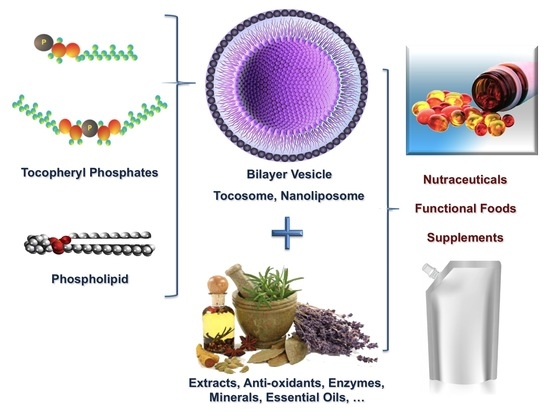
Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules
Abstract
:1. Introduction
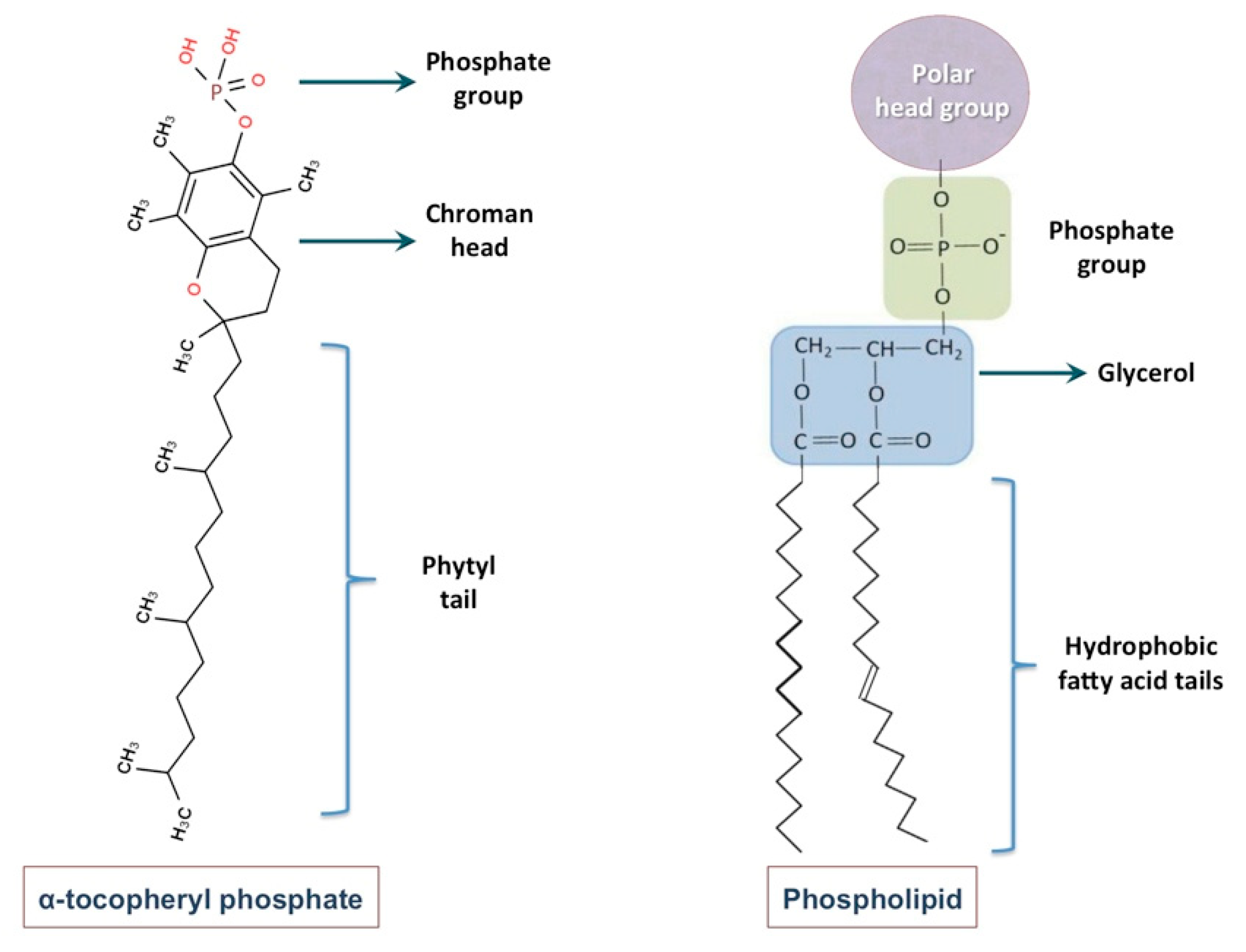
2. Nanoliposome Technology
3. Tocosomes
4. Differences between Tocosomes and Nanoliposomes
5. Mechanism of Formation of Nanoliposomes and Tocosomes
6. Applications in the Food Industry
6.1. Applications in Dairy Products
6.2. Encapsulation of Minerals
6.3. Encapsulation of Antioxidants
6.4. Encapsulation of Food Preservatives
6.5. Flavor and Aroma Encapsulation
6.6. Encapsulation of Essential Fatty Acids and Essential Oils
7. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bratovcic, A.; Suljagic, J. Micro-and nano-encapsulation in food industry. Croat. J. Food Sci. Technol. 2019, 11, 113–121. [Google Scholar] [CrossRef]
- Jafari, S.M. Lipid-Based Nanostructures for Food Encapsulation Purposes (Vol. 2); Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Costa, S.S.; Machado, B.A.S.; Martin, A.R.; Bagnara, F.; Ragadalli, S.A.; Alves, A.R.C. Drying by spray drying in the food industry: Micro-encapsulation, process parameters and main carriers used. Afr. J. Food Sci. 2015, 9, 462–470. [Google Scholar]
- Srivastava, Y.; Semwal, A.D.; Sharma, G.K. Application of various chemical and mechanical microencapsulation techniques in food sector-A. review. Int. J. Food Ferment. Technol. 2013, 3, 1–13. [Google Scholar] [CrossRef]
- Cerqueira, M.A.; Pinheiro, A.C.; Ramos, O.L.; Silva, H.; Bourbon, A.I.; Vicente, A.A. Advances in food nanotechnology. In Emerging Nanotechnologies in Food Science; Busquets, R., Ed.; Elsevier: Oxford, UK, 2017; pp. 11–38. [Google Scholar]
- Yurdugul, S.; Mozafari, M.R. Recent advances in micro-and nanoencapsulation of food ingredients. Cell. Mol. Biol. Lett. 2004, 9, 64–65. [Google Scholar]
- Mozafari, M.R. Commentary: Amphiphiles and their aggregates in basic and applied science. A post-conference thought on nomenclature. Cell. Mol. Biol. Lett. 2005, 10, 733–734. [Google Scholar] [PubMed]
- Mozafari, M.R.; Mortazavi, S.M. Nanoliposomes: From Fundamentals to Recent Developments; Trafford Pub. Ltd.: Oxford, UK, 2005. [Google Scholar]
- Mozafari, M.R.; Javanmard, R.; Raji, M. Tocosome: Novel drug delivery system containing phospholipids and tocopheryl phosphates. Int. J. Pharm. 2017, 528, 381–382. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Khosravi-Darani, K.; Borazan, G.G.; Cui, J.; Pardakhty, A.; Yurdugul, S. Encapsulation of food ingredients using nanoliposome technology. Int. J. Food Prop. 2008, 11, 833–844. [Google Scholar] [CrossRef]
- Khorasani, S.; Danaei, M.; Mozafari, M.R. Nanoliposome technology for the food and nutraceutical industries. Trends Food Sci. Technol. 2018, 79, 106–115. [Google Scholar] [CrossRef]
- Lokhande, S.S. Liposome drug delivery: An update review. Pharma Sci. Monit. 2018, 9, 188–202. [Google Scholar]
- Mozafari, M.R.; Zareie, M.H.; Piskin, E.; Hasirci, V. Formation of supramolecular structures by negatively charged liposomes in the presence of nucleic acids and divalent cations. Drug Deliv. 1998, 5, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Zareie, M.H.; Mozafari, M.R.; Hasirci, V.; Piskin, E. Scanning tunnelling microscopy investigation of liposome-DNA-Ca2+ complexes. J. Liposome Res. 1997, 7, 491–502. [Google Scholar] [CrossRef]
- Arya, M.; Yale University. Compositions and Methods for Treating Metabolic Disorders. U.S. Patent Application 15/781,606, 2017. [Google Scholar]
- Mozafari, M.R.; Omri, A. Importance of divalent cations in nanolipoplex gene delivery. J. Pharm. Sci. 2007, 96, 1955–1966. [Google Scholar] [CrossRef]
- Edris, A.E. Formulation and shelf life stability of water-borne lecithin nanoparticles for potential application in dietary supplements field. J. Diet. Suppl. 2012, 9, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Mozafari, M.R.; Johnson, C.; Hatziantoniou, S.; Demetzos, C. Nanoliposomes and their applications in food nanotechnology. J. Liposome Res. 2008, 18, 309–327. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.J. Pharmaceutical manufacturing of liposomes. Drugs Pharm. Sci. 1990, 41, 267–316. [Google Scholar]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. In Liposomes; Humana Press: Totowa, NJ, USA, 2010; pp. 29–50. [Google Scholar]
