Zuo, H.; Wang, Y.; Tian, L.; Xu, X.; Xiong, J.; Pei, X. Ethanol promotes apoptosis in rat ovarian granulosa cells via the Bcl-2 family dependent intrinsic apoptotic pathway. Cell. Mol. Biol. 2018, 64, 118–125. [Google Scholar] [CrossRef]
- Alemu, T.W.; Pandey, H.O.; Salilew, W.D.; Gebremedhn, S.; Neuhof, C.; Tholen, E.; Holker, M.; Schellander, K.; Tesfaye, D. Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 2018, 110, 130–141. [Google Scholar] [CrossRef]
- Hou, C.H.; Lin, F.L.; Hou, S.M.; Liu, J.F. Hyperthermia induces apoptosis through endoplasmic reticulum and reactive oxygen species in human osteosarcoma cells. Int. J. Mol. Sci. 2014, 15, 17380–17395. [Google Scholar] [CrossRef] [Green Version]
- Heads, R.J.; Yellon, D.M.; Latchman, D.S. Differential cytoprotection against heat stress or hypoxia following expression of specific stress protein genes in myogenic cells. J. Mol. Cell. Cardiol. 1995, 27, 1669–1678. [Google Scholar] [CrossRef]
- Huo, C.; Xiao, C.; She, R.; Liu, T.; Tian, J.; Dong, H.; Tian, H.; Hu, Y. Chronic heat stress negatively affects the immune functions of both spleens and intestinal mucosal system in pigs through the inhibition of apoptosis. Int. J. Mol. Sci. 2019, 136, 103672. [Google Scholar] [CrossRef]
- Mosser, D.D.; Caron, A.W.; Bourget, L.; Denis-Larose, C.; Massie, B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol. Cell Biol. 1997, 17, 5317–5327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutuk, S.G.; Naziroglu, M. Selenium Diminishes Docetaxel-Induced Cell Death, Oxidative Stress, and Inflammation in the Laryngotracheal Epithelium of the Mouse. Biol. Trace Elem. Res. 2019, 15. [Google Scholar] [CrossRef]
- Atef, M.M.; Abd-Ellatif, R.N.; Emam, M.N.; Abo, E.G.R.; Amer, A.I.; Hafez, Y.M. Therapeutic potential of sodium selenite in letrozole induced polycystic ovary syndrome rat model: Targeting mitochondrial approach (selenium in PCOS). Arch. Biochem. Biophys. 2019, 671, 245–254. [Google Scholar] [CrossRef]
- Ertilav, K.; Nazıroğlu, M.; Ataizi, Z.S.; Braidy, N. Selenium Enhances the Apoptotic Efficacy of Docetaxel Through Activation of TRPM2 Channel in DBTRG Glioblastoma Cells. Neurotox. Res. 2019, 35, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Wrobel, J.K.; Seelbach, M.J.; Chen, L.; Power, R.F.; Toborek, M. Supplementation with selenium-enriched yeast attenuates brain metastatic growth. Nutr. Cancer 2013, 65, 563–570. [Google Scholar] [CrossRef]
- Weiller, M.; Latta, M.; Kresse, M.; Lucas, R.; Wendel, A. Toxicity of nutritionally available selenium compounds in primary and transformed hepatocytes. Toxicology 2004, 201, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; An, Y.; Jiao, W.; Wang, J.; Li, S.; Teng, X. CHOP/caspase-3 signal pathway involves in mitigative effect of selenium on lead-induced apoptosis via endoplasmic reticulum pathway in chicken testes. Environ. Sci. Pollut. Res. Int. 2018, 25, 18838–18845. [Google Scholar] [CrossRef]
- Rosenfeld, C.S.; Wagner, J.S.; Roberts, R.M.; Lubahn, D.B. Intraovarian actions of oestrogen. Reproduction 2001, 122, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Bayne, S.; Li, H.; Jones, M.E.; Pinto, A.R.; van Sinderen, M.; Drummond, A.; Simpson, E.R.; Liu, J.P. Estrogen deficiency reversibly induces telomere shortening in mouse granulosa cells and ovarian aging in vivo. Protein Cell 2011, 2, 333–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozawa, M.; Tabayashi, D.; Latief, T.A.; Shimizu, T.; Oshima, I.; Kanai, Y. Alterations in follicular dynamics and steroidogenic abilities induced by heat stress during follicular recruitment in goats. Reproduction 2005, 129, 621–630. [Google Scholar] [CrossRef]
