Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse
Abstract
1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Liquid Hot Water Pretreated Sugarcane Bagasse
2.2. Structural Changes in LHW Pretreated Solids
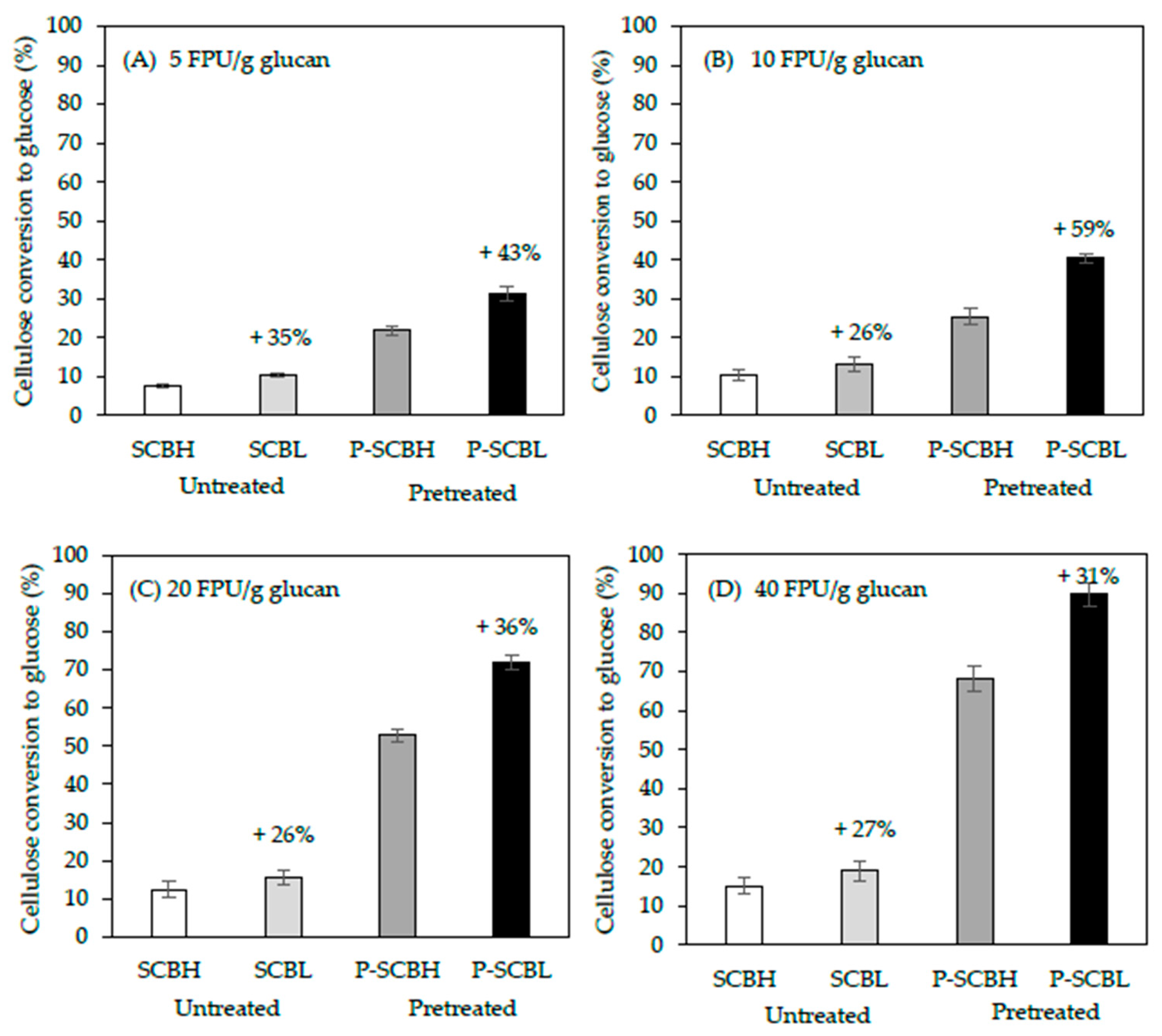
2.3. Cellulose Hydrolysis in LHW-Pretreated Sugarcane Bagasse
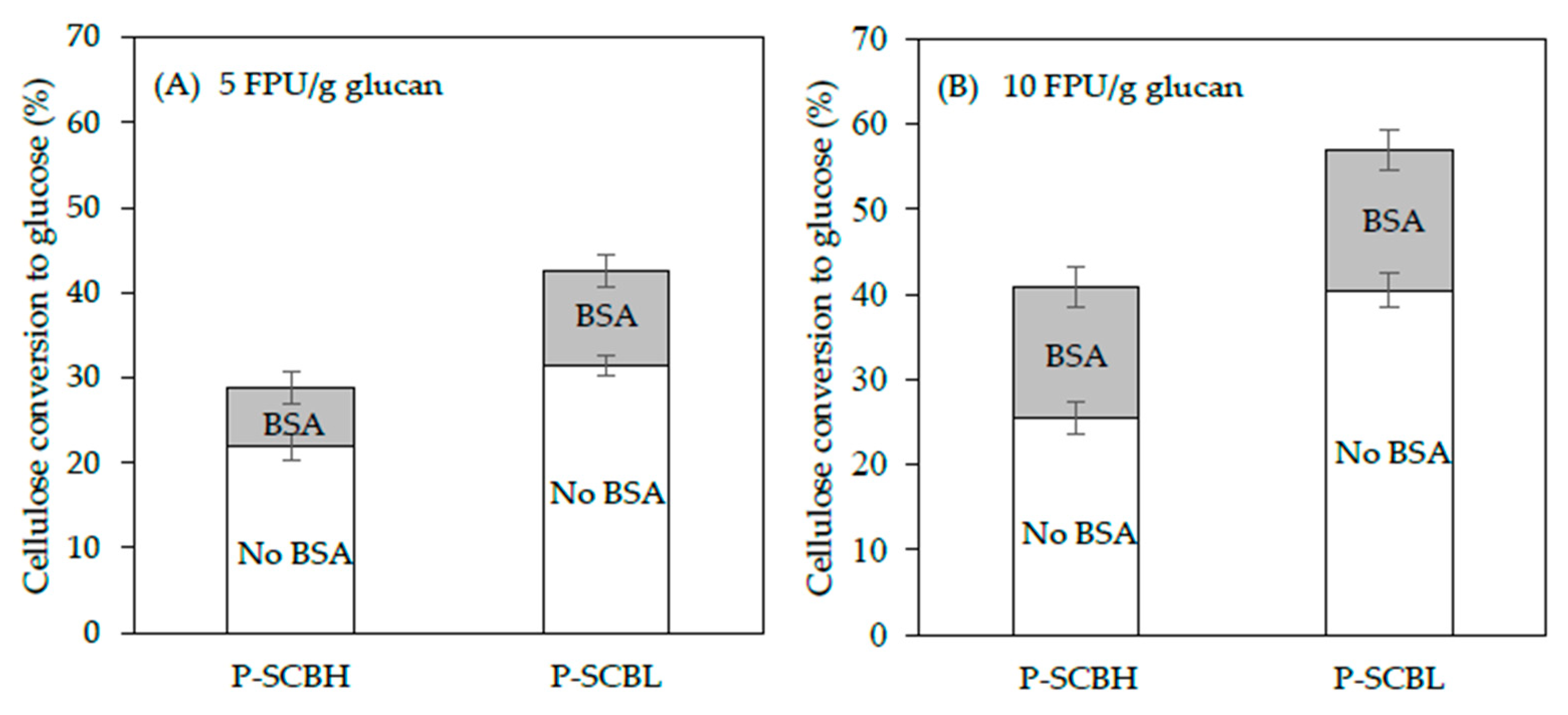
2.4. Effect of Bovine Serum Albumin (BSA) in Enzymatic Hydrolysis
3. Materials and Methods
3.1. Materials
3.2. Liquid Hot Water Pretreatment
3.3. Compositional Analysis
3.4. Enzymatic Hydrolysis of Sugarcane Bagasse
3.5. Scanning Electron Microscopy (SEM)
3.6. Analytical Assays
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ladeira Ázar, R.I.S.; Morgan, T.; dos Santos, A.C.F.; de Aquino Ximenes, E.; Ladisch, M.R.; Guimarães, V.M. Deactivation and activation of lignocellulose degrading enzymes in the presence of laccase. Enzym. Microb. Technol. 2018, 109, 25–30. [Google Scholar] [CrossRef]
- Ladeira-Ázar, R.I.S.; Morgan, T.; Maitan-Alfenas, G.P.; Guimarães, V.M. Inhibitors Compounds on Sugarcane Bagasse Saccharification: Effects of Pretreatment Methods and Alternatives to Decrease Inhibition. Appl. Biochem. Biotechnol. 2019, 188, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Orrego, D.; Zapata-Zapata, A.D.; Kim, D. Optimization and scale-up of coffee mucilage fermentation for ethanol production. Energies 2018, 11, 786. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Zanchetta, A.; dos Santos, A.C.F.; Ximenes, E.; da Costa Carreira Nunes, C.; Boscolo, M.; Gomes, E.; Ladisch, M.R. Temperature dependent cellulase adsorption on lignin from sugarcane bagasse. Bioresour. Technol. 2018, 252, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Ladisch, M.R. Biological Conversion of Plants to Fuels and Chemicals and the Effects of Inhibitors. In Aqueous Pretreatment of Plant Biomass for Biological and Chemical Conversion to Fuels and Chemicals; Wyman, C.E., Stevens, C.V., Eds.; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2013; pp. 39–60. [Google Scholar]
- dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef]
- Ázar, R.I.S.L.; Morgan, T.; Barbosa, M.H.P.; Guimarães, V.M.; Ximenes, E.; Ladisch, M. Impact of protein blocking on enzymatic saccharification of bagasse from sugarcane clones. Biotechnol. Bioeng. 2019, 116, 1584–1593. [Google Scholar] [CrossRef]
- Orrego, D.; Zapata-zapata, A.D.; Kim, D. Bioresource Technology Reports Ethanol production from co ff ee mucilage fermentation by S. cerevisiae immobilized in calcium-alginate beads. Bioresour. Technol. Rep. 2018, 3, 200–204. [Google Scholar] [CrossRef]
