Technological Application of Tannin-Based Extracts
Abstract
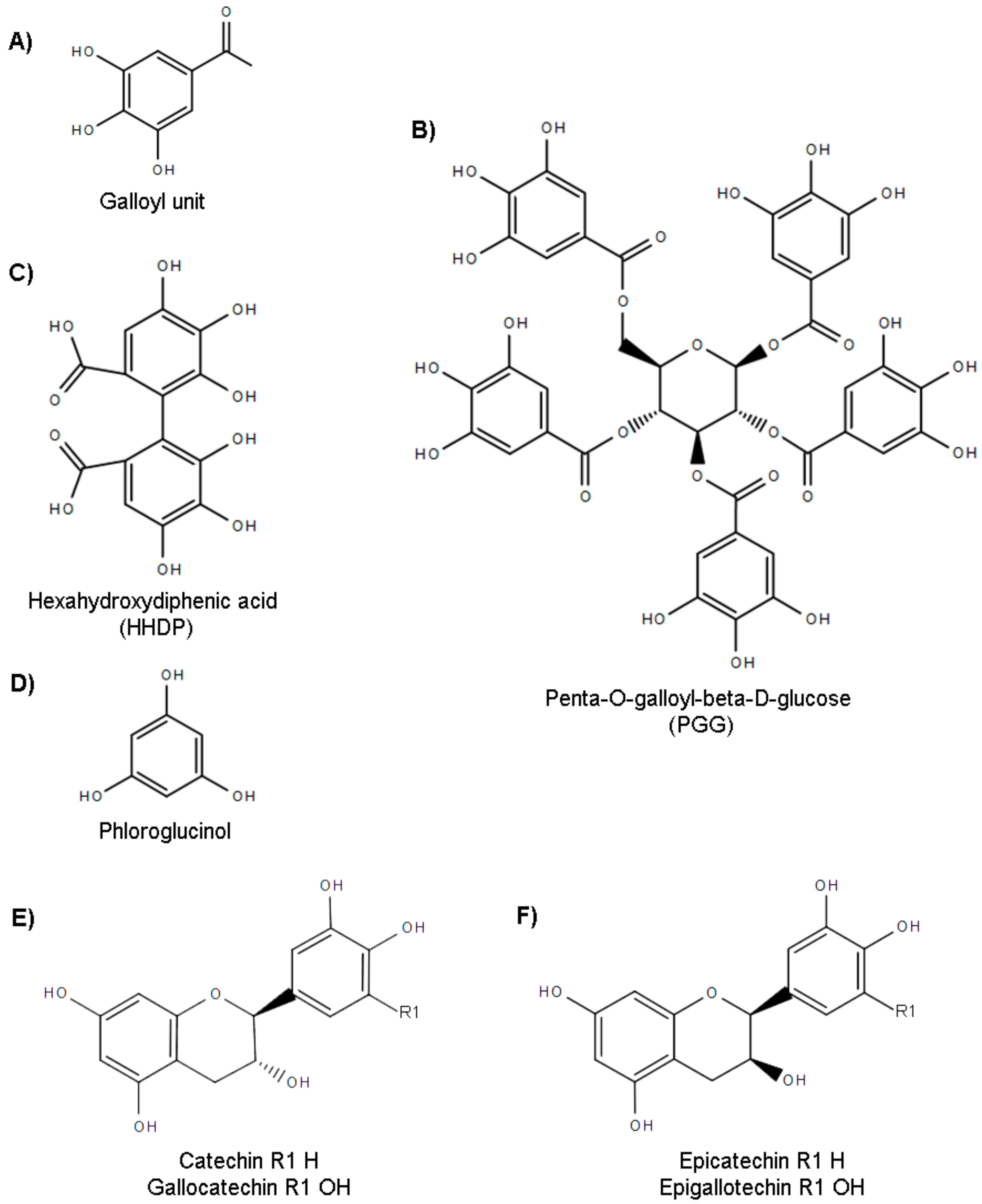
:1. Introduction
2. Major Sources of Tannins for Technological Applications
3. Efficient Extraction Procedures for Technological Application of Tannins: Rapid and Economic
3.1. Solid/Liquid Extraction
3.2. Supercritical Extraction
3.3. Pressurized Water Extraction
3.4. Microwave-Assisted Extraction
3.5. Ultrasound-Assisted Extraction
3.6. Comparison of Extraction Systems
4. Technological Applications
4.1. Coagulants: Environmental Application
4.2. Adhesives: Wood, Tires, Concrete
4.3. Ore Flotation Agents
4.4. Fabric Manufacture
4.4.1. Leather Industry
4.4.2. Dyeing Industry of Natural Fibres: Cotton, Wool, Silk
4.5. Food Additives
4.6. Medical, Pharmaceutical, and Veterinary Applications
4.6.1. Antioxidant
4.6.2. Antimicrobial
4.6.3. Anthelmintic
4.6.4. Antiviral
4.6.5. Anti-Inflammatory
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Haslam, E. Vegetable Tannins. In Biochemistry of Plant Phenolics; Swain, T., Harbone, J.B., Van Sumere, C.F., Eds.; Springer US: Boston, MA, USA, 1979; pp. 475–523. [Google Scholar]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzella, L.; Napolitano, A. Natural phenol polymers: Recent advances in food and health applications. Antioxidants 2017, 6, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano, J.; Puupponen-Pimiä, R.; Dauer, A.; Aura, A.-M.; Saura-Calixto, F. Tannins: Current knowledge of food sources, intake, bioavailability and biological effects. Mol. Nutr. Food Res. 2009, 53, 310–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Sieniawska, E.; Baj, T. Tannins. In Pharmacognosy; Elsevier: Amsterdam, The Netherlands, 2017; pp. 199–232. [Google Scholar]
- Hagerman, A.E. Hydrolyzable tannin structural chemistry. Tann. Handb. 2010, 1–8. [Google Scholar]
- Mueller-Harvey, I. Analysis of hydrolysable tannins. Anim. Feed Sci. Technol. 2001, 91, 3–20. [Google Scholar] [CrossRef]
- König, M.; Scholz, E.; Hartmann, R.; Lehmann, W.; Rimpler, H. Ellagitannins and complex tannins from Quercus petraea bark. J. Nat. Prod. 1994, 57, 1411–1415. [Google Scholar] [CrossRef]
- García, D.E.; Glasser, W.G.; Pizzi, A.; Paczkowski, S.P.; Laborie, M.-P. Modification of condensed tannins: From polyphenol chemistry to materials engineering. New J. Chem. 2016, 40, 36–49. [Google Scholar] [CrossRef]
- Pizzi, A. Tannins: Major sources, properties and applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 179–199. ISBN 978-0-08-045316-3. [Google Scholar]
- Venkatesan, J.; Keekan, K.K.; Anil, S.; Bhatnagar, I.; Kim, S.-K. Phlorotannins. Encycl. Food Chem. 2019, 515–527. [Google Scholar]
- Kim, S.-K.; Wijesekara, I. Role of marine nutraceuticals in cardiovascular health. Sustain. Energy Enhanc. Hum. Funct. Act. 2017, 273–279. [Google Scholar]
- Suvanto, J.; Nohynek, L.; Seppänen-Laakso, T.; Rischer, H.; Salminen, J.-P.; Puupponen-Pimiä, R. Variability in the production of tannins and other polyphenols in cell cultures of 12 Nordic plant species. Planta 2017, 246, 227–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kraus, T.E.C.; Dahlgren, R.A.; Zasoski, R.J. Tannins in nutrient dynamics of forest ecosystems–a review. Plant. Soil 2003, 256, 41–66. [Google Scholar] [CrossRef]
- Daniel, E.M.; Krupnick, A.S.; Heur, Y.-H.; Blinzler, J.A.; Nims, R.W.; Stoner, G.D. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J. Food Compos. Anal. 1989, 2, 338–349. [Google Scholar] [CrossRef]
- Alvarez-Parrilla, E.; Urrea-López, R.; Rosa, L.A. Bioactive components and health effects of pecan nuts and their byproducts: A review. J. Food Bioact. 2018. [Google Scholar] [CrossRef] [Green Version]
- Landete, J.M. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Giner-Chavez, B.I.; Van Soest, P.J.; Robertson, J.B.; Lascano, C.; Pell, A.N. Comparison of the precipitation of Alfalfa Leaf Protein and bovine serum albumin by tannins in the radial diffusion method. J. Sci. Food Agric. 1997, 74, 513–523. [Google Scholar] [CrossRef]
- Schofield, P.; Mbugua, D.; Pell, A. Analysis of condensed tannins: A review. Anim. Feed Sci. Technol. 2001, 91, 21–40. [Google Scholar] [CrossRef]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD–MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef]
- De Vasconcelos, M.C.B.M.; Bennett, R.N.; Rosa, E.A.S.; Ferreira-Cardoso, J. V Composition of European chestnut (Castanea sativa Mill.) and association with health effects: Fresh and processed products. J. Sci. Food Agric. 2010, 90, 1578–1589. [Google Scholar] [CrossRef]
- Pfundstein, B.; El Desouky, S.K.; Hull, W.E.; Haubner, R.; Erben, G.; Owen, R.W. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): Characterization, quantitation and determination of antioxidant capacities. Phytochemistry 2010, 71, 1132–1148. [Google Scholar] [CrossRef]