- Wolfenson, D.; Lew, B.J.; Thatcher, W.W.; Graber, Y.; Meidan, R. Seasonal and acute heat stress effects on steroid production by dominant follicles in cows. Anim. Reprod. Sci. 1997, 47, 9–19. [Google Scholar] [CrossRef]
- Shimizu, T.; Ohshima, I.; Ozawa, M.; Takahashi, S.; Tajima, A.; Shiota, M.; Miyazaki, H.; Kanai, Y. Heat stress diminishes gonadotropin receptor expression and enhances susceptibility to apoptosis of rat granulosa cells. Reproduction 2005, 129, 463–472. [Google Scholar] [CrossRef]
- Basini, G.; Tamanini, C. Selenium stimulates estradiol production in bovine granulosa cells: Possible involvement of nitric oxide. Domest. Anim. Endocrinol. 2000, 18, 1–17. [Google Scholar] [CrossRef]
- Yao, X.; EI-Samahy, M.A.; Fan, L.; Zheng, L.; Jin, Y.; Pang, J.; Zhang, G.; Liu, Z.; Wang, F. In vitro influence of selenium on the proliferation of and steroidogenesis in goat luteinized granulosa cells. Theriogenology 2018, 114, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Belani, M.; Purohit, N.; Pillai, P.; Gupta, S.; Gupta, S. Modulation of steroidogenic pathway in rat granulosa cells with subclinical Cd exposure and insulin resistance: An impact on female fertility. Biomed. Res. Int. 2014, 2014, 460251. [Google Scholar] [CrossRef] [Green Version]
- Wu, G.; Song, D.; Wei, Q.; Xing, J.; Shi, X.; Shi, F. Melatonin mitigates bisphenol A-induced estradiol production and proliferation by porcine ovarian granulosa cells in vitro. Anim. Reprod. Sci. 2018, 192, 91–98. [Google Scholar] [CrossRef]
- Chen, F.; Wan, N.; Yang, D.; Wen, X.; Mahmoud, T.N.; Zhou, D.; Tang, K.; Lin, P.; Wang, A.; Jin, Y. Herp depletion arrests the S phase of the cell cycle and increases estradiol synthesis in mouse granulosa cells. J. Reprod. Dev. 2016, 62, 159–166. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Wang, J.; Lang, H.; Wang, W.; Liu, X.; Liu, H.; Tan, C.; Li, X.; Zhao, Y.; Wu, X. Knockdown of CREB1 promotes apoptosis and decreases estradiol synthesis in mouse granulosa cells. Biomed. Pharmacother. 2018, 105, 1141–1146. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, K.; Li, C.; Li, L.; Wang, G. Heme oxygenase 1 regulates apoptosis induced by heat stress in bovine ovarian granulosa cells via the ERK1/2 pathway. J. Cell Physiol. 2019, 234, 3961–3972. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Li, J.Y.; Yang, W.X. Calcium/calmodulin-dependent protein kinase II regulates cyclooxygenase-2 expression and prostaglandin E2 production by activating cAMP-response element-binding protein in rat peritoneal macrophages. Immunology 2014, 143, 287–299. [Google Scholar] [CrossRef]