- Azzi, A. Tocopheryl phosphate, a novel natural form of vitamin E: In vitro and in vivo studies. FASEB J. 2006, 20, LB79–LB80. [Google Scholar]
- Gianello, R.; Libinaki, R.; Azzi, A.; Gavin, P.D.; Negis, Y.; Zingg, J.M.; West, S.M. α-Tocopheryl phosphate: A novel, natural form of vitamin E. Free Radic. Biol. Med. 2005, 39, 970–976. [Google Scholar] [CrossRef]
- Ogru, E.; Gianello, R.; Libinaki, R.; Smallridge, A.; Bak, R.; Geytenbeek, S.; West, S. Vitamin E phosphate: An endogenous form of vitamin E. In Free Radicals and Oxidative Stress: Chemistry, Biochemistry and Pathophysiological Implications; Galaris, D., Ed.; Medimond Pub. Inc.: Bologna, Italy, 2003; pp. 127–132. [Google Scholar]
- Munteanu, A.; Zingg, J.M.; Ogru, E.; Libinaki, R.; Gianello, R.; West, S.; Azzi, A. Modulation of cell proliferation and gene expression by α-tocopheryl phosphates: Relevance to atherosclerosis and inflammation. Biochem. Biophys. Res. Commun. 2004, 318, 311–316. [Google Scholar] [CrossRef]
- Libinaki, R.; Tesanovic, S.; Heal, A.; Nikolovski, B.; Vinh, A.; Widdop, R.E.; Ogru, E. Effect of tocopheryl phosphate on key biomarkers of inflammation: Implication in the reduction of atherosclerosis progression in a hypercholesterolaemic rabbit model. Clin. Exp. Pharmacol. Physiol. 2010, 37, 587–592. [Google Scholar] [CrossRef]
- Saitoh, Y.; Yumoto, A.; Miwa, N. α-tocopheryl phosphate suppresses tumor invasion concurrently with dynamic morphological changes and delocalization of cortactin from invadopodia. Int. J. Oncol. 2009, 35, 1277–1288. [Google Scholar]
- Nishio, K.; Ishida, N.; Saito, Y.; Ogawa-Akazawa, Y.; Shichiri, M.; Yoshida, Y.; Niki, E. α-Tocopheryl phosphate: Uptake, hydrolysis, and antioxidant action in cultured cells and mouse. Free Radic. Biol. Med. 2011, 50, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Lekli, I.; Das, M.; Azzi, A.; Das, D.K. Cardioprotection with α-tocopheryl phosphate: Amelioration of myocardial ischemia reperfusion injury is linked with its ability to generate a survival signal through Akt activation. Biochim. Biophys. Acta (BBA)–Mol. Basis Dis. 2008, 1782, 498–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Mohammadabadi, M.R.; Mozafari, M.R. Development of nanoliposome-encapsulated thymoquinone: Evaluation of loading efficiency and particle characterization. J. Biopharm. 2019, 11, 39–46. [Google Scholar]
- Aveling, E.; Zhou, J.; Lim, Y.F.; Mozafari, M.R. Targeting lipidic nanocarriers: Current strategies and problems. Pharmakeftiki 2006, 19, 101–109. [Google Scholar]
- Maherani, B.; Arab-Tehrany, E.; Mozafari, M.R.; Gaiani, C.; Linder, M. Liposomes: A review of manufacturing techniques and targeting strategies. Curr. Nanosci. 2011, 7, 436–452. [Google Scholar] [CrossRef]
- Kulkarni, C. Lipid self-assemblies and nanostructured emulsions for cosmetic formulations. Cosmetics 2016, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Lasic, D.D. Mechanisms of liposome formation. J. Liposome Res. 1995, 5, 431–441. [Google Scholar] [CrossRef]
- Lasic, D.D. Novel applications of liposomes. Trends Biotechnol. 1998, 16, 307–321. [Google Scholar] [CrossRef]
- Lasic, D.D.; Walker, S.; Bode, C.J.; Paquet, K.J. Kinetic and thermodynamic effects on the structure and formation of phosphatidylcholine vesicles. Hepatology 1991, 13, 1010–1013. [Google Scholar] [CrossRef] [PubMed]
- Katouzian, I.; Esfanjani, A.F.; Jafari, S.M.; Akhavan, S. Formulation and application of a new generation of lipid nano-carriers for the food bioactive ingredients. Trends Food Sci. Technol. 2017, 68, 14–25. [Google Scholar] [CrossRef]