- Cárdenas, E.L.M.; Zapata-Zapata, A.D.; Kim, D. Hydrogen Production from Coffee Mucilage in Dark Fermentation with Organic Wastes. Energies 2019, 12, 71. [Google Scholar] [CrossRef]
- Florencio, C.; Cunha, F.M.; Badino, A.C.; Farinas, C.S.; Ximenes, E.; Ladisch, M.R. Secretome analysis of Trichoderma reesei and Aspergillus niger cultivated by submerged and sequential fermentation process: Enzyme production for sugarcane bagasse hydrolysis. Enzym. Microb. Technol. 2016, 90, 53–56. [Google Scholar] [CrossRef]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Addition of Soybean Protein Improves Saccharification and Ethanol Production from Hydrothermally Pretreated Sugarcane Bagasse. Bioenergy Res. 2019, 12, 81–93. [Google Scholar] [CrossRef]
- Cao, G.; Ximenes, E.; Nichols, N.N.; Frazer, S.E.; Kim, D.; Cotta, M.A.; Ladisch, M. Bioabatement with hemicellulase supplementation to reduce enzymatic hydrolysis inhibitors. Bioresour. Technol. 2015, 190, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Orrego, D.; Ximenes, E.A.; Ladisch, M.R. Cellulose conversion of corn pericarp without pretreatment. Bioresour. Technol. 2017, 245, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ximenes, E.A.; Nichols, N.N.; Cao, G.; Frazer, S.E.; Ladisch, M.R. Maleic acid treatment of biologically detoxified corn stover liquor. Bioresour. Technol. 2016, 216, 437–445. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Ko, J.K.; Ladisch, M.R. Hydrolysis-determining substrate characteristics in liquid hot water pretreated hardwood. Biotechnol. Bioeng. 2015, 112, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Mosier, N.; Hendrickson, R.; Ho, N.; Sedlak, M.; Ladisch, M.R. Optimization of pH controlled liquid hot water pretreatment of corn stover. Bioresour. Technol. 2005, 96, 1986–1993. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, B.S.; Vinzant, T.B.; Elander, R.T.; Pallapolu, V.R.; Lee, Y.Y.; Garlock, R.J.; Balan, V.; Dale, B.E.; Kim, Y.; Mosier, N.S.; et al. Surface and ultrastructural characterization of raw and pretreated switchgrass. Bioresour. Technol. 2011, 102, 11097–11104. [Google Scholar] [CrossRef]
- Falls, M.; Shi, J.; Ebrik, M.A.; Redmond, T.; Yang, B.; Wyman, C.E.; Garlock, R.; Balan, V.; Dale, B.E.; Pallapolu, V.R.; et al. Investigation of enzyme formulation on pretreated switchgrass. Bioresour. Technol. 2011, 102, 11072–11079. [Google Scholar] [CrossRef]