- Prida, A.; Puech, J.-L. Influence of geographical origin and botanical species on the content of extractives in American, French, and East European oak woods. J. Agric. Food Chem. 2006, 54, 8115–8126. [Google Scholar] [CrossRef] [PubMed]
- Cadahía, E.; Varea, S.; Muñoz, L.; Fernández de Simón, B.; García-Vallejo, M.C. Evolution of Ellagitannins in Spanish, French, and American oak woods during natural seasoning and toasting. J. Agric. Food Chem. 2001, 49, 3677–3684. [Google Scholar] [CrossRef] [PubMed]
- Miladinović, B.; Kostić, M.; Šavikin, K.; Đorđević, B.; Mihajilov-Krstev, T.; Živanović, S.; Kitić, D. Chemical profile and antioxidative and antimicrobial activity of juices and extracts of 4 Black Currants varieties (Ribes nigrum L.). J. Food Sci. 2014, 79, 301–309. [Google Scholar]
- Bar-Ya’akov, I.; Tian, L.; Amir, R.; Holland, D. Primary metabolites, anthocyanins, and hydrolyzable tannins in the Pomegranate fruit. Front. Plant. Sci. 2019, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Mailoa, M.N.; Mahendradatta, M.; Laga, A.; Djide, N. Antimicrobial activities of tannins extract from guava leaves (Psidium guajava L) on pathogens microbial. Int. J. Sci. Technol. Res. 2014, 3, 236–241. [Google Scholar]
- Sriwilaijaroen, N.; Fukumoto, S.; Kumagai, K.; Hiramatsu, H.; Odagiri, T.; Tashiro, M.; Suzuki, Y. Antiviral effects of Psidium guajava Linn. (guava) tea on the growth of clinical isolated H1N1 viruses: Its role in viral hemagglutination and neuraminidase inhibition. Antivir. Res. 2012, 94, 139–146. [Google Scholar] [CrossRef]
- Marcela, M.; Santiago, Z.; Tania, R.J.; Stephania, R.; Andrés, F.A.; Maria, E.M.; Pedro, Z.; Benjamín, A.R. Mangiferin content, carotenoids, tannins and oxygen radical absorbance capacity (ORAC) values of six mango (Mangifera indica) cultivars from the Colombian Caribbean. J. Med. Plants Res. 2017, 11, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.; Roto, A.V.; Bolling, B.W. Characterization of Ellagitannins, Gallotannins, and bound Proanthocyanidins from California Almond (Prunus dulcis) Varieties. J. Agric. Food Chem. 2012, 60, 12151–12156. [Google Scholar] [CrossRef]
- Salminen, J.-P. Effects of sample drying and storage, and choice of extraction solvent and analysis method on the yield of Birch leaf hydrolyzable tannins. J. Chem. Ecol. 2003, 29, 1289–1305. [Google Scholar] [CrossRef]
- Ossipova, S.; Ossipov, V.; Haukioja, E.; Loponen, J.; Pihlaja, K. Proanthocyanidins of mountain birch leaves: Quantification and properties. Phytochem. Anal. 2001, 12, 128–133. [Google Scholar] [CrossRef]
- Roitto, M.; Rautio, P.; Markkola, A.; Julkunen-Tiitto, R.; Varama, M.; Saravesi, K.; Tuomi, J. Induced accumulation of phenolics and sawfly performance in Scots pine in response to previous defoliation. Tree Physiol. 2009, 29, 207–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulekbache-Makhlouf, L.; Meudec, E.; Mazauric, J.-P.; Madani, K.; Cheynier, V. Qualitative and semi-quantitative analysis of phenolics in Eucalyptus globulus leaves by high-performance liquid chromatography coupled with diode array detection and electrospray ionisation mass spectrometry. Phytochem. Anal. 2013, 24, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Rubanza, C.D.K.; Shem, M.N.; Otsyina, R.; Bakengesa, S.S.; Ichinohe, T.; Fujihara, T. Polyphenolics and tannins effect on in vitro digestibility of selected Acacia species leaves. Anim. Feed Sci. Technol. 2005, 119, 129–142. [Google Scholar] [CrossRef]
- Kardel, M.; Taube, F.; Schulz, H.; Schütze, W.; Gierus, M. Different approaches to evaluate tannin content and structure of selected plant extracts–review and new aspects. J. Appl. Bot. Food Qual. 2013, 86, 154–166. [Google Scholar]
- De Hoyos-Martínez, P.L.; Merle, J.; Labidi, J.; Charrier-El Bouhtoury, F. Tannins extraction: A key point for their valorization and cleaner production. J. Clean. Prod. 2019, 206, 1138–1155. [Google Scholar] [CrossRef] [Green Version]
- Cuong, D.X.; Hoan, N.X.; Dong, D.H.; Thuy, L.T.M.; Van Thanh, N.; Ha, H.T.; Tuyen, D.T.T.; Chinh, D.X. Tannins: Extraction from Plants. In Tannins- Structural properties, Biological Properties and Current Knowledge; IntechOpen: London, UK, 2019. [Google Scholar]
- Aires, A.; Carvalho, R.; Saavedra, M.J. Valorization of solid wastes from chestnut industry processing: Extraction and optimization of polyphenols, tannins and ellagitannins and its potential for adhesives, cosmetic and pharmaceutical industry. Waste Manag. 2016, 48, 457–464. [Google Scholar] [CrossRef]