- Wang, T.; Xue, J.; Hu, Q.; Zhou, M.; Luo, Y. Preparation of lipid nanoparticles with high loading capacity and exceptional gastrointestinal stability for potential oral delivery applications. J. Colloid Interface Sci. 2017, 507, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L. Nanobiotechnology advances in enzymatic biosensors for the agri-food industry. Environ. Chem. Lett. 2017, 15, 555–560. [Google Scholar] [CrossRef]
- Colas, J.C.; Shi, W.; Rao, V.M.; Omri, A.; Mozafari, M.R.; Singh, H. Microscopical investigations of nisin-loaded nanoliposomes prepared by Mozafari method and their bacterial targeting. Micron 2007, 38, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Poudel, A.; Gachumi, G.; Wasan, K.M.; Dallal Bashi, Z.; El-Aneed, A.; Badea, I. Development and Characterization of Liposomal Formulations Containing Phytosterols Extracted from Canola Oil Deodorizer Distillate along with Tocopherols as Food Additives. Pharmaceutics 2019, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.; Patel, R.; Dharamsi, A. A review: Proliposomes as a stable novel drug delivery. World J. Pharm. Res. 2019, 8, 638–651. [Google Scholar]
- Sanguansri, L.; Augustin, M.A. Microencapsulation in functional food product development. Funct. Food Prod. Dev. 2010, 1, 1–23. [Google Scholar]
- Khanniri, E.; Bagheripoor-Fallah, N.; Sohrabvandi, S.; Mortazavian, A.M.; Khosravi-Darani, K.; Mohammad, R. Application of liposomes in some dairy products. Crit. Rev. Food Sci. Nutr. 2016, 56, 484–493. [Google Scholar] [CrossRef]
- Koziolek, M.; Alcaro, S.; Augustijns, P.; Basit, A.W.; Grimm, M.; Hens, B.; Marciani, L. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur. J. Pharm. Sci. 2019, 134, 31–59. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, X.W.; Kong, X.J.; Qin, Z.; Li, S.H.; Jiao, Z.H.; Li, J.Y. An LC–MS/MS method for the quantification of diclofenac sodium in dairy cow plasma and its application in pharmacokinetics studies. Biomed. Chromatogr. 2019, 33, e4520. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Chavan, S.; Jain, U.; Tarwadi, K. Liposomes for Nanodelivery Systems in Food Products. In Nanoscience for Sustainable Agriculture; Springer: Cham, Switzerland, 2019; pp. 627–638. [Google Scholar]
- Law, B.A.; King, J.S. Use of liposomes for proteinase addition to Cheddar cheese. J. Dairy Res. 1985, 52, 183–188. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Park, H.J. Recent developments in microencapsulation of food ingredients. Drying Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Jahadi, M.; Khosravi-Darani, K. Liposomal encapsulation enzymes: From medical applications to kinetic characteristics. Mini Rev. Med. Chem. 2017, 17, 366–370. [Google Scholar] [CrossRef]
- Kheadr, E.E.; Vuillemard, J.C.; El Deeb, S.A. Accelerated Cheddar cheese ripening with encapsulated proteinases. Int. J. Food Sci. Technol. 2020, 35, 483–495. [Google Scholar] [CrossRef]
- Jackson, L.S.; Lee, K. Microencapsulation and the food industry. Lebensm. Wiss. Technol. 1991, 24, 289–297. [Google Scholar]
- Kirby, C. Microencapsulation and controlled delivery of food ingredients. Food Sci. Technol. Today 1991, 5, 74–78. [Google Scholar]
- Alkhalaf, W.; El Soda, M.; Gripon, J.C.; Vassal, L. Acceleration of cheese ripening with liposomes-entrapped proteinase: Influence of liposomes net charge. J. Dairy Sci. 1989, 72, 2233–2238. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mahmoudzadeh, M.; Atefi, M.; Khosravi-Darani, K.; Mozafari, M.R. Applications of nanoliposomes in cheese technology. Int. J. Dairy Technol. 2015, 68, 11–23. [Google Scholar] [CrossRef]
- Merz, M.; Eisele, T.; Berends, P.; Appel, D.; Rabe, S.; Blank, I.; Fischer, L. Flavourzyme, an enzyme preparation with industrial relevance: Automated nine-step purification and partial characterization of eight enzymes. J. Agric. Food Chem. 2015, 63, 5682–5693. [Google Scholar] [CrossRef]