- Kim, D.; Ku, S. Bacillus cellulase molecular cloning, expression, and surface display on the outer membrane of Escherichia coli. Molecules 2018, 23, 503. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef]
- Larsson, S.; Cassland, P.; Jönsson, L.J. Development of a Saccharomyces cerevisiae Strain with Enhanced Resistance to Phenolic Fermentation Inhibitors in Lignocellulose Hydrolysates by Heterologous Expression of Laccase. Appl. Env. Microbiol. 2001, 67, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Palmqvist, E. Fermentation of lignocellulosic hydrolysates. I: Inhibition and detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Palmqvist, E. Fermentation of lignocellulosic hydrolysates. II: Inhibitors and mechanisms of inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzym. Microb. Technol. 2011, 48, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Technol. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Alriksson, B.; Cavka, A.; Jönsson, L.J. Bioresource Technology Improving the fermentability of enzymatic hydrolysates of lignocellulose through chemical in-situ detoxification with reducing agents. Bioresour. Technol. 2011, 102, 1254–1263. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ladisch, M.R.; Engelberth, A.S. Acetic acid removal from corn stover hydrolysate using ethyl acetate and the impact on Saccharomyces cerevisiae bioethanol fermentation. Biotechnol. Prog. 2016, 32, 929–937. [Google Scholar] [CrossRef]
- Ko, J.K.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Adsorption of enzyme onto lignins of liquid hot water pretreated hardwoods. Biotechnol. Bioeng. 2015, 112, 447–456. [Google Scholar] [CrossRef]
- Ko, J.K.; Kim, Y.; Ximenes, E.; Ladisch, M.R. Effect of liquid hot water pretreatment severity on properties of hardwood lignin and enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2015, 112, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Soybean protein as a cost-effective lignin-blocking additive for the saccharification of sugarcane bagasse. Bioresour. Technol. 2016, 221, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.; Ladisch, M.; Meilan, R. Loosening lignin’s grip on biofuel production. Nat. Biotechnol. 2007, 25, 746–748. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ximenes, E.; Kim, Y.; Slininger, M.; Meilan, R.; Ladisch, M.; Chapple, C. Lignin monomer composition affects Arabidopsis cell-wall degradability after liquid hot water pretreatment. Biotechnol. Biofuels 2010, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Mielenz, J.R.; Xiao, X.; Ge, Y.; Hamilton, C.Y.; Rodriguez, M.; Chen, F.; Foston, M.; Ragauskas, A.; Bouton, J.; et al. Genetic manipulation of lignin reduces recalcitrance and improves ethanol production from switchgrass. Proc. Natl. Acad. Sci. USA 2011, 108, 3803–3808. [Google Scholar] [CrossRef]