- Seabra, I.J.; Chim, R.B.; Salgueiro, P.; Braga, M.E.M.; de Sousa, H.C. Influence of solvent additives on the aqueous extraction of tannins from pine bark: Potential extracts for leather tanning. J. Chem. Technol. Biotechnol. 2018, 93, 1169–1182. [Google Scholar] [CrossRef]
- Rajha, H.N.; Darra, N.; Hobaika, Z.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Extraction of total phenolic compounds, flavonoids, anthocyanins and tannins from Grape byproducts by response surface methodology. Influence of solid-liquid ratio, particle size, time, temperature and solvent mixtures on the optimization process. Food Nutr. Sci. 2014, 5, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, H.; Lv, W.; Ma, C.; Lou, Z.; Xie, J.; Liu, B. Ionic liquid-based ultrasonic/microwave-assisted extraction combined with UPLC-MS-MS for the determination of tannins in Galla chinensis. Nat. Prod. Res. 2012, 26, 1842–1847. [Google Scholar] [CrossRef]
- Chowdhury, S.A.; Vijayaraghavan, R.; MacFarlane, D.R. Distillable ionic liquid extraction of tannins from plant materials. Green Chem. 2010, 12, 1023–1028. [Google Scholar] [CrossRef]
- Talmaciu, A.I.; Ravber, M.; Volf, I.; Knez, Ž.; Popa, V.I. Isolation of bioactive compounds from spruce bark waste using sub- and supercritical fluids. J. Supercrit. Fluids 2016, 117, 243–251. [Google Scholar] [CrossRef]
- Ashraf-Khorassani, M.; Taylor, L.T. Sequential fractionation of grape seeds into oils, polyphenols, and procyanidins via a single system employing CO2-based fluids. J. Agric. Food Chem. 2004, 52, 2440–2444. [Google Scholar] [CrossRef] [PubMed]
- Sapkale, G.N.; Patil, S.M.; Surwase, U.; Bhatbhage, P.K. Supercritical Fluid Extraction. Int. J. Chem. Sci. 2010, 8, 729–743. [Google Scholar]
- Prado, J.M.; Vardanega, R.; Debien, I.C.N.; de Meireles, M.A.A.; Gerschenson, L.N.; Sowbhagya, H.B.; Chemat, S. Conventional extraction. In Food Waste Recovery; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 127–148. ISBN 9780128003510. [Google Scholar]
- Erşan, S.; Güçlü, Ü.Ö.; Carle, R.; Schweiggert, R.M. Subcritical water extraction of phenolic and antioxidant constituents from pistachio (Pistacia vera L.) hulls. Food Chem. 2018, 253, 46–54. [Google Scholar]
- Vergara-Salinas, J.R.; Bulnes, P.; Zúñiga, M.C.; Pérez-Jiménez, J.; Torres, J.L.; Mateos-Martín, M.L.; Agosin, E.; Pérez-Correa, J.R. Effect of pressurized hot water extraction on antioxidants from grape pomace before and after enological fermentation. J. Agric. Food Chem. 2013, 61, 6929–6936. [Google Scholar] [CrossRef]
- Ravber, M.; Knez, Ž.; Škerget, M. Isolation of phenolic compounds from larch wood waste using pressurized hot water: Extraction, analysis and economic evaluation. Cellulose 2015, 22, 3359–3375. [Google Scholar] [CrossRef]
- Chao, B.; Liu, R.; Zhang, X.; Zhang, X.; Tan, T. Tannin extraction pretreatment and very high gravity fermentation of acorn starch for bioethanol production. Bioresour. Technol. 2017, 241, 900–907. [Google Scholar] [CrossRef]
- Ćurko, N.; Tomašević, M.; Bubalo, M.C.; Gracin, L.; Redovniković, I.R.; Ganić, K.K. Extraction of proanthocyanidins and anthocyanins from grape skin by using ionic liquids. Food Technol. Biotechnol. 2017, 55, 429–437. [Google Scholar] [CrossRef]
- Maran, J.P.; Manikandan, S.; Priya, B.; Gurumoorthi, P. Box-Behnken design based multi-response analysis and optimization of supercritical carbon dioxide extraction of bioactive flavonoid compounds from tea (Camellia sinensis L.) leaves. J. Food Sci. Technol. 2015, 52, 92–104. [Google Scholar] [CrossRef]
- Conde, E.; Hemming, J.; Smeds, A.; Reinoso, B.D.; Moure, A.; Willför, S.; Domínguez, H.; Parajó, J.C. Extraction of low-molar-mass phenolics and lipophilic compounds from Pinus pinaster wood with compressed CO2. J. Supercrit. Fluids 2013, 81, 193–199. [Google Scholar] [CrossRef]
- Cavalcanti, R.N.; Navarro-Díaz, H.J.; Santos, D.T.; Rostagno, M.A.; Meireles, M.A.A. Supercritical carbon dioxide extraction of polyphenols from pomegranate (Punica granatum L.) leaves: Chemical composition, economic evaluation and chemometric approach. J. Food Res. 2012, 1, 282. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.; Casal, S.I.P.; Pereira, J.A.; Ramalhosa, E.; Saraiva, J.A. Optimization of high pressure bioactive compounds extraction from pansies (Viola × wittrockiana) by response surface methodology. High. Press. Res. 2017, 37, 415–429. [Google Scholar] [CrossRef]
- Mašković, P.Z.; Veličković, V.; Đurović, S.; Zeković, Z.; Radojković, M.; Cvetanović, A.; Švarc-Gajić, J.; Mitić, M.; Vujić, J. Biological activity and chemical profile of Lavatera thuringiaca L. extracts obtained by different extraction approaches. Phytomedicine 2018, 38, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Skouroumounis, G.K.; Elsey, G.M.; Taylor, D.K. Microwave-assistance provides very rapid and efficient extraction of grape seed polyphenols. Food Chem. 2011, 129, 570–576. [Google Scholar] [CrossRef]