- Jahadi, M.; Khosravi-Darani, K.; Ehsani, M.R.; Mozafari, M.R.; Saboury, A.A.; Pourhosseini, P.S. The encapsulation of flavourzyme in nanoliposome by heating method. J. Food Sci. Technol. 2015, 52, 2063–2072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahadi, M.; Khosravi-Darani, K.; Ehsani, M.R.; Mozafari, M.R.; Saboury, A.A.; Zoghi, A.; Mohammadi, M. Modelling of proteolysis in Iranian brined cheese using proteinase-loaded nanoliposome. Int. J. Dairy Technol. 2016, 69, 57–62. [Google Scholar] [CrossRef]
- Maurya, V.K.; Bashir, K.; Aggarwal, M. Vitamin D microencapsulation and fortification: Trends and technologies. J. Steroid Biochem. Mol. Biol. 2020, 196, 105489. [Google Scholar] [CrossRef] [PubMed]
- Rovoli, M.; Pappas, I.; Lalas, S.; Gortzi, O.; Kontopidis, G. In vitro and in vivo assessment of vitamin A encapsulation in a liposome–protein delivery system. J. Liposome Res. 2019, 29, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Banville, C.; Vuillemard, J.C.; Lacroix, C. Comparison of different methods for fortifying Cheddar cheese with vitamin D. Int. Dairy J. 2000, 10, 375–382. [Google Scholar] [CrossRef]
- Mozafari, M.R.; Flanagan, J.; Matia-Merino, L.; Awati, A.; Omri, A.; Suntres, Z.E.; Singh, H. Recent trends in the lipid-based nanoencapsulation of antioxidants and their role in foods. J. Sci. Food Agric. 2006, 86, 2038–2045. [Google Scholar] [CrossRef]
- Pothakamury, U.; Barbosa-Canovas, G. Fundamental aspects of controlled release in foods. Trends Food Sci. Technol. 1996, 6, 397–406. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Jafari, S.M. The importance of minerals in human nutrition: Bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci. Technol. 2017, 62, 119–132. [Google Scholar] [CrossRef]
- Roohinejad, S.; Greiner, R.; Oey, I.; Wen, J. (Eds.) Emulsion-based Systems for Delivery of Food Active Compounds: Formation, Application, Health and Safety; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Horton, S.; Ross, J. The economics of iron deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Gaucheron, F. Iron fortification in dairy industry. Trends Food Sci. Technol. 2000, 11, 403–409. [Google Scholar] [CrossRef]
- Ziegler, E.E. Consumption of cow’s milk as a cause of iron deficiency in infants and toddlers. Nutr. Rev. 2011, 69, S37–S42. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Sanguansri, L.; Margetts, C.; Young, B. Microencapsulating food ingredients. Food Aust. 2001, 53, 220–223. [Google Scholar]
- El-Salam, M.A.; El-Shibiny, S.; Mehanna, N. Iron fortification of milk and dairy products. Egypt. J. Dairy Sci. 2017, 45, 1–16. [Google Scholar]
- Arnaud, J.P. Pro-liposomes for the food industry. Food Technol. Eur. 1995, 2, 30–34. [Google Scholar]
- Simiqueli, A.A.; Lima Filho, T.; Minim, L.A.; de Oliveira, E.B.; Torres, I.V.; Vidigal, M.C.T.R.; Minim, V.P.R. The W/O/W emulsion containing FeSO4 in the different phases alters the hedonic thresholds in milk-based dessert. LWT 2019, 99, 98–104. [Google Scholar] [CrossRef]
- Donsi, F.; Velikov, K.P. Encapsulation of food ingredients by single O/W and W/O nanoemulsions. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 37–87. [Google Scholar]
- Xia, S.; Xu, S. Ferrous sulfate liposomes: Preparation, stability and application in fluid milk. Food Res. Int. 2005, 38, 289–296. [Google Scholar] [CrossRef]
- Bonnet, M.; Cansell, M.; Berkaoui, A.; Ropers, M.H.; Anton, M.; Leal-Calderon, F. Release rate profiles of magnesium from multiple W/O/W emulsions. Food Hydrocolloids 2009, 23, 92–101. [Google Scholar] [CrossRef]
- Shahidi, F. (Ed.) Handbook of Antioxidants for Food Preservation; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- Gutteridge, J.M.; Halliwell, B. Free radicals and antioxidants in the year 2000: A historical look to the future. Ann. N. Y. Acad. Sci. 2000, 899, 136–147. [Google Scholar] [CrossRef]
- Gordon, M.H. The development of oxidative rancidity in foods. In Antioxidants in Food; Woodhead Publishing: Cambridge, UK, 2011; pp. 7–21. [Google Scholar]