- Banerjee, C.; Khatri, P.; Raman, R.; Bhatia, H.; Datta, M.; Mazumder, S. Role of calmodulin-calmodulin kinase II, cAMP/protein kinase A and ERK 1/2 on aeromonas hydrophila-induced apoptosis of head kidney macrophages. PLoS Pathog. 2014, 10, e1004018. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; Bian, Z.; Xie, D.; Peng, Q. A selenium-modified ginseng polysaccharide promotes the apoptosis in human promyelocytic leukemia (HL-60) cells via a mitochondrial-mediated pathway. Biol. Trace. Elem. Res. 2017, 177, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lim, Y.; Lee, D.; Elvira, R.; Lee, J.M.; Lee, M.R.; Han, J. Modulation of protein synthesis by eIF2α phosphorylation protects cell from heat stress-mediated apoptosis. Cells 2018, 7, 254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozpedek, W.; Pytel, D.; Mucha, B.; Leszczynska, H.; Diehl, J.A.; Majsterek, I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Curr. Mol. Med. 2016, 16, 533–544. [Google Scholar] [CrossRef]
- Chen, M.T.; Huang, R.L.; Ou, L.J.; Chen, Y.N.; Men, L.; Chang, X.; Wang, L.; Yang, Y.Z.; Zhang, Z. Pollen typhae total flavone inhibits endoplasmic reticulum stress-induced apoptosis in human aortic-vascular smooth muscle cells through down-regulating PERK-eIF2alpha-ATF4-CHOP pathway. Chin. J. Integr. Med. 2019, 25, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Chen, H.; Lin, P.; Wang, A.; Wang, L.; Jin, Y. ATF6 knock-down decreases apoptosis, arrests the S phase of the cell cycle and increases steroid hormone production in mouse granulosa cells. Am. J. Physiol. Cell Physiol. 2017, 312, C341–C353. [Google Scholar] [CrossRef]
- Sirotkin, A.V. Effect of two types of stress (heat shock/high temperature and malnutrition/serum deprivation) on porcine ovarian cell functions and their response to hormones. J. Exp. Biol. 2010, 213, 2125–2130. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.; Jiang, J.; Lu, K.; Chen, Q.; Tao, D.; Chen, Z. Therapeutic potential of ADAM17 modulation in gastric cancer through regulation of the EGFR and TNF-α signaling pathways. Mol. Cell. Biochem. 2017, 426, 17–26. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Q.X.; Lu, R.R. Surface layer protein from Lactobacillus acidophilus NCFM inhibit intestinal pathogen-induced apoptosis in HT-29 cells. Int. J. Biol. Macromol. 2017, 96, 766–774. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, H.; Wang, Y.; Liu, C.; Zhu, W.; Zheng, S.; Wan, F. Taurine induces the apoptosis of breast cancer cells by regulating apoptosis-related proteins of mitochondria. Int. J. Mol. Med. 2015, 35, 218–226. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available on request to the authors. |






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, Y.; Yin, Q.; Jin, E.; Chen, H.; He, S. Selenium Attenuates Chronic Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in Mouse Granulosa Cells. Molecules 2020, 25, 557. https://doi.org/10.3390/molecules25030557
Xiong Y, Yin Q, Jin E, Chen H, He S. Selenium Attenuates Chronic Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in Mouse Granulosa Cells. Molecules. 2020; 25(3):557. https://doi.org/10.3390/molecules25030557
Chicago/Turabian StyleXiong, Yongjie, Qirun Yin, Erhui Jin, Huatao Chen, and Shaojun He. 2020. "Selenium Attenuates Chronic Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in Mouse Granulosa Cells" Molecules 25, no. 3: 557. https://doi.org/10.3390/molecules25030557
APA StyleXiong, Y., Yin, Q., Jin, E., Chen, H., & He, S. (2020). Selenium Attenuates Chronic Heat Stress-Induced Apoptosis via the Inhibition of Endoplasmic Reticulum Stress in Mouse Granulosa Cells. Molecules, 25(3), 557. https://doi.org/10.3390/molecules25030557