- Ćurko, N.; Kelšin, K.; Dragović-Uzelac, V.; Tomašević, M.; Ganić, K.K. Microwave-assisted extraction of tannins from grape skin pomaces. In Proceedings of the 9th International CONGRESS of Food Technologists, Biotechnologists and Nutritionists, Zagreb, Croatia, 3–6 October 2018. [Google Scholar]
- Huma, Z.E.; Jayasena, V.; Nasar-Abbas, S.M.; Imran, M.; Khan, M.K. Process optimization of polyphenol extraction from carob (Ceratonia siliqua) kibbles using microwave-assisted technique. J. Food Process. Preserv. 2018, 42, 1–10. [Google Scholar] [CrossRef]
- Naima, R.; Oumam, M.; Hannache, H.; Sesbou, A.; Charrier, B.; Pizzi, A.; Charrier–El Bouhtoury, F. Comparison of the impact of different extraction methods on polyphenols yields and tannins extracted from Moroccan Acacia mollissima barks. Ind. Crop. Prod. 2015, 70, 245–252. [Google Scholar] [CrossRef]
- Tabaraki, R.; Safari, A.; Yeganeh, A.F. Ultrasonic-assisted extraction of condensed tannin from acron, gland, leaf and gall of oak using response surface methodology. J. Appl. Chem. Res. 2013, 77, 67–77. [Google Scholar]
- Poveda, J.M.; Loarce, L.; Alarcón, M.; Díaz-Maroto, M.C.; Alañón, M.E. Revalorization of winery by-products as source of natural preservatives obtained by means of green extraction techniques. Ind. Crop. Prod. 2018, 112, 617–625. [Google Scholar] [CrossRef]
- Sousa, A.D.; Maia, A.I.V.; Rodrigues, T.H.S.; Canuto, K.M.; Ribeiro, P.R.V.; de Cassia, A.P.R.; Vieira, R.F.; de Brito, E.S. Ultrasound-assisted and pressurized liquid extraction of phenolic compounds from Phyllanthus amarus and its composition evaluation by UPLC-QTOF. Ind. Crop. Prod. 2016, 79, 91–103. [Google Scholar] [CrossRef]
- Yin, C.Y. Emerging usage of plant-based coagulants for water and wastewater treatment. Process. Biochem. 2010, 45, 1437–1444. [Google Scholar] [CrossRef] [Green Version]
- Graham, N.; Gang, F.; Fowler, G.; Watts, M. Characterisation and coagulation performance of a tannin-based cationic polymer: A preliminary assessment. Colloids Surf. A Physicochem. Eng. Asp. 2008, 327, 9–16. [Google Scholar] [CrossRef]
- Grehs, B.W.N.; Lopes, A.R.; Moreira, N.F.F.; Fernandes, T.; Linton, M.A.O.; Silva, A.M.T.; Manaia, C.M.; Carissimi, E.; Nunes, O.C. Removal of microorganisms and antibiotic resistance genes from treated urban wastewater: A comparison between aluminium sulphate and tannin coagulants. Water Res. 2019, 166, 115056. [Google Scholar] [CrossRef] [PubMed]
- Dela Justina, M.; Rodrigues Bagnolin Muniz, B.; Mattge Bröring, M.; Costa, V.J.; Skoronski, E. Using vegetable tannin and polyaluminium chloride as coagulants for dairy wastewater treatment: A comparative study. J. Water Process. Eng. 2018, 25, 173–181. [Google Scholar] [CrossRef]
- Lopes, E.C.; Santos, S.C.R.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Evaluation of a tannin-based coagulant on the decolorization of synthetic effluents. J. Environ. Chem. Eng. 2019, 7, 103125. [Google Scholar] [CrossRef]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Dávila-Acedo, M.A. Optimization of the synthesis of a new coagulant from a tannin extract. J. Hazard. Mater. 2011, 186, 1704–1712. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; Yaser, A.Z. Colour removal from biologically treated landfill leachate with tannin-based coagulant. J. Environ. Chem. Eng. 2019, 7, 103483. [Google Scholar] [CrossRef]
- Pizzi, A.; Horak, R.M.; Ferreira, D.; Roux, D.G. Condensates of phenol, resorcinol, phloroglucinol, and pyrogallol as model compounds of flavonoid A- and B-rings with formaldehyde. J. Appl. Polym. Sci. 1979, 24, 1571–1578. [Google Scholar] [CrossRef]
- Pichelin, F.; Nakatani, M.; Pizzi, A.; Wieland, S.; Despres, A.; Rigolet, S. Structural beams from thick wood panels bonded industrially with formaldehyde-free tannin adhesives. For. Prod. J. 2006, 56, 31. [Google Scholar]
- Faris, A.H.; Ibrahim, M.N.M.; Rahim, A.A. Preparation and characterization of green adhesives using modified tannin and hyperbranched poly (amine-ester). Int. J. Adhes. Adhes. 2016, 71, 39–47. [Google Scholar] [CrossRef]
- Zhang, J.; Xi, X.; Liang, J.; Pizzi, A.; Du, G.; Deng, S. Tannin-based adhesive cross-linked by furfuryl alcohol-glyoxal and epoxy resins. Int. J. Adhes. Adhes. 2019, 94, 47–52. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Hamed, G.R.; Chung, K.H.; Hemingway, R.W. Condensed tannins as substitutes for resorcinol in bonding polyester and nylon cord to rubber. Acs Symp. Ser. 1989, 18, 242–253. [Google Scholar]