- Stahl, W.; Junghans, A.; de Boer, B.; Driomina, E.S.; Briviba, K.; Sies, H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: Synergistic effects of lycopene and lutein. FEBS Lett. 1998, 427, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Ultrastructural architecture of liposome-entrapped glutathione: A cryo-SEM study. Cell. Mol. Biol. Lett. 2004, 9, 101–103. [Google Scholar]
- Suntres, Z.E.; Shek, P.N. Alleviation of paraquat-induced lung injury by pretreatment with bifunctional liposomes containing α-tocopherol and glutathione. Biochem. Pharmacol. 1996, 52, 1515–1520. [Google Scholar] [CrossRef]
- Marsanasco, M.; Marquez, A.L.; Wagner, J.R.; Alonso, S.D.V.; Chiaramoni, N.S. Liposomes as vehicles for vitamins E and C: An alternative to fortify orange juice and offer vitamin C protection after heat treatment. Food Res. Int. 2011, 44, 3039–3046. [Google Scholar] [CrossRef]
- Chatzidaki, M.D.; Papadimitriou, V.; Xenakis, A. Encapsulation of food ingredients by microemulsions. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 129–149. [Google Scholar]
- Chatzidaki, M.D.; Mitsou, E.; Yaghmur, A.; Xenakis, A.; Papadimitriou, V. Formulation and characterization of food-grade microemulsions as carriers of natural phenolic antioxidants. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 130–136. [Google Scholar] [CrossRef]
- Laguerre, M.; Lopez-Giraldo, L.J.; Lecomte, J.; Barea, B.; Cambon, E.; Tchobo, P.F.; Villeneuve, P. Conjugated autoxidizable triene (CAT) assay: A novel spectrophotometric method for determination of antioxidant capacity using triacylglycerol as ultraviolet probe. Anal. Biochem. 2008, 380, 282–290. [Google Scholar] [CrossRef]
- Panya, A.; Temthawee, W.; Phonsatta, N.; Charoensuk, D.; Deetae, P.; Visessanguan, W.; Decker, E.A. Apolar radical initiated conjugated autoxidizable triene (ApoCAT) assay: Effects of oxidant locations on antioxidant capacities and interactions. J. Agric. Food Chem. 2015, 63, 7546–7555. [Google Scholar] [CrossRef] [PubMed]
- Phonsatta, N.; Deetae, P.; Luangpituksa, P.; Grajeda-Iglesias, C.; Figueroa-Espinoza, M.C.; Le Comte, J.; Panya, A. Comparison of antioxidant evaluation assays for investigating antioxidative activity of gallic acid and its alkyl esters in different food matrices. J. Agric. Food Chem. 2017, 65, 7509–7518. [Google Scholar] [CrossRef] [PubMed]
- Artiga-Artigas, M.; Guerra-Rosas, M.I.; Morales-Castro, J.; Salvia-Trujillo, L.; Martin-Belloso, O. Influence of essential oils and pectin on nanoemulsion formulation: A ternary phase experimental approach. Food Hydrocolloids 2018, 81, 209–219. [Google Scholar] [CrossRef] [Green Version]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Karimian, M.S.; Majeed, M.; Sahebkar, A. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: A randomized controlled trial. Inflammopharmacology 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Donsi, F.; Ferrari, G. Essential oil nanoemulsions as antimicrobial agents in food. J. Biotechnol. 2016, 233, 106–120. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic antimicrobial potential of essential oils in combination with nanoparticles: Emerging trends and future perspectives. Inter. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ghaderi-Ghahfarokhi, M.; Barzegar, M.; Sahari, M.A.; Azizi, M.H. Nanoencapsulation approach to improve antimicrobial and antioxidant activity of thyme essential oil in beef burgers during refrigerated storage. Food Bioprocess. Technol. 2016, 9, 1187–1201. [Google Scholar] [CrossRef]
- Lopes, N.A.; Brandelli, A. Nanostructures for delivery of natural antimicrobials in food. Crit. Rev. Food Sci. Nut. 2018, 58, 2202–2212. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Ung, P.; Uemura, K.; Takahashi, C.; Kobayashi, I.; Romano, P.; Nakajima, M. Antimicrobial oil-in-water nanoemulsions: Synergistic effect of nisin and carvacrol against Bacillus subtilis. J. Food Sci. Eng. 2016, 6, 63–74. [Google Scholar]