- Bonawitz, N.D.; Kim, J.I.; Tobimatsu, Y.; Ciesielski, P.N.; Anderson, N.A.; Ximenes, E.; Maeda, J.; Ralph, J.; Donohoe, B.S.; Ladisch, M.; et al. Disruption of Mediator rescues the stunted growth of a lignin-deficient Arabidopsis mutant. Nature 2014, 509, 376–380. [Google Scholar] [CrossRef]
- Lai, C.; Yang, B.; Lin, Z.; Jia, Y.; Huang, C.; Li, X.; Song, X.; Yong, Q. New strategy to elucidate the positive effects of extractable lignin on enzymatic hydrolysis by quartz crystal microbalance with dissipation. Biotechnol. Biofuels 2019, 12, 1–12. [Google Scholar] [CrossRef]
- Lai, C.; Tu, M.; Li, M.; Yu, S. Remarkable solvent and extractable lignin effects on enzymatic digestibility of organosolv pretreated hardwood. Bioresour. Technol. 2014, 156, 92–99. [Google Scholar] [CrossRef]
- Lai, C.; Yang, B.; He, J.; Huang, C.; Li, X.; Song, X.; Yong, Q. Enhanced enzymatic digestibility of mixed wood sawdust by lignin modification with naphthol derivatives during dilute acid pretreatment. Bioresour. Technol. 2018, 269, 18–24. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Bioresource Technology Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Baxter, H.L.; Mazarei, M.; Labbe, N.; Kline, L.M.; Cheng, Q.; Windham, M.T.; Mann, D.G.J.; Fu, C.; Ziebell, A.; Sykes, R.W.; et al. Two-year field analysis of reduced recalcitrance transgenic switchgrass. Plant. Biotechnol. J. 2014, 12, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Vermerris, W.; Gallo, M.; Fedenko, J.R.; Erickson, J.E.; Altpeter, F. RNA interference suppression of lignin biosynthesis increases fermentable sugar yields for biofuel production from field-grown sugarcane. Plant. Biotechnol. J. 2013, 11, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.K.; Lee, S.M. Advances in cellulosic conversion to fuels: Engineering yeasts for cellulosic bioethanol and biodiesel production. Curr. Opin. Biotechnol. 2018, 50, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dien, B.S.; Ximenes, E.A.; O’Bryan, P.J.; Moniruzzaman, M.; Li, X.L.; Balan, V.; Dale, B.; Cotta, M.A. Enzyme characterization for hydrolysis of AFEX and liquid hot-water pretreated distillers’ grains and their conversion to ethanol. Bioresour. Technol. 2008, 99, 5216–5225. [Google Scholar] [CrossRef]