- Chung, K.H.; Hamed, G.R. Adhesives containing pine bark tannin for bonding nylon cord to rubber. In Chemistry and Significance of Condensed Tannins; Hemingway, R.W., Karchesy, J.J., Branham, S.J., Eds.; Springer: Boston, MA, USA, 1989; pp. 479–492. [Google Scholar]
- Kaspar, H.R.E.; Pizzi, A. Industrial plasticizing/dispersion aids for cement based on polyflavonoid tannins. J. Appl. Polym. Sci. 1996, 59, 1181–1190. [Google Scholar] [CrossRef]
- Kreibich, R.E. Tannin-based wood adhesives. In Chemistry and Significance of Condensed Tannins; Springer: Boston, MA, USA, 1989; pp. 457–478. [Google Scholar]
- Rutledge, J. Fundamental surface chemistry and froth flotation behavior using quebracho tannins. Master’s Thesis, Colorado School of Mines, Golden, CO, USA, 2016. [Google Scholar]
- Matveeva, T.N.; Gromova, N.K.; Lantsova, L.B. Effect of Tannin on compound collector adsorption and stibnite and arsenopyrite flotation from complex ore. J. Min. Sci. 2017, 53, 1108–1115. [Google Scholar] [CrossRef]
- Matveeva, T.N.; Gromova, N.K.; Lantsova, L.B. Adsorption of tannin-bearing organic reagents on stibnite, arsenopyrite and chalcopyrite in complex gold ore flotation. J. Min. Sci. 2016, 52, 551–558. [Google Scholar] [CrossRef]
- Bacelo, H.A.M.; Santos, S.C.R.; Botelho, C.M.S. Tannin-based biosorbents for environmental applications–A review. Chem. Eng. J. 2016, 303, 575–587. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Long, Q.; Wei, Z.; Chen, Y. Improving the selective flotation of jamesonite using tannin extract. Int. J. Miner. Process. 2011, 100, 54–56. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Rutledge, J.; Anderson, C.G. Tannins in mineral processing and extractive metallurgy. Metals 2015, 5, 1520–1542. [Google Scholar] [CrossRef] [Green Version]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Matveyeva, T.; Gromova, N.; Ivanova, E. Research on adsorption and flotation properties of herbal reagents in processing of refractory ores. Inz. Miner. 2015, 2015, 57–62. [Google Scholar]
- Falcão, L.; Araújo, M.E.M. Vegetable tannins used in the manufacture of historic leathers. Molecules 2018, 23, 1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CAPPEQ. Tannin in the Leather Tanning Industry. Available online: https://www.tannins.org/leather-tanning/ (accessed on 19 December 2019).
- Falcão, L.; Araújo, M.E.M. Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests. J. Cult. Herit. 2011, 12, 149–156. [Google Scholar] [CrossRef]
- Pizzi, A.; Simon, C.; George, B.; Perrin, D.; Triboulot, M.C. Tannin antioxidant characteristics in leather versus leather light stability: Models. J. Appl. Polym. Sci. 2004, 91, 1030–1040. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Sousa, G.; Crispim, F.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus bark as source of tannin extracts for application in leather industry. Acs Sustain. Chem. Eng. 2013, 1, 950–955. [Google Scholar] [CrossRef]
- Mongkholrattanasit, R.; Kryštůfek, J.; Wiener, J.; Studničková, J. Properties of wool and cotton fabrics dyed with eucalyptus, tannin and flavonoids. Fibres Text. East. Eur. 2011, 85, 90–95. [Google Scholar]
- Pisitsak, P.; Hutakamol, J.; Thongcharoen, R.; Phokaew, P.; Kanjanawan, K.; Saksaeng, N. Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind. Crop. Prod. 2016, 79, 47–56. [Google Scholar] [CrossRef]
- Ghaheh, F.S.; Nateri, A.S.; Mortazavi, S.M.; Abedi, D.; Mokhtari, J. The effect of mordant salts on antibacterial activity of wool fabric dyed with pomegranate and walnut shell extracts. Color. Technol. 2012, 128, 473–478. [Google Scholar] [CrossRef]
- Prabhu, K.H.; Teli, M.D.; Waghmare, N.G. Eco-friendly dyeing using natural mordant extracted from Emblica officinalis G. Fruit on cotton and silk fabrics with antibacterial activity. Fibers Polym. 2011, 12, 753–759. [Google Scholar] [CrossRef]
- Rather, L.J.; Akhter, S.; Padder, R.A.; Hassan, Q.P.; Hussain, M.; Khan, M.A.; Mohammad, F. Colorful and semi durable antioxidant finish of woolen yarn with tannin rich extract of Acacia nilotica natural dye. Dye. Pigment. 2017, 139, 812–819. [Google Scholar] [CrossRef]
- Yang, T.T.; Guan, J.P.; Tang, R.C.; Chen, G. Condensed tannin from Dioscorea cirrhosa tuber as an eco-friendly and durable flame retardant for silk textile. Ind. Crop. Prod. 2018, 115, 16–25. [Google Scholar] [CrossRef]
- Hollman, P.C.H.; Katan, M.B. Dietary flavonoids: Intake, health effects and bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jiménez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [Google Scholar] [CrossRef] [PubMed]