- Thomas, V.M.; Brown, R.M.; Ashcraft, D.S.; Pankey, G.A. Synergistic effect between nisin and polymyxin B against pandrug-resistant and extensively drug-resistant Acinetobacter baumannii. Int. J. Antimicrobial Agents 2019, 53, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Sarabandi, K.; Rafiee, Z.; Khodaei, D.; Jafari, S.M. Encapsulation of food ingredients by nanoliposomes. In Lipid-Based Nanostructures for Food Encapsulation Purposes; Academic Press: Cambridge, MA, USA, 2019; pp. 347–404. [Google Scholar]
- Prombutara, P.; Kulwatthanasal, Y.; Supaka, N.; Sramala, I.; Chareonpornwattana, S. Production of nisin-loaded solid lipid nanoparticles for sustained antimicrobial activity. Food Control. 2012, 24, 184–190. [Google Scholar] [CrossRef]
- Da Silva Malheiros, P.; Daroit, D.J.; Brandelli, A. Food applications of liposome-encapsulated antimicrobial peptides. Trends Food Sci. Technol. 2010, 21, 284–292. [Google Scholar] [CrossRef]
- Bouksaim, M.; Lacroix, C.; Audet, P.; Simard, R.E. Effects of mixed starter composition on nisin Z production by Lactococcus lactis subsp. lactis biovar. diacetylactis UL 719 during production and ripening of Gouda cheese. Int. J. Food Microbiol. 2000, 59, 141–156. [Google Scholar] [CrossRef]
- Assadpour, E.; Jafari, S.M. A systematic review on nanoencapsulation of food bioactive ingredients and nutraceuticals by various nanocarriers. Crit. Rev. Food Sci. Nutr. 2018, 1–23. [Google Scholar] [CrossRef]
- Da Silva Malheiros, P.; Sant’Anna, V.; de Souza Barbosa, M.; Brandelli, A.; de Melo Franco, B.D.G. Effect of liposome-encapsulated nisin and bacteriocin-like substance P34 on Listeria monocytogenes growth in Minas frescal cheese. Int. J. Food Microbiol. 2012, 156, 272–277. [Google Scholar] [CrossRef]
- Pinilla, C.M.B.; Brandelli, A. Antimicrobial activity of nanoliposomes co-encapsulating nisin and garlic extract against Gram-positive and Gram-negative bacteria in milk. Innovative Food Sci. Emerg. Technol. 2016, 36, 287–293. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control. 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, H.; Lin, L. The specific antibacterial effect of the Salvia oil nanoliposomes against Staphylococcus aureus biofilms on milk container. Food Control. 2016, 61, 92–98. [Google Scholar] [CrossRef]
- Peng, S.; Zou, L.; Liu, W.E.I.; Gan, L.U.; Liu, W.; Liang, R.; Chen, X. Storage stability and antibacterial activity of eugenol nanoliposomes prepared by an ethanol injection–dynamic high-pressure microfluidization method. J. Food Prot. 2015, 78, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Khatibi, S.A.; Misaghi, A.; Moosavy, M.H.; Akhondzadeh Basti, A.; Mohamadian, S.; Khanjari, A. Effect of nanoliposomes containing Zataria multiflora Boiss. essential oil on gene expression of Shiga toxin 2 in Escherichia coli O157: H7. J. Applied Microbiol. 2018, 124, 389–397. [Google Scholar] [CrossRef]
- Best, D. Ingredient trends alert. Food Process. 2000, 2000, 57–62. [Google Scholar]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Nanoencapsulation of d-limonene within nanocarriers produced by pectin-whey protein complexes. Food Hydrocolloids 2018, 77, 152–162. [Google Scholar] [CrossRef]
- Zuidam, N.J.; Heinrich, E. Encapsulation of aroma. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Springer: New York, NY, USA, 2010; pp. 127–160. [Google Scholar]
- Trifkovic, K.; Dordevic, V.; Balanc, B.; Kalusevic, A.; Levic, S.; Bugarski, B.; Nedovic, V. Novel approaches in nanoencapsulation of aromas and flavors. In Encapsulations; Academic Press: Cambridge, MA, USA, 2016; pp. 363–419. [Google Scholar]
- Van Nieuwenhuyzen, W.; Szuhaj, B. Effects of lecithins and proteins on the stability of emulsions. Lipid/Fett 1998, 100, 282–291. [Google Scholar] [CrossRef]
- Kaddah, S.; Khreich, N.; Kaddah, F.; Charcosset, C.; Greige-Gerges, H. Cholesterol modulates the liposome membrane fluidity and permeability for a hydrophilic molecule. Food Chem. Toxicol. 2018, 113, 40–48. [Google Scholar] [CrossRef]