- Kim, Y.; Mosier, N.S.; Ladisch, M.R. Enzymatic Digestion of Liquid Hot Water Pretreated Hybrid Poplar. Biotechnol. Progr. 2009, 25, 340–348. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Mosier, N.S.; Ladisch, M.R. Severity Factor Coefficients for Subcritical Liquid Hot Water Pretreatment of Hardwood Chips. Biotechnol. Bioeng. 2014, 111, 254–263. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, D.C. Determination of Structural Carbohydrates and Lignin in Biomass Determination of Structural Carbohydrates and Lignin in Biomass; Alliance for Sustainable Energy, LLC: Golden, CO, USA, 2012. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

| Composition (%) | Bagasse High Lignin | Bagasse Low Lignin | ||
|---|---|---|---|---|
| Raw SCB, Untreated | LHW-Pretreated | Raw SCB, Untreated | LHW-Pretreated | |
| Glucan | 37.94 ± 0.2 | 60.14 ± 0.42 | 36.44 ± 0.03 | 63.44 ± 0.03 |
| Xylan | 18.39 ± 0.15 | 8.38 ± 0.19 | 17.91 ± 0.06 | 6.88 ± 0.07 |
| Arabinan | 3.18 ± 0.02 | 2.05 ± 0.01 | 3.21 ± 0.05 | 1.98 ± 0.02 |
| Lignin | 27.2 ± 0.39 | 22.28 ± 0.24 | 26.14 ± 0.04 | 20.55 ± 0.34 |
| Acetyl | 9.84 ± 0.15 | 4.65 ± 0.07 | 9.97 ± 0.19 | 4.53 ± 0.29 |
| Ash | 2.93 ± 0.2 | 3.45 ± 0.12 | 4.29 ± 0.08 | 2.8 ± 0.52 |
| Total | 99.48 ± 0.19 | 100.95 ± 0.18 | 97.96 ± 0.08 | 100.18 ± 0.21 |
| Soluble Inhibitors | Composition of Vacuum Filtrate | |
|---|---|---|
| a Furfural (g/L) | 2.8 ± 0.48 | 2.4 ± 0.74 |
| a Hydroxymethylfurfural (HMF) (g/L) | 0.12 ± 0.02 | 0.11 ± 0.03 |
| a Acetic acid (g/L) | 1.82 ± 0.31 | 1.74 ± 0.39 |
| b Total phenols (mg/L) | 655.76 ± 3.39 | 552.73 ± 6.41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ladeira Ázar, R.I.S.; Bordignon-Junior, S.E.; Laufer, C.; Specht, J.; Ferrier, D.; Kim, D. Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse. Molecules 2020, 25, 623. https://doi.org/10.3390/molecules25030623
Ladeira Ázar RIS, Bordignon-Junior SE, Laufer C, Specht J, Ferrier D, Kim D. Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse. Molecules. 2020; 25(3):623. https://doi.org/10.3390/molecules25030623
Chicago/Turabian StyleLadeira Ázar, Rafaela I. S., Sidnei Emilio Bordignon-Junior, Craig Laufer, Jordan Specht, Drew Ferrier, and Daehwan Kim. 2020. "Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse" Molecules 25, no. 3: 623. https://doi.org/10.3390/molecules25030623
APA StyleLadeira Ázar, R. I. S., Bordignon-Junior, S. E., Laufer, C., Specht, J., Ferrier, D., & Kim, D. (2020). Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse. Molecules, 25(3), 623. https://doi.org/10.3390/molecules25030623