- Luciano, G.; Vasta, V.; Monahan, F.J.; López-Andrés, P.; Biondi, L.; Lanza, M.; Priolo, A. Antioxidant status, colour stability and myoglobin resistance to oxidation of longissimus dorsi muscle from lambs fed a tannin-containing diet. Food Chem. 2011, 124, 1036–1042. [Google Scholar] [CrossRef]
- López-Andrés, P.; Luciano, G.; Vasta, V.; Gibson, T.M.; Biondi, L.; Priolo, A.; Mueller-Harvey, I. Dietary quebracho tannins are not absorbed, but increase the antioxidant capacity of liver and plasma in sheep. Br. J. Nutr. 2013, 110, 632–639. [Google Scholar] [CrossRef] [Green Version]
- Marshall, T.A.; Roberts, R.J. In vitro and in vivo assessment of lipid peroxidation of infant nutrient preparations: Effect of nutrition on oxygen toxicity. J. Am. Coll. Nutr. 1990, 9, 190–199. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Chowdhury, M.A.K.; Hou, Y.; Gong, J. Phytogenic compounds as alternatives to in-feed antibiotics: Potentials and challenges in application. Pathogens 2015, 4, 137–156. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Turchi, B.; Mancini, S.; Pastorelli, R.; Viti, C.; Tronconi, L.; Bertelloni, F.; Felicioli, A.; Cerri, D.; Fratini, F.; Paci, G. Dietary supplementation of chestnut and quebracho tannins mix: Effect on caecal microbial communities and live performance of growing rabbits. Res. Vet. Sci. 2019, 124, 129–136. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the safety and efficacy of tannic acid when used as feed flavouring for all animal species. Efsa J. 2014, 12, 3828. [CrossRef]
- EC Commission Implementing Regulation (EU) 2017/66 of 14 December 2016 concerning the authorisation of tannic acid as a feed additive for all animal species 2017. OJ L 2017, 13, 259–262.
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam.–Part. A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1761–1774. [Google Scholar] [CrossRef]
- EC Commission implementing Regulation (EU) No 872/2012 of 1 October 2012 adopting the list of flavouring substances provided for by Regulation (EC) No 2232/96 of the European Parliament and of the Council, introducing it in Annex I to Regulation (EC) No 1334. OJ L 2012, 267, 1–161.
- Ariga, T.; Hamano, M. Antioxidative properties of proanthocyanidins. Part II. Radical scavenging action and its mode in procyanidins B-1 and B-3 from azuki beans to peroxyl radicals. Agric. Biol. Chem. 1990, 54, 2499–2504. [Google Scholar] [CrossRef] [Green Version]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Wilms, L.C.; Beetstra, S.A.J.N.; Heijnen, C.G.M.; Voss, H.P.; Bast, A. Interactions between flavonoids and proteins: Effect on the total antioxidant capacity. J. Agric. Food Chem. 2002, 50, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Tam, N.F.Y.; Lin, Y.M.; Ding, Z.H.; Chai, W.M.; Wei, S.D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar] [CrossRef]
- Liu, X.L.; Hao, Y.Q.; Jin, L.; Xu, Z.J.; McAllister, T.A.; Wang, Y. Anti-Escherichia coli O157:H7 properties of purple prairie clover and sainfoin condensed tannins. Molecules 2013, 18, 2183–2199. [Google Scholar] [CrossRef]
- Doss, A.; Mohammed, M.H.; Dhanabalan, R. Antibacterial activity of tannins from the leaves of Solanum trilobatum Linn. Indian J. Sci. Technol. 2009, 2, 41–43. [Google Scholar]
- Banso, A.; Adeyemo, S.O. Evaluation of antibacterial properties of tannins isolated from Dichrostachys cinerea. Afr. J. Biotechnol. 2007, 6. [Google Scholar]
- Wang, Y.; Xu, Z.; Bach, S.J.; McAllister, T.A. Sensitivity of Escherichia coli to seaweed (Ascophyllum nodosum) phlorotannins and terrestrial tannins. Asian-Australas. J. Anim. Sci. 2009, 22, 238–245. [Google Scholar] [CrossRef]
- Sakanaka, S.; Kim, M.; Taniguchi, M.; Yamamoto, T. Antibacterial substances in japanese green tea extract against Streptococcus Mutans, a cariogenic bacterium. Agric. Biol. Chem. 1989, 53, 2307–2311. [Google Scholar] [CrossRef]
- Mueller-Harvey, I. Unravelling the conundrum of tannins in animal nutrition and health. J. Sci. Food Agric. 2006, 86, 2010–2037. [Google Scholar] [CrossRef]
- Hoste, H.; Jackson, F.; Athanasiadou, S.; Thamsborg, S.M.; Hoskin, S.O. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006, 186, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H.; Martinez-Ortiz-De-Montellano, C.; Manolaraki, F.; Brunet, S.; Ojeda-Robertos, N.; Fourquaux, I.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Vet. Parasitol. 2012, 22, 253–261. [Google Scholar] [CrossRef]
- Thi, M.N.; Van Binh, D.; Ørskov, E.R. Effect of foliages containing condensed tannins and on gastrointestinal parasites. Anim. Feed Sci. Technol. 2005, 121, 77–87. [Google Scholar]