- Peschka, R.; Dennehy, C.; Szoka Jr, F.C. A simple in vitro model to study the release kinetics of liposome encapsulated material. J. Control. Release 1998, 56, 41–51. [Google Scholar] [CrossRef]
- Mehrad, B.; Shabanpour, B.; Jafari, S.M.; Pourashouri, P. Characterization of dried fish oil from Menhaden encapsulated by spray drying. Aquac. Aquar. Conserv. Legis. 2015, 8, 57–69. [Google Scholar]
- Di Pasquale, M.G. The essentials of essential fatty acids. J. Dietary Suppl. 2009, 6, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Plourde, M.; Cunnane, S.C. Extremely limited synthesis of long chain polyunsaturates in adults: Implications for their dietary essentiality and use as supplements. Applied Physiol. Nutr. Metab. 2007, 32, 619–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, U.N. Essential fatty acids-a review. Current Pharm. Biotechnol. 2006, 7, 467–482. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Nahvi, Z.; Zandi, M. Antioxidant peptide-loaded electrospun chitosan/poly (vinyl alcohol) nanofibrous mat intended for food biopackaging purposes. Food Hydrocolloids 2019, 89, 637–648. [Google Scholar] [CrossRef]
- Cantwell, M.M.; Flynn, M.A.T.; Cronin, D.; O’Neill, J.P.; Gibney, M.J. Contribution of foods to trans unsaturated fatty acid intake in a group of Irish adults. J. Human Nutr. Dietetics 2005, 18, 377–385. [Google Scholar] [CrossRef]
- Lopez-Garcia, E.; Schulze, M.B.; Meigs, J.B.; Manson, J.E.; Rifai, N.; Stampfer, M.J.; Hu, F.B. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J. Nutr. 2005, 135, 562–566. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Esfahani, R.; Jafari, S.M.; Jafarpour, A.; Dehnad, D. Loading of fish oil into nanocarriers prepared through gelatin-gum Arabic complexation. Food Hydrocoll. 2019, 90, 291–298. [Google Scholar] [CrossRef]
- Jafari, S.M. (Ed.) Nanoencapsulation of Food Bioactive Ingredients: Principles and Applications; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Ojagh, S.M.; Hasani, S. Characteristics and oxidative stability of fish oil nano-liposomes and its application in functional bread. J. Food Meas. Charact. 2018, 12, 1084–1092. [Google Scholar] [CrossRef]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Yazid, A.M. Comparative study of the oxidative and physical stability of liposomal and nanoliposomal polyunsaturated fatty acids prepared with conventional and Mozafari methods. Food Chem. 2012, 135, 2761–2770. [Google Scholar] [CrossRef] [PubMed]
- Ghorbanzade, T.; Jafari, S.M.; Akhavan, S.; Hadavi, R. Nano-encapsulation of fish oil in nano-liposomes and its application in fortification of yogurt. Food Chem. 2017, 216, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Rasti, B.; Jinap, S.; Mozafari, M.R.; Abd-Manap, M.Y. Optimization on preparation condition of polyunsaturated fatty acids nanoliposome prepared by Mozafari method. J. Liposome Res. 2014, 24, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Sahari, M.A.; Moghimi, H.R.; Hadian, Z.; Barzegar, M.; Mohammadi, A. Improved physical stability of docosahexaenoic acid and eicosapentaenoic acid encapsulated using nanoliposome containing α-tocopherol. Int. Food Sci. Technol. 2016, 51, 1075–1086. [Google Scholar] [CrossRef]
- Hadian, Z.; Sahari, M.A.; Moghimi, H.R.; Barzegar, M. Formulation, characterization and optimization of liposomes containing eicosapentaenoic and docosahexaenoic acids; a methodology approach. Iranian J. Pharm. Res. IJPR 2014, 13, 393. [Google Scholar]
- Kubo, K.; Sekine, S.; Saito, M. Docosahexaenoic acid-containing phosphatidylethanolamine in the external layer of liposomes protects docosahexaenoic acid from 2, 2′-azobis (2-aminopropane) dihydrochloride-mediated lipid peroxidation. Arch. Biochem. Biophys. 2003, 410, 141–148. [Google Scholar] [CrossRef]
- Rasti, B.; Erfanian, A.; Selamat, J. Novel nanoliposomal encapsulated omega-3 fatty acids and their applications in food. Food Chem. 2017, 230, 690–696. [Google Scholar] [CrossRef]