- Brunet, S.; Montellano, C.M.O.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Aguilar-Caballero, A.J.; Capetillo-Leal, C.; Hoste, H. Effect of the consumption of Lysiloma latisiliquum on the larval establishment of gastrointestinal nematodes in goats. Vet. Parasitol. 2008, 157, 81–88. [Google Scholar] [CrossRef]
- Pathak, A.K.; Dutta, N.; Banerjee, P.S.; Goswami, T.K.; Sharma, K. Effect of condensed tannins supplementation through leaf meal mixture on voluntary feed intake, immune response and worm burden in Haemonchus contortus infected sheep. J. Parasit. Dis. 2016, 40, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Molan, A.L.; Waghorn, G.C.; McNabb, W.C. Effect of condensed tannins on egg hatching and larval development of Trichostrongylus colubriformis in vitro. Vet. Rec. 2002, 150, 65–69. [Google Scholar] [CrossRef]
- Molan, A.L.; Hoskin, S.O.; Barry, T.N.; McNabb, W.C. Effect of condensed tannins extracted front four forages on the viability of the larvae of deep lungworms and gastrointestinal nematodes. Vet. Rec. 2000, 147, 44–48. [Google Scholar] [CrossRef]
- Molan, A.L.; Sivakumaran, S.; Spencer, P.A.; Meagher, L.P. Green tea flavan-3-ols and oligomeric proanthocyanidins inhibit the motility of infective larvae of Teladorsagia circumcincta and Trichostrongylus colubriformis in vitro. Res. Vet. Sci. 2004, 77, 239–243. [Google Scholar] [CrossRef]
- Heckendorn, F.; Häring, D.A.; Maurer, V.; Senn, M.; Hertzberg, H. Individual administration of three tanniferous forage plants to lambs artificially infected with Haemonchus contortus and Cooperia curticei. Vet. Parasitol. 2007, 146, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Athanasiadou, S.; Kyriazakis, I.; Jackson, F.; Coop, R.L. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep: In vitro and in vivo studies. Vet. Parasitol. 2001, 99, 205–219. [Google Scholar] [CrossRef]
- Paolini, V.; Frayssines, A.; De La Farge, F.; Dorchies, P.; Hoste, H. Effects of condensed tannins on established populations and on incoming larvae of Trichostrongylus colubriformis and Teladorsagia circumcincta in goats. Vet. Res. 2003, 34, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Xiu, J.; Liu, J.; Zhang, L.; Li, X.; Xu, Y.; Qin, C.; Zhang, L. Chebulagic acid, a hydrolyzable tannin, exhibited antiviral activity in vitro and in vivo against human enterovirus 71. Int. J. Mol. Sci. 2013, 14, 9618–9627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, T.K.; Lam, T.L.; Ng, T.B.; Fong, W.P.; Wan, D.C.C. A comparison of HIV-1 integrase inhibition by aqueous and methanol extracts of Chinese medicinal herbs. Life Sci. 2001, 68, 1687–1694. [Google Scholar] [CrossRef]
- Calland, N.; Albecka, A.; Belouzard, S.; Wychowski, C.; Duverlie, G.; Descamps, V.; Hober, D.; Dubuisson, J.; Rouillé, Y.; Séron, K. (-)-Epigallocatechin-3-gallate is a new inhibitor of hepatitis C virus entry. Hepatology 2012, 55, 720–729. [Google Scholar] [CrossRef]
- Di Pasquale, G.; Ostedgaard, L.; Vermeer, D.; Swaim, W.D.; Karp, P.; Chiorini, J.A. Bovine AAV transcytosis inhibition by tannic acid results in functional expression of CFTR in vitro and altered biodistribution in vivo. Gene Ther. 2012, 19, 576. [Google Scholar] [CrossRef]
- Zhang, X.F.; Dai, Y.C.; Zhong, W.; Tan, M.; Lv, Z.P.; Zhou, Y.C.; Jiang, X. Tannic acid inhibited norovirus binding to HBGA receptors, a study of 50 Chinese medicinal herbs. Bioorganic Med. Chem. 2012, 20, 1616–1623. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef]
- Park, M.; Cho, H.; Jung, H.; Lee, H.; Hwang, K.T. Antioxidant and anti-inflammatory activities of tannin fraction of the extract from black raspberry seeds compared to grape seeds. J. Food Biochem. 2014, 38, 259–270. [Google Scholar] [CrossRef]
- Dutot, M.; Fagon, R.; Hemon, M.; Rat, P. Antioxidant, anti-inflammatory, and anti-senescence activities of a phlorotannin-rich natural extract from Brown Seaweed Ascophyllum nodosum. Appl. Biochem. Biotechnol. 2012, 167, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.W.; Kang, M.C.; Kim, E.A.; Jeon, Y.J. Anti-inflammatory activity of phlorotannin-rich fermented Ecklonia cava processing by-product extract in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Liu, J.B.; Ding, Y.S.; Zhang, Y.; Chen, J.B.; Cui, B.S.; Bai, J.Y.; Lin, M.B.; Hou, Q.; Zhang, P.C.; Li, S. Anti-inflammatory hydrolyzable tannins from Myricaria bracteata. J. Nat. Prod. 2015, 78, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Nahler, G.; Nahler, G. Committee for Veterinary Medicinal Products (CVMP). Dict. Pharm. Med. 2009, 32. [Google Scholar]
- European Union. Reglamento (CE) No 451/2008 del Parlamento Europeo y del Consejo. 2008. Available online: https://op.europa.eu/en/publication-detail/-/publication/1ce90714-803a-4aba-b9a1-bd74b8f9b30e/language-es (accessed on 19 December 2019).
Sample Availability: No samples have been used for the performance of this review. |
| Species | Tissue | Type | Method | Concentration (mg/g dw) | Reference |
|---|---|---|---|---|---|
| Carya illinoinensis | Nut | H and C | HPLC | 0.22 ± 0.11 H; 4.66 ± 2.28 C | [2,16] |
| Kernel (K) and nutshell (N) | C | Vanillin-HCl | 0.4–5.3 C (K); 0.5–876 C (N) | [17] | |
| Juglans regia | Nut | H and C | HPLC | 0.05 ± 0.01 H; 0.06 ± 0.03 C | [2,16] |
| Ribes nigrum | Juice and extracts | H and C | ST | 0.04 ± 0.01 H; 1.14 ± 0.50 C | [2,26] |
| Fragaria sp. | Fruit | H and C | HPLC | 0.77 ± 0.006 H; 0.99 ± 0.08 C | [2,16] |
| Rubus fruticosus | Fruit | H and C | HPLC | 2.10 ± 0.60 H; 0.25 ± 0.20 C | [2,16] |
| Rubus occidentalis | Fruit | H and C | HPLC | 2.43 ± 0.83 H; 0.38 ± 0.35 C | [2,16] |
| Punica granatum | Fruit, seeds, juice (J) | H | HPLC | 0.12 ± 0.06 H; 7–1169 mg/L (J) | [2,27] |
| Psidium spp. | Fruit | H | HPLC, ST | 2.35–55.5 H | [2,28,29] |
| Mangifera indica | Fruit | H and C | ST | 0.55–0.95 H | [2,30] |
| Prunus dulcis | Fruit | H | HPLC | 0.27 ± 0.07 H; 1.62 ± 0.95 C | [2,31] |
| Desmodium ovalifolium | Leaves and twigs | C | RDABA | 57–273 C | [19,20] |
| Gliricidia sepium | Leaves | C | RDABA | 25–186 C | [19,20] |
| Manihot esculenta | Leaves and stems | C | RDABA | 26–169 C | [19,20] |
| Arachis pintoi | Leaves and twigs | C | RDABA | 40–186 C | [19,20] |
| Castanea sativa | Fruits and bark | H and C | HPLC | 0.7–89 H; 0.0001–167 H and C | [2,21,22] |
| Terminalia sp. | Fruits | H and C | HPLC | 126–822 H and C | [23] |
| Quercus | Wood | H | HPLC, GC | 19.26–47.26 H Q. robur; 11.55–30.88 H Q. petraea; 8.18 ± 0.18 H Q. alba. | [24,25] |
| Betula spp. | Leaves | H and C | HPLC | 0.72–60.67 HT; 47–103C | [32,33] |
| Pinus sylvestris | Needles | C | HPLC | 70–80 C | [34] |
| Eucalyptus globulus | Leaves | H and C | HPLC | 93.57 H + Phloroglucinol | [35] |
| Acacia sp. | Leaves | H and C | ST | 185 H and C A. angustissima; 84 H and C A. drepanolobium, 256 H and C A. nilotica; 98 H and C A. polyacantha; 217 H and C A. tortilisand; 226 H and C A. Senegal | [36] |
| A. mearnsii | Bark | C | Butanol-HCl | 235 C | [37] |
| HPLC | 108 C | ||||
| Caesalpinia spinosa | Pod | H and C | Butanol-HCl | 4.6 C | [37] |
| HPLC | 647 H | ||||
| Schinopsis lorentzii | Heartwood | C | Butanol-HCl | 123 C | [37] |
| HPLC | 164 C |
| Method | Conditions | Source | Recovery (mg/g) | Relative Cost (L, M, H) | Reference |
|---|---|---|---|---|---|
| SLE | 1% NaOH (aq) | Castanea dentata peels | 4.071 | L | [40] |
| 1% Na2SO3 (aq) | 0.609 | L | |||
| 1.5% EtOH (aq) | Pinus pinaster barks | 62.8 CME | L | [41] | |
| 66% EtOH (aq) | Grape by-products | 12.3 g/L | L | [42] | |
| 40% EtOH (aq) | Acorn | 80% | L | [52] | |
| Ionic liquid A | Acacia catechu | 85% | M/H | [44] | |
| Ionic liquid B 0.5 M | Grape skin | 60.1 | M/H | [53] | |
| SFE | CO2 + EtOH 70%; 40 °C; 10 MPa | Picea abies bark | 26.38 | H | [45] |
| CO2 + MeOH 40%; 80 °C; 65 MPa | Grape seeds | 770 | H | [46] | |
| CO2 + EtOH; 50 °C; 18.80 MPa | Camellia sinensis leaves | 499.90 | H | [54] | |
| CO2 + EtOH 10%; 50 °C; 10 MPa | Pinus pinaster wood | 75.61 | H | [55] | |
| CO2, 50 °C, 30 MPa | Punica granatum leaves | 340 | H | [56] | |
| PWE | H2O; 50 °C; 150 MPa | Pistacia vera by-products | 70.90 | H | [49] |
| H2O; RT; 250 MPa | Viola × wittrockiana | 93.86 | H | [57] | |
| H2O; 100 °C; 2 MPa | Larch wood | 381.90 | H | [51] | |
| H2O; 100 °C; 10,34 MPa | Grape pomace | 52.90 | H | [50] | |
| H2O; 140 °C; 4 MPa | Lavatera thuringiaca | 72.23 GAE | H | [58] | |
| ME | EtOH 70%; 125 W | Grape seeds | 528 | H | [59] |
| MeOH 60% | Grape by-products | 22.27 mg/L | H | [60] | |
| EtOH 45%; 340 W | Ceratonia siliqua kibbles | 4.11 | H | [61] | |
| H2O; 150 W | Acacia mollissima barks | 47.64 | H | [62] | |
| EtOH; 150 W | 30.29 | ||||
| EtOH (aq); 400 W | Galla chinensis | 528.5 | H | [43] | |
| EtOH 96%; | Lavatera thuringiaca | 71.15 GAE | H | [58] | |
| UE | MeOH 90%; 140 W | Quercus sp. | 127 | L | [63] |
| EtOH 44%; 500 W | Grape by-products | 86.67 | L | [64] | |
| dH2O; 301 W | Phyllanthus amarus | 27.23 | L | [65] | |
| EtOH (aq); 1200 W | Galla chinensis | 491.20 | L | [43] | |
| EtOH (aq) + ME; 1200 W | 543.50 | ||||
| Ionic liquid B 2.5 M + ME; 1200 W | 630.2 | ||||
| EtOH 96%; 216 W | Lavatera thuringiaca | 71.78 GAE | L | [58] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraga-Corral, M.; García-Oliveira, P.; Pereira, A.G.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Prieto, M.A.; Simal-Gandara, J. Technological Application of Tannin-Based Extracts. Molecules 2020, 25, 614. https://doi.org/10.3390/molecules25030614
Fraga-Corral M, García-Oliveira P, Pereira AG, Lourenço-Lopes C, Jimenez-Lopez C, Prieto MA, Simal-Gandara J. Technological Application of Tannin-Based Extracts. Molecules. 2020; 25(3):614. https://doi.org/10.3390/molecules25030614
Chicago/Turabian StyleFraga-Corral, Maria, Paula García-Oliveira, Antia G. Pereira, Catarina Lourenço-Lopes, Cecilia Jimenez-Lopez, Miguel Angel Prieto, and Jesus Simal-Gandara. 2020. "Technological Application of Tannin-Based Extracts" Molecules 25, no. 3: 614. https://doi.org/10.3390/molecules25030614
APA StyleFraga-Corral, M., García-Oliveira, P., Pereira, A. G., Lourenço-Lopes, C., Jimenez-Lopez, C., Prieto, M. A., & Simal-Gandara, J. (2020). Technological Application of Tannin-Based Extracts. Molecules, 25(3), 614. https://doi.org/10.3390/molecules25030614