- Yaghmur, A.; Tran, B.V.; Moghimi, S.M. Non-lamellar liquid crystalline nanocarriers for thymoquinone encapsulation. Molecules 2020, 25, 16. [Google Scholar] [CrossRef] [Green Version]
- Azmi, I.D.; Wibroe, P.P.; Wu, L.P.; Kazem, A.I.; Amenitsch, H.; Moghimi, S.M.; Yaghmur, A. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. J. Controlled Release 2016, 239, 1–9. [Google Scholar] [CrossRef]




| Method | Advantages | Disadvantages |
|---|---|---|
| Thin-film hydration method | High solubility of ingredients in the initial stage of the process | Use of potentially toxic solvents, time consuming, difficult to scale-up |
| Ethanol/ether injection | Simple procedure | Organic solvent residue, nozzle blockage in ether system, time consuming, sterilization issue |
| Reverse phase evaporation | Simple design, acceptable encapsulation efficiency | Not suitable for the encapsulation of sensitive material due to large quantity of organic solvent use, time consuming, sterilization issue |
| Microfluidisation | Control of particle size, large volume manufacture in a continuous and reproducible manner | Employment of high pressures (up to l0,000 psi) |
| Supercritical Fluid Process (SFP) | Control of particle size, possibility of in situ sterilization, low organic solvent consumption | High cost, low yield, high pressure up to 350 bar used |
| Dual asymmetric centrifugation | Simple method, yields products with narrow size distribution, high encapsulation efficiency | Not suitable for bulk production, high pressure and high shear force |
| Sonication | Simple and fast technique | Overheating of the sample causing degradation, sonicator tips releases metal particles into the product |
| Heating Method | Organic solvent free, scalable | High temperature requirement |
| Mozafari Method | Simple design, safe and mild procedure, organic solvent free, easily scalable | New method, Reproducibility need to be attested under different conditions |
| Binary Nanodispersions | Organic solvent free, not requiring secondary emulsifier | Requires ultrasonication |
| Formulation/Method | Targeted Microorganisms | Encapsulated Antimicrobial |
|---|---|---|
| Dynamic high-pressure microfluidization | L. monocytogenes, S. aureus | Eugenol |
| Ethanol injection | Escherichia coli | Garlic essential oil |
| Freeze-thaw method | Microsporum gypseum, Microsporum canis, Trichophyton verrucosum, Trichophyton rubrum | Essential oil of Eucalyptus camaldulensis leaf |
| Mozafari method | Bacillus subtilis, Pseudomonas aeruginosa | Nisin |
| Proliposome | L. monocytogenes | Nisin |
| Sonication | L. monocytogenes, E. coli, Penicillium chrysogenum, Botrytis cinerea | d-limonene |
| Thin-film hydration method | S. aureus, E. coli | Clove oil |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarrabi, A.; Alipoor Amro Abadi, M.; Khorasani, S.; Mohammadabadi, M.-R.; Jamshidi, A.; Torkaman, S.; Taghavi, E.; Mozafari, M.R.; Rasti, B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules 2020, 25, 638. https://doi.org/10.3390/molecules25030638
Zarrabi A, Alipoor Amro Abadi M, Khorasani S, Mohammadabadi M-R, Jamshidi A, Torkaman S, Taghavi E, Mozafari MR, Rasti B. Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules. 2020; 25(3):638. https://doi.org/10.3390/molecules25030638
Chicago/Turabian StyleZarrabi, Ali, Mandana Alipoor Amro Abadi, Sepideh Khorasani, M.-Reza Mohammadabadi, Aniseh Jamshidi, Sarabanou Torkaman, Elham Taghavi, M.R. Mozafari, and Babak Rasti. 2020. "Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules" Molecules 25, no. 3: 638. https://doi.org/10.3390/molecules25030638
APA StyleZarrabi, A., Alipoor Amro Abadi, M., Khorasani, S., Mohammadabadi, M.-R., Jamshidi, A., Torkaman, S., Taghavi, E., Mozafari, M. R., & Rasti, B. (2020). Nanoliposomes and Tocosomes as Multifunctional Nanocarriers for the Encapsulation of Nutraceutical and Dietary Molecules. Molecules, 25(3), 638. https://doi.org/10.3390/molecules25030638